In this issue of Blood, Zheng and colleagues demonstrate that the Notch signaling pathway is active in lymphatic endothelial cells and that inhibition of Notch signaling, in concert with vascular endothelial growth factor (VEGF) stimulation, promotes lymphatic endothelial cell sprouting.1 This work suggests that Notch signaling plays a key role in regulating the switch between lymphatic endothelial cell quiescence and sprouting, akin to the role played by Notch signaling during angiogenic sprouting of the blood vasculature, and has important implications for therapeutic targeting of the Notch pathway as a treatment for vascular disorders and cancer.
Notch signaling plays a variety of key roles in vascular development and physiologic and pathologic angiogenesis (the sprouting growth of blood vessels).2,3 The role of Notch signaling in lymphangiogenesis is just beginning to be explored; conflicting results in various model systems have made it difficult to ascribe a definitive role to Notch pathway activity in lymphatic endothelial cells. Recent work in zebrafish suggested that Notch signaling is important for the initial sprouting of lymphangioblasts from the axial vein; ablating Notch activity via a number of approaches interrupted formation of the thoracic duct.4 Moreover, work performed in that study suggested that after lymphatic endothelial cell fate specification, Notch signaling was important for guiding the navigation of lymphatic vessels along the intersomitic arteries.4
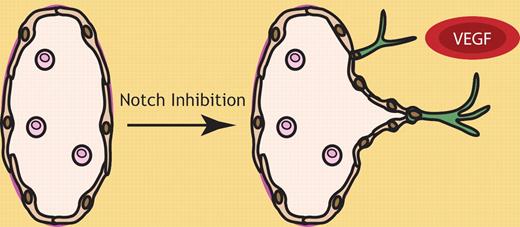
Notch inhibition, in concert with VEGF stimulation, promotes lymphatic vessel sprouting. Notch signaling regulates quiescence of the lymphatic vasculature. On exposure to lymphangiogenic stimuli including VEGF and VEGF-C, Dll4 levels are elevated in lymphatic endothelial tip cells (green), presumably activating Notch signaling in neighboring lymphatic endothelial stalk cells and thereby preventing their sprouting. Notch pathway inhibition promotes lymphatic vascular sprouting induced by VEGF.
Notch inhibition, in concert with VEGF stimulation, promotes lymphatic vessel sprouting. Notch signaling regulates quiescence of the lymphatic vasculature. On exposure to lymphangiogenic stimuli including VEGF and VEGF-C, Dll4 levels are elevated in lymphatic endothelial tip cells (green), presumably activating Notch signaling in neighboring lymphatic endothelial stalk cells and thereby preventing their sprouting. Notch pathway inhibition promotes lymphatic vascular sprouting induced by VEGF.
In contrast to the study by Guedens et al,4 work in a mouse model demonstrated that Notch signaling is not required for the specification of Prox1-positive lymphatic endothelial progenitor cells in the embryonic cardinal veins.5 Srinivasan and colleagues inactivated Rbpj, a major transcriptional effector of Notch signaling, specifically in endothelial cells. While few embryos survived to embryonic day 10.5, reflecting the fact that Notch is required for early aspects of vascular development, the specification of lymphatic endothelial cells in the embryonic cardinal veins was not impeded. In this model, embryonic lethality precluded the analysis of the role of Notch signaling in later stages of lymphatic vascular development.
In the article by Zheng et al, the use of novel human lymphatic endothelial cell sprouting assays, together with a mouse model of in vivo sprouting lymphangiogenesis, has revealed a role for Notch signaling in maintaining quiescence of the lymphatic vasculature by suppressing lymphatic endothelial cell sprouting.1 Inhibition of Notch signaling using a soluble Dll4-Fc protein, or a chemical inhibitor of Notch cleavage and activation, promoted lymphatic vascular sprouting induced by VEGF in vitro and in vivo (see figure). Interestingly, in an in vitro sprouting assay, Notch inhibition selectively promoted sprouting induced by VEGF and low doses of VEGF-C, but at high doses of VEGF-C, lymphatic vascular sprouting was not further increased by Notch pathway inhibition. This observation goes some way toward explaining the enhanced effects of VEGF-C compared with VEGF in the promotion of lymphangiogenesis, although additional factors such as cell surface VEGFR-3 levels on lymphatic endothelial cells, together with the levels of other factors involved in Notch, VEGF and VEGF-C signal transduction may contribute to this effect. An intriguing finding of this work is that VEGFR-2 inhibition in vitro, using either a blocking antibody or a soluble receptor acting as a VEGF trap, ablated lymphatic endothelial cell sprouting induced by Dll4-Fc, while VEGFR-3 inhibition did not.1
Lymphatic vascular sprouting induced by Notch inhibition in vivo was prevented by blockade of VEGFR-2 or VEGFR-3 signaling using soluble VEGF and VEGF-C traps, implying that signals transduced via VEGFR-2 and VEGFR-3 are important for D114-stimulated lymphatic sprouting in the tissue environment. VEGF treatment has previously been shown to promote lymphatic endothelial cell proliferation but not sprouting, suggesting that the signaling pathways activated downstream of VEGFR-2 are likely to be modulated by Notch pathway activity. The cross-talk between VEGFR-2 and VEGFR-3 signaling pathways and the increased complexity of the in vivo environment could also potentially contribute to these differences.
Finally, Zheng and colleagues demonstrate that lymphatic endothelial cells with high levels of DLL4 won the competition for tip cell position, suggesting that, akin to angiogenic blood vascular sprouting, Notch signaling regulates tip and stalk cell identity/selection.1 Previous work by Shawber and colleagues demonstrated that Notch signaling was active in primary lymphatic endothelial cells and in lymphatic vessels in vivo, and that Notch signaling increased VEGFR-3 levels in endothelial cells, making them more responsive to VEGF-C.6 In contrast, the work performed by Zheng et al did not find any alterations in VEGFR-2 or VEGFR-3 mRNA or protein levels after Notch inhibition in lymphatic endothelial cells,1 although treatment with VEGF or VEGF-C resulted in increased levels of DLL4 and the Notch target genes HES1, HEY1, and NRARP. These findings suggest a mechanism by which tip cell identity could be induced in response to lymphangiogenic stimuli.
Why don't high Dll4 levels in tip cells activate Notch signaling in an autocrine manner? Does signaling by Jagged ligands impact on lymphangiogenesis and the choice between sprouting and quiescence? How does Notch signaling regulate VEGFR-2/VEGFR-3 signal transduction? What other players are involved in the Notch/VEGFR signaling complex in lymphatic endothelial cells? Answers to these questions will further our understanding of the role of Notch signaling in lymphangiogenesis. The conditional inactivation of Notch pathway components in a temporal and spatial context in mouse models will be vital in determining the precise role of Notch signaling in lymphatic vascular development in vivo and is no doubt the focus of studies currently in progress. This work by Zheng and colleagues provides novel and fundamental information regarding the role of Notch signaling in lymphatic endothelial cells and has important implications for targeting the Notch pathway as a means to treat cancer; Notch inhibition could potentially promote tumor lymphangiogenesis and metastasis. The selective targeting of Notch pathway inhibitors to blood vessels or lymphatic vessels may prove a useful rationale to generate more effective cancer therapeutics.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal