Abstract
MicroRNAs (miRNAs) are pivotal for regulation of hematopoiesis but their critical targets remain largely unknown. Here, we show that ectopic expression of miR-17, -20,-93 and -106, all AAAGUGC seed-containing miRNAs, increases proliferation, colony outgrowth and replating capacity of myeloid progenitors and results in enhanced P-ERK levels. We found that these miRNAs are endogenously and abundantly expressed in myeloid progenitors and down-regulated in mature neutrophils. Quantitative proteomics identified sequestosome 1 (SQSTM1), an ubiquitin-binding protein and regulator of autophagy-mediated protein degradation, as a major target for these miRNAs in myeloid progenitors. In addition, we found increased expression of Sqstm1 transcripts during CSF3-induced neutrophil differentiation of 32D-CSF3R cells and an inverse correlation of SQSTM1 protein levels and miR-106 expression in AML samples. ShRNA-mediated silencing of Sqstm1 phenocopied the effects of ectopic miR-17/20/93/106 expression in hematopoietic progenitors in vitro and in mice. Further, SQSTM1 binds to the ligand-activated colony-stimulating factor 3 receptor (CSF3R) mainly in the late endosomal compartment, but not in LC3 positive autophagosomes. SQSTM1 regulates CSF3R stability and ligand-induced mitogen-activated protein kinase signaling. We demonstrate that AAAGUGC seed-containing miRNAs promote cell expansion, replating capacity and signaling in hematopoietic cells by interference with SQSTM1-regulated pathways.
Introduction
MiRNAs are transcribed as long primary transcripts that are processed by RNaseIII endonucleases DROSHA and DICER into single-stranded RNAs of ∼ 22nt.1 The nucleotides 2-7 at the 5′-end of miRNAs, referred to as the miRNA seed region, are important for miRNA target recognition.2 MiRNAs regulate gene expression by pairing with the seed complementary sequences in the 3′ untranslated region (UTR) of mRNAs. Most mammalian miRNAs both repress translation and enhance decay of their target transcript.3,4 MiRNAs containing homologous seeds such as, for example, the Let-7 family of miRNAs, are believed to regulate the same targets.2
Involvement of miRNAs in hematopoiesis is strongly suggested by the position of miRNA genes near translocation breakpoints and by their presence in loci targeted for deletion in human leukemias.5 Furthermore, expression profiling data suggest a major role for miRNAs in regulation of hematopoietic cell commitment, proliferation, apoptosis, survival, and differentiation.6-9 The importance of miRNAs during hematopoiesis has been shown by disruption of miRNA biogenesis in mice. For instance, Dicer-deleted hematopoietic stem cells are unable to reconstitute the hematopoietic system.10 Further, conditional deletion of Dicer in T and B cells results in strong reduction of lymphocytes and diminished cell survival and functions.11-13 Argonaute-2 knock-out in hematopoiesis results in impaired differentiation of B-lymphocytes and erythroid cells.14,15
MiRNAs can be expressed in a cell type or tissue specific manner. For instance, miR-223 and miR-142 are almost exclusively expressed in hematopoietic cells.16 MiR-223 is transcriptionally controlled by CCAAT/enhancer-binding protein α (CEBPA) and suppresses the myeloid transcription factor MEF2C, a major regulator of progenitor cell proliferation and granulocyte specific functions.17,18 In addition, specific miRNAs control cellular processes important for proliferation, survival, cytokine production and cell lineage decisions of developing T and B cells.8,12 In hematopoietic stem cells, sustained expression of miR-155 causes a myeloproliferative disorder in mice.19 Furthermore, forced miR-29a in hematopoietic precursors induces aberrant self-renewal and acute myeloid leukemia by still unidentified mechanisms.20 These examples illustrate the role of miRNAs as regulators of critical pathways determining normal hematopoietic cell fate and differentiation. However, there is a lack of data concerning the mRNA targets of miRNAs that are expressed in hematopoiesis.
Here, we have used a newly developed barcoded miRNA expression library to screen for miRNAs that control normal granulopoiesis and found that AAAGUGC seed-containing miRNAs potently enhance expansion of myeloid 32D cells and primary hematopoietic progenitors. Using quantitative proteomics, we identified Sequestosome 1 (Sqstm1) as a prominent target of the AAAGUGC seed-containing miRNAs in myeloid cells. We show that SQSTM1 controls myeloid cell expansion and replating capacity, mitogen-activated protein (MAP) kinase activity and CSF3R stability.
Methods
Generation of a barcoded retroviral expression vector (MSCV-BC-miRNA)
The MSCV-BC constructs were generated using standard molecular biology techniques. Maps, sequences and cloning information are available on the Hynes laboratory web site. Ninety-six unique BC sequences were cloned in the MSCV vector, which are anti-sense to the sequences coupled to xMAP beads (Luminex). MiRNA plus ∼ 250 flanking sequences were amplified by PCR and cloned in the MSCV-BC vector.
Cell culture and gene transfer
The IL-3–dependent murine myeloid cell lines 32D and Ba/F3 containing the human wild-type CSF3R were expanded, differentiated, and analyzed as described.21 Stem cells were expanded as described.22 The Angiopoietin-like–2 vector was a kind gift from Cheng Cheng Zhang (Whitehead Institute, Cambridge, MA) and the RAB7-GFP was a gift of Peter van der Sluijs (Department of Cell Biology, University Medical Center Utrecht, The Netherlands).23 MSCV virus particles were generated as described.24 The 32D cells, Ba/F3 cells, and BM-derived progenitors and hematopoietic stem cells were infected with pMSCV-BC-miRNA virus using retroNectin (Takara Bio Inc) according to the manufacturer's instruction and selected for GFP expression with FACSaria cell sorter (Becton Dickinson). Hek293 and HeLa cells were grown in DMEM supplemented with FCS (10%) and under standard conditions. For SQSTM1 knockdown SQSTM1 ON-targetplus smartpool siRNAs, SiGLO-CyclophilinB, and control siRNA pool (Dharmacon), at a final concentration of 10nM, were transfected into HeLa cells with Dharmafect-I (Dharmacon) according to the manufacturer's protocols.
Luminex experiments
Sorted MSCV-BC-miRNA 32D cell populations were mixed with empty MSCV-BC control cells in a 1:1 ratio and switched to CSF3-containing medium. Genomic DNA was isolated at different time points and BC sequences were amplified by PCR with primers: reverse primer 5′-Bio-CAGAGAACTATCATTGCATATACAC-3′ and forward primer 5′-CTAATACGACTCACTATAGGGA-GAACGC-3′, labeled with streptavidin-Phycoerythrin (2 μg/mL) and analyzed on a Luminex machine according to the manufacturer's instruction (Luminex).
Colony assays and competitive reconstitution in mice
Lineage negative hematopoietic cells were harvested from the femurs and tibiae of 8- to 12-week-old C57BL/6 mice (The Jackson Laboratory) as described.16 For the colony assays, 104 MSCV transduced BM cells were plated in triplicate in methocult (M3231; StemCell Technologies), either supplemented with human CSF3 (100 ng/mL) and puromycin (1.5 μg/mL) for CFU-G assay or IL-6 (10 ng/mL), IL-3 (supernatant 1/1000), SCF (10 ng/mL), GM-CSF (10 ng/mL and puromycin (1.5 μg/mL) for replating assays. For competitive reconstitution experiments, 8- to 10-week-old recipient mice were lethally irradiated with 8.5 Gy and tail vein injected with 1-5 × 105 transduced Lin− cells. Peripheral blood was obtained by tail and total BM was analyzed as described.25 All animal experiments were approved by the Animal Experimental Farewal Committee.
Luciferase experiments
The wild-type and mutant full-length human Sqstm1 3′UTRs were cloned behind the Firefly Luciferase gene of the pGL3-Promoter vector (Promega). HEK293 cells were transfected with pGL3-SQSTM1 3′UTR and Renilla control vectors with Fugene6 transfection reagent (Roche). For dual Luciferase reporter assay, cells were lysed and analyzed according to the manufacturer's instruction (Promega) with a VICTOR multilabel counter.
Real-time quantitative PCR
Human AML cells were obtained after informed consent was granted and were purified as previously described.26 BM samples were collected from healthy individuals following the declaration of Helsinki principles. Different stages of neutrophil differentiation, that is, myeloblasts/promyelocytes, metamyelocytes, and neutrophils, were FACS-sorted using cell type-specific markers,27 that is, CD10-APC, CD11b-APC-Cy7, CD34-Pe-Cy7, CD45-PerCP, CD117-PE (Becton Dickinson), and CD36-FITC (Beckman Coulter). The purity of sorted samples was determined by immunophenotyping and morphology of the cytospins, stained with May-Grünwald-Giemsa. Total RNA was extracted and miRNAs were detected as described.28
For details on quantitative mass spectrometry (SILAC samples), mass spectrometric analysis (Bio-CSF3 pull-down samples), Western blotting, and confocal and spinning-disk live cell imaging, see supplemental Methods (available on Blood Web site; see the Supplemental Materials link at the top of the online article)
Results
Ectopic expression of miR-292 or miR-93 interferes with CSF3-induced myelopoiesis
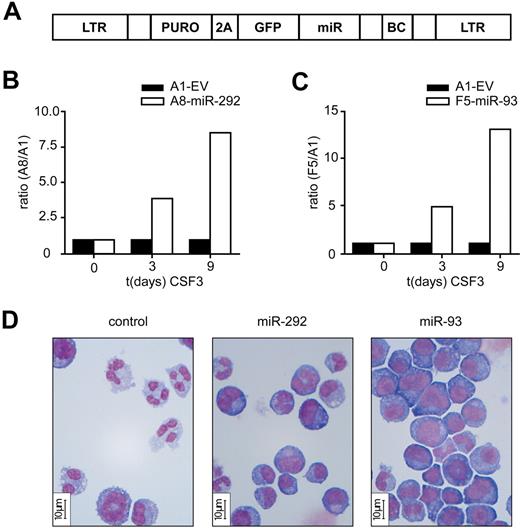
We developed a barcoded (BC) retroviral (Murine Stem Cell Virus, MSCV) miRNA expression library, MSCV-BC-miRNA, that allows for functional analysis of miRNAs on a large scale (Figure 1A, supplemental Table 1). We have previously developed 32D clones expressing human colony-stimulating factor 3 receptor (CSF3R) as a model to study neutrophilic differentiation.21 32D-CSF3R cells remain immature when cultured in IL-3–containing medium. However, on transfer to CSF3-containing medium these cells initially proliferate and then undergo terminal neutrophilic differentiation.21 We used the 32D-CSF3R cell line as a model to identify miRNAs that control granulopoiesis and to examine whether particular miRNAs when overexpressed would interfere with the balance of cell expansion and differentiation. We infected 32D-CSF3R cells with retroviruses expressing miRNAs as well as an empty vector (EV) MSCV-BC-A1 (BCA1) as control. Up to 10 cell populations, each expressing a different miRNA from a BC retroviral vector and a BCA1 vector control, were mixed in a 1:1 ratio and transferred to CSF3-containing medium. While expression of most miRNAs tested (see supplemental Table 1) did not change the miRNA BC/EV BC ratios after 9 days of CSF3 treatment, BC signals A8 (miR-292) and F5 (miR-93) increased relative to the BCA1 (Figure 1B-C), indicating an enhanced cell expansion when miR-292 or miR-93 are expressed. To substantiate the effect of these miRNAs on myelopoiesis, we repeated the experiment with individual miR-292 and miR-93 expressing cell populations and compared growth and differentiation capacities of these cells with EV infected controls. When 32D-miR-292 and 32D-miR-93 cells were switched to CSF3-containing medium, cells with a blast-like morphology persisted in culture and expanded continuously under CSF3 conditions, whereas the wild type 32D-CSF3R cells and empty vector infected control cells stopped dividing and differentiated after 7 days of culture (Figure 1D). Thus, ectopic expression of miR-292 and miR-93 enhances the expansion of blast-like 32D-CSF3R cells at the expense of myeloid differentiation.
Functional investigation of miRNAs in 32D cells. (A) Overview of the barcoded retroviral miRNA expression vector MSCV-BC-miRNA. MiRNA expression is driven by the viral LTR promoter. This vector contains the miRNA and ∼ 250 bp endogenous flanking sequences. To allow selection of infected cells, we incorporated a dual selection cassette that consist of the puromycin-N-acetyltransferase gene fused to a segment of the FMDV 2A peptide followed by the gene coding for GFP and a 24 BP barcode (BC) sequence. (B-C) Murine myeloid 32D cells were infected with MSCV-BC vectors containing different miRNAs or no miRNA as control (A1, EV) and sorted for GFP expression by flow cytometry. Equal number of cells were mixed and switched from IL-3- to CSF3-containing medium. Cell samples were taken at indicated time points and genomic DNA was isolated. The abundance of the different BC sequences was measured with the Luminex technology. The ratios of the barcode A8 (miR-292) and barcode F5 (miR-93) signals to the A1 barcode (EV) signal of a representative result (of 2 experiments for miR-93 and 3 experiments for miR-292) are shown. (D) Micrographs showing morphology of control 32D cells (A1-EV), 32D-A8-miR-292 and 32D-F5-miR-93 cells on day 7 of CSF3 treatment. Bar indicates 10 μm.
Functional investigation of miRNAs in 32D cells. (A) Overview of the barcoded retroviral miRNA expression vector MSCV-BC-miRNA. MiRNA expression is driven by the viral LTR promoter. This vector contains the miRNA and ∼ 250 bp endogenous flanking sequences. To allow selection of infected cells, we incorporated a dual selection cassette that consist of the puromycin-N-acetyltransferase gene fused to a segment of the FMDV 2A peptide followed by the gene coding for GFP and a 24 BP barcode (BC) sequence. (B-C) Murine myeloid 32D cells were infected with MSCV-BC vectors containing different miRNAs or no miRNA as control (A1, EV) and sorted for GFP expression by flow cytometry. Equal number of cells were mixed and switched from IL-3- to CSF3-containing medium. Cell samples were taken at indicated time points and genomic DNA was isolated. The abundance of the different BC sequences was measured with the Luminex technology. The ratios of the barcode A8 (miR-292) and barcode F5 (miR-93) signals to the A1 barcode (EV) signal of a representative result (of 2 experiments for miR-93 and 3 experiments for miR-292) are shown. (D) Micrographs showing morphology of control 32D cells (A1-EV), 32D-A8-miR-292 and 32D-F5-miR-93 cells on day 7 of CSF3 treatment. Bar indicates 10 μm.
Multivariate analysis for overall survival (OS) in cytogenetically normal AML
| Variables . | HR . | 95% CI . | P* . |
|---|---|---|---|
| Overall survival | |||
| miRNA-106† | 1.69 | 1.09-2.62 | .018* |
| FLT3ITD‡ | 2.34 | 1.16-4.69 | .017* |
| NPM1§ | 0.21 | 0.09-0.48 | <.0001 |
| CEBPA‖ | 0.92 | 0.44-1.92 | .82 |
| WBC count¶ (×109/L) | 1.52 | 1.14-2.02 | .004* |
| Age# | 1.03 | 1.00-1.06 | .036* |
| Variables . | HR . | 95% CI . | P* . |
|---|---|---|---|
| Overall survival | |||
| miRNA-106† | 1.69 | 1.09-2.62 | .018* |
| FLT3ITD‡ | 2.34 | 1.16-4.69 | .017* |
| NPM1§ | 0.21 | 0.09-0.48 | <.0001 |
| CEBPA‖ | 0.92 | 0.44-1.92 | .82 |
| WBC count¶ (×109/L) | 1.52 | 1.14-2.02 | .004* |
| Age# | 1.03 | 1.00-1.06 | .036* |
Cox proportional hazard model for multivariable analyses of miRNA-106 as prognostic marker for overall survival. Analyses included 85 cytogenetically normal acute myeloid leukemia patients with age ≤ 60 years.
HR indicates hazard ratio; CI, confidence interval; FLT3ITD, FLT3 Internal Tandem Duplications; NPM1, Nucleophosmin; CEBPA, CCAAT-enhancer binding protein alpha; and WBC, white blood cell.
P ≤ .05.
miRNA-106 expression is used as a continuous variable.
FLT3ITD versus no FLT3ITD.
NPM1 mutation versus no NPM1 mutation.
CEBPA mutation versus no CEBPA mutation.
WBC count > 20 × 109/L versus < 20 × 109/L.
Age is used as a continuous variable.
MiR-93-related miRNAs are endogenously expressed in myeloid cells
MiR-292 (AAAGUGCCGCCAGGUUUUGAGUGU) and miR-93 (CAAAGUGCUGUUCGUGCAGGUAG) contain remarkably similar seeds. The previous experiments triggered the question whether miR-292 and miR-93 or other sequence related miRNAs are expressed in myelopoiesis. Sixteen murine miRNAs containing (A)AAGUGC seed sequences were found in the miRbase database (http://www.mirbase.org/) and are listed in supplemental Table 2. Notably, expression of miR-292–related miRNAs (miR-302a-d, -290-3p, -291a-3p, -291b-3p, -294, -295 and human homologues miRNAs miR-302a-d, -372, -373, -512, -515-3p, -519a-e, -520a-e, -526b, could not be detected by qPCR in normal hematopoietic cells (data not shown). Only the miR-17, -20, -93 and -106, which are highly conserved between species, are expressed in hematopoietic cells at different stages of myeloid development from both mouse and human origin (Figure 2). MiR-17 and miR-20a were the most abundant miRNAs followed by miR-106a/b and miR-93 with a ∼ 2- to -4 fold lower expression. Strikingly, miR-20b was clearly the lowest expressed in 32D progenitors. Expression levels of miR-17, -20a -20b, -106a, and -106b were reduced during differentiation of 32D-CSF3R cells (Figure 2A) and miR-17, -20a, and -20b significantly declined in human mature neutrophils (Figure 2B). Collectively, miR-17, -20, -93, and -106 contain the same seed sequence, are conserved across species and are expressed in hematopoietic cells. Therefore, these miRNAs were selected for further analysis.
MiR-17/20/93/106 expression levels during mouse and human neutrophil differentiation. (A) 32D-CSF3R cells were switched from IL-3 to CSF3-containing medium on day t = 0. Micrographs show the morphology of 32D-CSF3R cells on indicated time points of CSF3 treatment. Bars indicate 10 μm. Total RNA was isolated at indicated time points. Expression of indicated mature miRNAs was measured in triplicate with quantitative RT-PCR. The miRNA expressions relative to SnoRNA-234 were measured from immature (steady state, IL-3) samples and set to 1. The average fold expression (n = 3) at indicated time points relative to the IL-3 condition are shown. Error bars represent SD. Significance was calculated by comparing the samples of steady state and the different time points of CSF3 treatment with the Mann-Whitney test (asymptotic significance [2-tailed]). *P < .05. (B) Indicated myeloid cell fractions from human cord blood were stained with cell type specific antibodies and isolated using FACS sort. Micrographs show morphology of sorted cell fractions. Bars indicate 10 μm. MiRNA levels and significance were measured and calculated as described in panel A.
MiR-17/20/93/106 expression levels during mouse and human neutrophil differentiation. (A) 32D-CSF3R cells were switched from IL-3 to CSF3-containing medium on day t = 0. Micrographs show the morphology of 32D-CSF3R cells on indicated time points of CSF3 treatment. Bars indicate 10 μm. Total RNA was isolated at indicated time points. Expression of indicated mature miRNAs was measured in triplicate with quantitative RT-PCR. The miRNA expressions relative to SnoRNA-234 were measured from immature (steady state, IL-3) samples and set to 1. The average fold expression (n = 3) at indicated time points relative to the IL-3 condition are shown. Error bars represent SD. Significance was calculated by comparing the samples of steady state and the different time points of CSF3 treatment with the Mann-Whitney test (asymptotic significance [2-tailed]). *P < .05. (B) Indicated myeloid cell fractions from human cord blood were stained with cell type specific antibodies and isolated using FACS sort. Micrographs show morphology of sorted cell fractions. Bars indicate 10 μm. MiRNA levels and significance were measured and calculated as described in panel A.
Ectopic expression of miR-17, -20, -93, and -106 promotes expansion and replating capacity of myeloid progenitors and enhances MAP kinase signaling
Next, we tested whether ectopic expression of these miRNAs would also affect the outgrowth of myelo-neutrophilic progenitor cells (granulocyte-colony-forming unit [CFU-G]). Mouse lineage marker negative (Lin−) hematopoietic progenitor cells isolated from the bone marrow (BM) were transduced with MSCV–miR-17, -20, -93, -106 or MSCV-EV control viruses. We observed an up to 2.5-fold increase in the number of CSF3-induced colonies that are formed by Lin− cells transduced with MSCV–miR-17, -20, -93, and -106 containing vectors compared with control infected cells (Figure 3A). Notably, not only the number of colonies, also colony size increased markedly when these miRNAs are overexpressed (Figure 3A). These differentiated cells appeared morphologically normal (supplemental Figure 1A). Next, we asked whether enhanced expression of miR-17, -20, -93, and -106 has an effect on replating capacity of hematopoietic cells. To study this, we transduced Lin− cells isolated from the BM and performed a progenitor replating assay. We observed markedly more and bigger colonies of MSCV–miR-17, -20, -93, and -106 transduced cells compared with control cells after the second plating (Figure 3B), indicating an enhanced self-renewal capacity of cells that ectopically express the latter AAAGUGC-seed miRNAs. No colony formation was observed after the third plating of 104 cells, demonstrating that these cells do not self-renew ad infinitum under these conditions. To assess the effects of AAAGUGC seed miRNAs expression also in vivo, MSCV–miR-17, -20, -93, -106 and MSCV-EV virus–infected Lin− GFP+ BM cells (∼ 20%) mixed with wt Lin− cells (∼ 80%) were transplanted in lethally irradiated recipient mice. There was a considerable proliferation advantage for the multipotent progenitors (Lin−, Sca-I+, c-Kit+/−) expressing miR-17, -20, -93, and -106 over wt cells compared with EV-transduced cells at 6 weeks after transplantation as is evident from the fold induction of miRNA containing GFP+ cells (Figure 3C). Immunophenotypic analyses of miR-17, -20, -93, and -106 expressing BM and blood cells showed no aberrant myeloid differentiation (supplemental Figure 1B-C). To investigate whether AAAGUGC seed-containing miRNAs control expansion of granulocytic progenitors through the regulation of cytokine-induced signaling, we analyzed CSF3-induced phosphorylation of several signaling intermediates in presence and absence of AAAGUGC miRNAs. Hematopoietic Ba/F3 cells coexpressing the CSF3R and miR-17, -20, -93, and -106 were factor deprived and restimulated with CSF3. Phosphorylation of extracellular signal-regulated kinase (ERK) was enhanced in cells expressing the different AAAGUGC seed-containing miRNAs compared with control cells (Figure 3D). Strikingly, enhanced phosphorylation levels were not observed for other signaling molecules such as STAT5, STAT3, and AKT (data not shown), suggesting the specific regulation of mitogen-activated protein (MAP) kinase activity. Collectively, increased expression of AAAGUGC-seed containing miRNAs in Lin− BM cells promotes replating capacity and expansion of myeloid progenitors and CSF3-induced MAP kinase signaling.
Functional analysis of miR-17/20/93/106 in primary mouse Lin− BM cells. (A) CFU-G assay of mouse lin− BM progenitors infected with MSCV–miR-17, -miR-20, -miR-93, miR-106 or empty vector control virus. Cells were plated in triplicate at densities of 1 × 104 cells per dish in 1 mL methylcellulose medium containing CSF3 (100 ng/mL). Colonies consisting of more than 50 cells were counted after 7 days of growth. Micrographs show size of CFU-CSF3 on day 7 after plating. Bar indicates 100 μm. Significance was calculated by comparing the samples with EV control and the different miRNAs with the Mann-Whitney test (asymptotic significance [2-tailed]). *P < .05. (B) Replating assay of mouse lin− BM progenitors infected with MSCV–miR-17, -miR-20, -miR-93, miR-106 or empty vector control virus. Cells were plated in triplicate at densities of 1 × 104 cells per dish in 1 mL methylcellulose medium containing IL-6 (10 ng/mL), IL-3 (supernatant 1/1000), SCF (10 ng/mL), CSF2 (10 ng/mL). Cells were isolated from dishes, counted and replated under the same conditions. Colonies from the second plating were counted after 7 days of growth. Micrographs show size of CFU's on day 7 after plating. Bar indicates 100 μm. Significance was calculated as described in panel A. (C) Mouse lineage negative progenitor cells isolated from the BM of C57BL/6 mice were infected with MSCV–miR-17, -miR-20, -miR-93, miR-106 or empty vector control virus. Recipient C57BL/6 mice were irradiated (8.5 Gy) and reconstituted with ∼ 20% GFP positive cells and ∼ 80% WT cells. Six weeks after transplantation, mice were killed and BM cells were isolated. Lin− cells were stained for flow cytometry analysis. The fold induction of the percentage GFP+ control (n = 4), miR-17 (n = 6), miR-20 (n = 4), miR-93 (n = 4), miR-106 (n = 6) Lin−; Sca-I+; c-Kit+/− cells in the BM compared with the input are shown. Significance was calculated by comparing the samples of mice transplanted with EV control and the different miRNAs with the Mann-Whitney test (asymptotic significance [2-tailed]). *P < .05 and **P = .057. (D) Ba/F3-CSF3R cells were infected with MSCV–miR-17, miR-20, miR-93, miR-106 or EV control viruses. Cells were factor deprived for 4 hours (t = 0) followed by CSF3 (100 ng/mL) stimulation for 10 minutes. Cells were washed 2 times with PBS and incubated in RPMI medium for 60 or 120 minutes. Samples for cell lysates were taken at indicated time points and analyzed by Western blotting using total and phospho-specific antibodies against ERK.
Functional analysis of miR-17/20/93/106 in primary mouse Lin− BM cells. (A) CFU-G assay of mouse lin− BM progenitors infected with MSCV–miR-17, -miR-20, -miR-93, miR-106 or empty vector control virus. Cells were plated in triplicate at densities of 1 × 104 cells per dish in 1 mL methylcellulose medium containing CSF3 (100 ng/mL). Colonies consisting of more than 50 cells were counted after 7 days of growth. Micrographs show size of CFU-CSF3 on day 7 after plating. Bar indicates 100 μm. Significance was calculated by comparing the samples with EV control and the different miRNAs with the Mann-Whitney test (asymptotic significance [2-tailed]). *P < .05. (B) Replating assay of mouse lin− BM progenitors infected with MSCV–miR-17, -miR-20, -miR-93, miR-106 or empty vector control virus. Cells were plated in triplicate at densities of 1 × 104 cells per dish in 1 mL methylcellulose medium containing IL-6 (10 ng/mL), IL-3 (supernatant 1/1000), SCF (10 ng/mL), CSF2 (10 ng/mL). Cells were isolated from dishes, counted and replated under the same conditions. Colonies from the second plating were counted after 7 days of growth. Micrographs show size of CFU's on day 7 after plating. Bar indicates 100 μm. Significance was calculated as described in panel A. (C) Mouse lineage negative progenitor cells isolated from the BM of C57BL/6 mice were infected with MSCV–miR-17, -miR-20, -miR-93, miR-106 or empty vector control virus. Recipient C57BL/6 mice were irradiated (8.5 Gy) and reconstituted with ∼ 20% GFP positive cells and ∼ 80% WT cells. Six weeks after transplantation, mice were killed and BM cells were isolated. Lin− cells were stained for flow cytometry analysis. The fold induction of the percentage GFP+ control (n = 4), miR-17 (n = 6), miR-20 (n = 4), miR-93 (n = 4), miR-106 (n = 6) Lin−; Sca-I+; c-Kit+/− cells in the BM compared with the input are shown. Significance was calculated by comparing the samples of mice transplanted with EV control and the different miRNAs with the Mann-Whitney test (asymptotic significance [2-tailed]). *P < .05 and **P = .057. (D) Ba/F3-CSF3R cells were infected with MSCV–miR-17, miR-20, miR-93, miR-106 or EV control viruses. Cells were factor deprived for 4 hours (t = 0) followed by CSF3 (100 ng/mL) stimulation for 10 minutes. Cells were washed 2 times with PBS and incubated in RPMI medium for 60 or 120 minutes. Samples for cell lysates were taken at indicated time points and analyzed by Western blotting using total and phospho-specific antibodies against ERK.
MiR-17, -20, -93, and -106 regulate SQSTM1 levels in hematopoietic cells
We investigated how miR-93 promotes cell expansion by identification of the target transcripts in 32D-CSF3R cells. To this end, we analyzed the proteome of 32D-CSF3R cells expressing miR-93 compared with EV control cells using the SILAC quantitative proteomics approach. 32D-CSF3R-EV control cells were grown in medium containing 13C6-labeled (heavy) Lysine (Lys6) and Arginine (Arg6) and 32D-CSF3R-miR-93 cells in medium containing 12C6 (regular) Lys and Arg. In addition, these cells were switched to CSF3-containing medium for 4 days. For all the samples measured we identified at least 3400 unique proteins with 2 or more peptides per protein (for top 100 down-regulated proteins see supplemental Table 3). Most predicted targets for miR-93 by Target scan (http://www.targetscan.org) are not or slightly down-regulated (supplemental Table 3). SQSTM1 was identified as the only protein from the top 20 down-regulated hits for miR-93 with a predicted recognition site for miR-17/20/93/106/519 in the 3′UTR (supplemental Table 3 and Figure 4A). SQSTM1 was identified based on 9 unique peptides. In addition to reduced protein levels, diminished Sqstm1 mRNA was detected by QPCR in cells expressing miR-93 (Figure 4B).
Identification of SQSTM1 as a target for AAAGUGC seed-containing miRNAs. The myeloid cell line 32D was infected with miR-93 or empty vector (EV) control viruses. The EV control cells were grown in RPMI media containing 13C-labeled Lys and Arg (heavy) and 32D–miR-93 cells in medium containing regular Lys and Arg (light). Cells were counted and miR-93 containing cells were either mixed with EV control cells in a 1:1 ratio for proteomics analysis or subjected for mRNA isolation. (A) Samples were prepared and analyzed by quantitative proteomics. The ratios of protein expression in steady state (ss) conditions and at 4 days of CSF3 treatment are plotted. The correlation of the average log (L/H) values (n = 2 independent measurements) of a biologic duplicate (rep1 = x-axis and rep2 = y-axis) of all the identified proteins are indicated by the black dots. Single identifications from one experiment are indicated by the green dots. SQSTM1 is indicated by the red arrow and dot. (B) The average SQSTM1 protein levels (n = 2 independent measurements) of a biologic duplicate and the average mRNA abundances relative to GAPDH and EV control cells in steady state (ss) conditions and at 4 days of CSF3 treatment are shown (n = 3). Error bars represent SD (mRNA). Significance was calculated by comparing the EV control with the steady state and CSF3 condition with the Mann-Whitney test (asymptotic significance [2-tailed]). *P < .05. (C) Luciferase reporter plasmids containing the full-length 3′UTR sequence of Sqstm1 with a wt or mutated miRNA binding site were generated and transfected with the Firefly control vector into HEK293 cells. Two days after transfection, cells were lysed and assayed for Luciferase activity. Luciferase values were normalized against Firefly activity. Normalized values for wt 3′UTR were put to 100%. The Luciferase activity values of the mutant 3′UTR relative to the wt is shown. Error bars represent SD of 3 experiments. (D) The Luciferase reporter plasmids containing the full-length 3′UTR sequence of Sqstm1 were cotransfected with indicated MSCV-miRs. The Luciferase activity values of the miRNA expressing cells relative to the Empty vector control are shown. Error bars represent SD of 3 experiments. (E) The Luciferase reporter plasmids containing the mutant 3′UTR sequence of Sqstm1 were cotransfected with indicated MSCV-miRs. (F) 32D-CSF3R cells were switched from IL-3 to CSF3-containing medium on t = 0 days (steady state, SS). Total RNA was isolated at indicated time points. Sqstm1 levels were measured by qPCR in triplicate. Sqstm1 expression relative to Gapdh and to SS condition is shown. (G) Western blot analysis of normal karyotype AML samples exhibiting low and high miR-106 with monoclonal anti-SQSTM1 antibodies. Actin was stained for loading control.
Identification of SQSTM1 as a target for AAAGUGC seed-containing miRNAs. The myeloid cell line 32D was infected with miR-93 or empty vector (EV) control viruses. The EV control cells were grown in RPMI media containing 13C-labeled Lys and Arg (heavy) and 32D–miR-93 cells in medium containing regular Lys and Arg (light). Cells were counted and miR-93 containing cells were either mixed with EV control cells in a 1:1 ratio for proteomics analysis or subjected for mRNA isolation. (A) Samples were prepared and analyzed by quantitative proteomics. The ratios of protein expression in steady state (ss) conditions and at 4 days of CSF3 treatment are plotted. The correlation of the average log (L/H) values (n = 2 independent measurements) of a biologic duplicate (rep1 = x-axis and rep2 = y-axis) of all the identified proteins are indicated by the black dots. Single identifications from one experiment are indicated by the green dots. SQSTM1 is indicated by the red arrow and dot. (B) The average SQSTM1 protein levels (n = 2 independent measurements) of a biologic duplicate and the average mRNA abundances relative to GAPDH and EV control cells in steady state (ss) conditions and at 4 days of CSF3 treatment are shown (n = 3). Error bars represent SD (mRNA). Significance was calculated by comparing the EV control with the steady state and CSF3 condition with the Mann-Whitney test (asymptotic significance [2-tailed]). *P < .05. (C) Luciferase reporter plasmids containing the full-length 3′UTR sequence of Sqstm1 with a wt or mutated miRNA binding site were generated and transfected with the Firefly control vector into HEK293 cells. Two days after transfection, cells were lysed and assayed for Luciferase activity. Luciferase values were normalized against Firefly activity. Normalized values for wt 3′UTR were put to 100%. The Luciferase activity values of the mutant 3′UTR relative to the wt is shown. Error bars represent SD of 3 experiments. (D) The Luciferase reporter plasmids containing the full-length 3′UTR sequence of Sqstm1 were cotransfected with indicated MSCV-miRs. The Luciferase activity values of the miRNA expressing cells relative to the Empty vector control are shown. Error bars represent SD of 3 experiments. (E) The Luciferase reporter plasmids containing the mutant 3′UTR sequence of Sqstm1 were cotransfected with indicated MSCV-miRs. (F) 32D-CSF3R cells were switched from IL-3 to CSF3-containing medium on t = 0 days (steady state, SS). Total RNA was isolated at indicated time points. Sqstm1 levels were measured by qPCR in triplicate. Sqstm1 expression relative to Gapdh and to SS condition is shown. (G) Western blot analysis of normal karyotype AML samples exhibiting low and high miR-106 with monoclonal anti-SQSTM1 antibodies. Actin was stained for loading control.
Sqstm1 is a predicted target of miR-17/20/93/106 by target scan as its transcript contains a well-conserved 8-mer site at position 650 (AGCACUUU) of the Sqstm1 3′UTR (Figure 4C). To test whether this putative miRNA binding site controls SQSTM1 expression, we cloned the full-length Sqstm1 3′UTR behind a Luciferase reporter and compared Luciferase activity of the reporters containing a wt Sqstm1 3′UTR with a mutated 3′ UTR lacking the 8-mer miRNA recognition site. Indeed, a 2 nucleotide mutation of the miRNA binding site resulted in 60% increased Luciferase activity compared with the wt control 3′UTR (Figure 4C), indicating regulatory functions of this sequence on protein expression. Enhanced expression of miR-17, -20, -93, and -106 resulted in an additional 15% down-regulation of Luciferase activity using the wt Sqstm1 3′UTR (Figure 4D), which was not observed with the mutant 3′UTR (Figure 4E). These results indicate the predicted target-site as the important determinant for miR-17/20/93/106–mediated regulation of SQSTM1 expression. However, the 3′-parts of the miRNAs tested might still modulate the action of the miRNAs. In addition, we found increased expression of Sqstm1 transcripts during CSF3-induced neutrophil differentiation of 32D-CSF3R cells (Figure 4F) and an inverse correlation of SQSTM1 protein levels and miR-106 expression in AML samples (Figure 4G).
We reasoned that, if AAAGUGC miRNAs promote cell expansion by repressing the expression of SQSTM1, this effect should be at least partially pheno-copied by shRNA-mediated knockdown of SQSTM1. Indeed, we observed bigger colonies and an enhanced replating capacity, using 2 different specific shRNAs against Sqstm1 compared with control cells (Figure 5A). In addition, we noted an enhanced cell expansion of Sqstm1-shRNA expressing progenitor cells compared with controls in mice (Figure 5B). These results reveal SQSTM1 as a key effector of AAAGUGC-seed containing miRNAs in myeloid cells at the advantage of cell expansion.
ShRNA-mediated silencing of SQSTM1 phenocopies miRNA overexpression. (A) Replating assay of mouse lin− BM progenitors infected with 2 different hairpin vectors against SQSTM1, pSM2C-SH2024 and pSM2C-SH-2219, or empty vector control virus. Cells were plated in triplicate at densities of 1 × 104 cells per dish in 1 mL methylcellulose medium containing IL-6 (10 ng/mL), IL-3 (supernatant 1/1000), SCF (10 ng/mL), CSF2 (10 ng/mL). Cells were grown for a week, isolated and replated in a secondary CFU-assay (104 cells per plate). Colonies from the second plating were counted after 7 days of growth. Micrographs show size of CFUs on day 7 after plating. Black bar indicates 100 μm, red bars indicate 200 μm. Significance was calculated by comparing the samples with EV control and the different shRNAs with the Mann-Whitney test (asymoptotic significance [2-tailed]). *P < .05. (B) Mouse lineage negative progenitor cells isolated from the BM of C57BL/6 mice were infected with pSM2C-sh-2024 and pSM2C-sh-2219, empty vector control virus or with GFP containing control virus. Cells were selected in PURO 1.5 (μg/mL) containing expansion medium for 2 days. Recipient C57BL/6 mice were lethally irradiated (8.5 Gy) and reconstituted with 45% GFP positive cells mixed with shRNA or EV control infected cells. Six weeks after transplantation, mice were killed and BM cells were isolated. Lin− cells were stained for flow cytometry analysis. The fold induction of the percentage of GFP− Lin−; Sca-I+; c-Kit+ cells in the BM of pSM2C-EV (n = 4), sh-2024 (n = 5) and sh-2219 (n = 4) relative to the input are shown. Significance was calculated by comparing the samples of mice transplanted with EV control and the different shRNAs with the Mann-Whitney test (asymptotic significance [2-tailed]). *P < .05.
ShRNA-mediated silencing of SQSTM1 phenocopies miRNA overexpression. (A) Replating assay of mouse lin− BM progenitors infected with 2 different hairpin vectors against SQSTM1, pSM2C-SH2024 and pSM2C-SH-2219, or empty vector control virus. Cells were plated in triplicate at densities of 1 × 104 cells per dish in 1 mL methylcellulose medium containing IL-6 (10 ng/mL), IL-3 (supernatant 1/1000), SCF (10 ng/mL), CSF2 (10 ng/mL). Cells were grown for a week, isolated and replated in a secondary CFU-assay (104 cells per plate). Colonies from the second plating were counted after 7 days of growth. Micrographs show size of CFUs on day 7 after plating. Black bar indicates 100 μm, red bars indicate 200 μm. Significance was calculated by comparing the samples with EV control and the different shRNAs with the Mann-Whitney test (asymoptotic significance [2-tailed]). *P < .05. (B) Mouse lineage negative progenitor cells isolated from the BM of C57BL/6 mice were infected with pSM2C-sh-2024 and pSM2C-sh-2219, empty vector control virus or with GFP containing control virus. Cells were selected in PURO 1.5 (μg/mL) containing expansion medium for 2 days. Recipient C57BL/6 mice were lethally irradiated (8.5 Gy) and reconstituted with 45% GFP positive cells mixed with shRNA or EV control infected cells. Six weeks after transplantation, mice were killed and BM cells were isolated. Lin− cells were stained for flow cytometry analysis. The fold induction of the percentage of GFP− Lin−; Sca-I+; c-Kit+ cells in the BM of pSM2C-EV (n = 4), sh-2024 (n = 5) and sh-2219 (n = 4) relative to the input are shown. Significance was calculated by comparing the samples of mice transplanted with EV control and the different shRNAs with the Mann-Whitney test (asymptotic significance [2-tailed]). *P < .05.
Role of SQSTM1 in regulation of ligand-induced CSF3R routing, stability and signaling
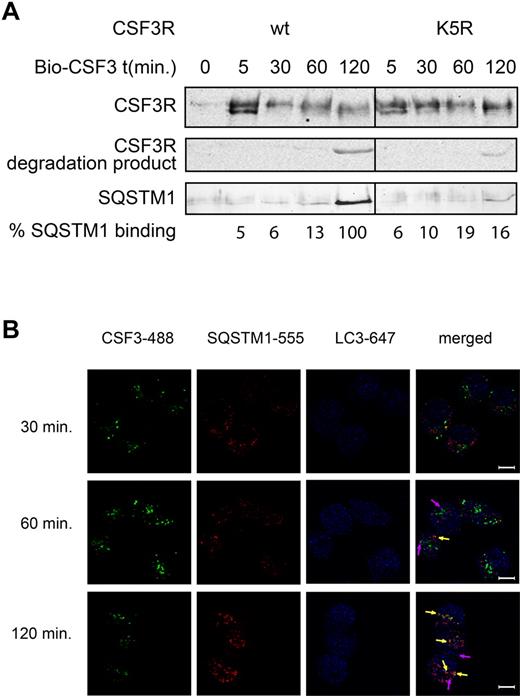
Signaling kinetics of the CSF3R govern the balance between cellular expansion and differentiation of progenitors.29 Ubiquitination of activated CSF3R controls intracellular receptor routing to lysosomal degradation and signal duration.30 To identify proteins that bind to the ligand-activated CSF3R complex, we performed a proteomics analysis of CSF3R complexes pulled down on streptavidin-coated magnetic beads at 30 minutes after stimulation with biotinylated CSF3 (Bio-CSF3; A.M. and I.P.T., unpublished data, September 2007). SQSTM1 was one of the proteins that were consistently present in the activated CSF3R complex. CSF3R-SQSTM1 interaction was confirmed by Western blot analysis in HeLa cells that stably express hu-CSF3Rs (HeLa-CSF3R cells; Figure 6A). SQSTM1 binding to the CSF3R was not detectable above background by Western blotting at early time points but was obvious at 2 hours after stimulation. Notably, the enhanced binding of SQSTM1 to the receptor correlated with CSF3R degradation (Figure 6A). SQSTM1 binding to the CSF3R was dependent on receptor lysines, as the CSF3R lysine null mutant (K5R)31 showed a strongly reduced SQSTM1 binding capacity compared with the wt CSF3R (Figure 6A). In full agreement with these observations, confocal microscopy analyses showed increasing CSF3R colocalization with endogenously expressed SQSTM1 at 2 hours after stimulation compared with earlier time points (Figure 6B). Although SQSTM1 has been associated with autophagy, no colocalization has been observed between the CSF3R and the LC3 marker for autophagosomes at different time points after stimulation (Figure 6B). To study the effects of SQSTM1 binding for CSF3R routing we silenced SQSTM1 expression by RNAi and analyzed receptor localization in living cells up to 2 hours after stimulation. We detected a 100% colocalization of CSF3Rs with RAB7, an intracellular marker for late endosomes after 2 hours (supplemental Figure 2), indicating that siRNA-mediated knockdown of SQSTM1 has no effect on normal routing of the CSF3R to the endosomal compartment after ligand stimulation in our model. Because SQSTM1 targets ubiquitinated proteins for degradation, we asked whether SQSTM1 controls CSF3R stability. Similar ligand-activated CSF3R protein levels were detected at 15 minutes after treatment with Bio-CSF3 (Figure 7B). In contrast, a ∼ 75% siRNA-mediated silencing of SQSTM1 in HeLa-CSF3R cells resulted in small, but consistently enhanced CSF3R levels (∼ 30%) at 2 hours after stimulation, compared with control siRNA treated cells (Figure 7A-B). The enhanced CSF3R level was concomitant with increased ligand-induced P-ERK levels (Figure 7C). Thus, the interaction of SQSTM1 to the stimulated CSF3R adjusts receptor degradation via the late endosomal compartment and regulates cellular P-ERK levels.
SQSTM1 binds to the CSF3R. (A) HeLa-CSF3R and HeLa-K5R cells were stimulated with Bio-CSF3 or left unstimulated (t = 0) for 10 minutes at room temperature, washed with RPMI and transferred to 37°C for indicated time points. CSF3R binding proteins were pulled down with streptavidin-coated beads from cell lysates and analyzed by Western blotting using monoclonal antibodies against CSF3R and SQSTM1. The percentage SQSTM1 binding relative to the amount of CSF3Rs is given. (B) HeLa-CSF3R cells were stimulated with CSF3-488 for 10 minutes at room temperature, washed and transferred to 37°C for indicated time-points. Subsequently, cells were fixed and stained for SQSTM1 (red) and LC3 (blue). Colocalization of CSF3R and SQSTM1 is indicated by the orange-yellow dots and yellow arrows. Colocalization of SQSTM1 with LC3 is indicated by the pink dots and arrows.
SQSTM1 binds to the CSF3R. (A) HeLa-CSF3R and HeLa-K5R cells were stimulated with Bio-CSF3 or left unstimulated (t = 0) for 10 minutes at room temperature, washed with RPMI and transferred to 37°C for indicated time points. CSF3R binding proteins were pulled down with streptavidin-coated beads from cell lysates and analyzed by Western blotting using monoclonal antibodies against CSF3R and SQSTM1. The percentage SQSTM1 binding relative to the amount of CSF3Rs is given. (B) HeLa-CSF3R cells were stimulated with CSF3-488 for 10 minutes at room temperature, washed and transferred to 37°C for indicated time-points. Subsequently, cells were fixed and stained for SQSTM1 (red) and LC3 (blue). Colocalization of CSF3R and SQSTM1 is indicated by the orange-yellow dots and yellow arrows. Colocalization of SQSTM1 with LC3 is indicated by the pink dots and arrows.
Role of SQSTM1 in regulation of ligand-induced CSF3R routing, stability and signaling. (A) HeLa-CSF3R cells were transfected with siRNAs against Sqstm1 or control siRNAs. Western blotting showed a ∼ 75% knock-down (KD) in cells transfected with Sqstm1 siRNAs compared with cells transfected with control siRNAs. (B) Transfected cells were stimulated with Bio-CSF3 or left unstimulated for 10 minutes at RT (t = 0), washed with RPMI and transferred to 37°C for indicated time points. CSF3R proteins were pulled down and analyzed as described in panel A. The ratio of detected CSFR protein levels siSQSTM1/siControl samples (n = 3) was calculated for 15 and 120 minutes CSF3 treatment and is depicted. The significance of the difference between the calculated ratios of time point 120 minutes and 15 minutes was calculated with the Mann-Whitney test (asymptotic significance [2-tailed]; P < .10). (C) HeLa-CSF3R cells were transfected with siRNAs against Sqstm1 (+) or control siRNAs (−). Western blotting showed a > 95% knockdown (KD) in cells transfected with siRNAs targeting Sqstm1 transcripts compared with cells transfected with control siRNAs. Cells were serum starved for 24 hours followed by CSF3 (100 ng/mL) treatment as described in panel A. Samples for cell lysates were taken at indicated time points and analyzed by Western blotting with total and/or phospho-specific antibodies against ERK.
Role of SQSTM1 in regulation of ligand-induced CSF3R routing, stability and signaling. (A) HeLa-CSF3R cells were transfected with siRNAs against Sqstm1 or control siRNAs. Western blotting showed a ∼ 75% knock-down (KD) in cells transfected with Sqstm1 siRNAs compared with cells transfected with control siRNAs. (B) Transfected cells were stimulated with Bio-CSF3 or left unstimulated for 10 minutes at RT (t = 0), washed with RPMI and transferred to 37°C for indicated time points. CSF3R proteins were pulled down and analyzed as described in panel A. The ratio of detected CSFR protein levels siSQSTM1/siControl samples (n = 3) was calculated for 15 and 120 minutes CSF3 treatment and is depicted. The significance of the difference between the calculated ratios of time point 120 minutes and 15 minutes was calculated with the Mann-Whitney test (asymptotic significance [2-tailed]; P < .10). (C) HeLa-CSF3R cells were transfected with siRNAs against Sqstm1 (+) or control siRNAs (−). Western blotting showed a > 95% knockdown (KD) in cells transfected with siRNAs targeting Sqstm1 transcripts compared with cells transfected with control siRNAs. Cells were serum starved for 24 hours followed by CSF3 (100 ng/mL) treatment as described in panel A. Samples for cell lysates were taken at indicated time points and analyzed by Western blotting with total and/or phospho-specific antibodies against ERK.
Discussion
Role of AAAGUGC seed-containing miRNAs in hematopoiesis
miRNAs play pivotal regulatory roles in homeostatic physiologic processes including hematopoiesis. Hematopoiesis is characterized by the tight regulation of cellular renewal, proliferation and differentiation with the objective of adjusting blood cell production according daily needs. Here, we present a series of experiments that identify a set of miRNAs that share identical AAAGUGC seed sequences and also share functions in hematopoietic progenitor cells. Our data from 32D cells, mouse progenitor assays and in vivo transplantation studies consistently indicate a role for miR-17/20/93/106 in hematopoietic cell expansion and replating capacity. We show that miR-17, -20, -93, and -106 are abundantly expressed in hematopoietic progenitors of human and mouse origin and their expression declines considerably during myeloid differentiation. Consistent with this, expression of miR-17 and miR-106 were previously detected in human CD34+ progenitor cells and were shown to be significantly down-regulated during in vitro differentiation toward mature megakaryocytes and monocytes.7 The miR-17-5p, -20, and -106 were also found to be down-regulated in monocytopoiesis.32 Collectively, these results suggest a more general role for these miRNAs at an early stage of myeloid development. Perturbed maturation of primary hematopoietic progenitors was not observed in CFU assays and in transplantation experiments in mice, indicating that the effect of miRNAs is a genuinely enhanced expansion of hematopoietic precursors, and not proliferation because of impaired differentiation.
The AAAGUGC seed-containing miRNAs target SQSTM1 and control CSF3R stability, MAP kinase activity and cell expansion
Whereas most proteins detected by quantitative proteomics were not or moderately changed in their expression by miR-93 in 32D cells, SQSTM1 expression was strongly decreased. SQSTM1 is a multifunctional signal adaptor protein controlling a variety of cellular events such as, for example, osteoclastogenesis, T-cell and adipocyte differentiation, regulation of the NF-κB pathway, nerve growth factor receptor (NGFR) internalization, and transportation of poly-ubiquitinated proteins destined for degradation by the proteasome and autophagy systems.33-37 We show for the first time that SQSTM1 expression is posttranscriptionally regulated by miRNAs.
SQSTM1 was formerly found to colocalize robustly with the EGFR and the late endosomal markers RAB7 and LAMP1, implicating functions of SQSTM1 predominantly in late endosomes and lysosomes.38 Ubiquitination of the lysine in the juxtamembrane part of the CSF3R is pivotal for trafficking of the receptor from early to late endosomes, and impaired lysosomal targeting results in enhanced proliferation of progenitors.30 We observed SQSTM1 binding to the activated CSF3R complex 30 minutes after stimulation and this interaction was the most abundant after 2 hours. This interaction was concomitant with the amount of CSF3R degradation products on the gel. The K5R mutant of the CSF3R binds reduced levels of SQSTM1 compared with wt CSF3R. This receptor mutant accumulates in the early endosomes.31 Therefore, it is still unclear, whether ubiquitination of the CSF3R itself is crucial for SQSTM1-mediated functions. Does SQSTM1 binding affect CSF3R localization? SiRNA-mediated silencing of SQSTM1 expression showed no role of SQSTM1 in CSF3R routing to the late endosomal compartment. However, knock-down of SQSTM1 resulted in enhanced CSF3R stability, which can be explained by inefficient receptor routing to the lysosomes. Studies with SQSTM1 mutants are needed to elucidate the binding specificities of SQSTM1 to the CSF3R complex.
We showed enhanced P-ERK levels when SQSTM1 expression is silenced. This could be explained by 2 possible mechanisms which are not mutually exclusive. First, several studies demonstrate the importance of SQSTM1 in regulation of the activity of MAPK family members by inhibition and direct sequestration of ERKs.39-41 Second, recent data show that the MEK-ERK pathway is anchored to the late endosomes.42 Defective routing of the CSF3R to lysosomal degradation presumably results in prolonged localization of active receptors in late endosomal compartment, and is a plausible explanation of the enhanced ERK activation.
The question emerging from these data are, whether the proliferative effects of SQSTM1 silencing could be explained by enhanced P-ERK levels? In fact, CSF3-induced activation of the ERK kinases is known to be involved in stimulation of proliferation.29 In addition, the tyrosine at amino acid position 764 of the CSF3R is a docking site for signaling proteins implicated in the activation of P21RAS/MAP kinase pathway and controls cell expansion.43 Thus, the proliferative phenotype of hematopoietic cells with enhanced levels of AAAGUGC seed-containing miRNAs could be at least partially explained by the enhanced activation of MAP kinases caused by reduced SQSTM1 action. In conclusion, AAAGUGC-seed containing miRNAs in hematopoietic cells control cell expansion, self-renewal, and MAP-kinase signaling by interference with SQSTM1-regulated pathways.
Role of AAAGUGC seed-containing miRNAs in myeloid leukemia
There is strong evidence for a role of AAAGUGC seed-containing miRNAs in cancer. For instance, the miR-372 and miR-373 have been implicated as oncogenes in testicular germ cell tumors through reduction of LATS2 tumor suppressor levels.44 Elevated expression of miR-17 was found in tumors of different origins, for example, hematopoietic, colorectal, and lung tumors and transgenic mice that overexpress the miR-17∼92 polycistron are more susceptible to cancer.45-47 Further, increased miR-106 level is significantly correlated with tumor stage, metastasis and invasion in gastric carcinoma and cancer cell lines.48 MiR-93, miR-17, miR-20, and miR-106 are abundantly expressed in a large subset of AML samples.28 In a genome wide miRNA expression analysis of 52 AML samples with common translocations, miR-17 and miR-20 in combination with 5 other miRNAs could discriminate MLL-rearrangement AMLs from CBFs and t(15;17) rearrangement positive leukemias.49 Similar to our observations in normal progenitors, forced expression of the miR-17∼92 cluster, which contain miR-17 and miR-20, in MLL-transformed cells results in enhanced colony forming capacity of BM progenitors, particularly in cooperation with MLL fusions.50 Accordingly, leukemic stem cell potential and self-renewal is enhanced in MLL transformed cells when miR-17∼92 is abundantly expressed, in part by the modulation of P21 expression.51 We identified P21 by mass spectrometry, but unfortunately these results did not match the selection criteria as described in supplemental Methods. Therefore, this protein had been discarded for further analysis. Strikingly, SQSTM1 has not been identified as a target in the study of Mi et al.50 There are two explanations feasible for this discrepancy: (1) SQSTM1 is because of still unidentified mechanisms not regulated in MLL transformed tumor stem cells. (2) SQSTM1 protein expression is down-regulated by the miRNAs, but the difference in mRNA expression in the MLL transformed cells compared with controls might be minor and too small to be picked up by the microarrays used in the study. The second hypothesis is in full agreement to our data, because we found that overexpression of AAAGUGC-seed miRNAs strongly affects SQSTM1 protein expression and not so much the mRNA stability (< 40%). In our opinion, it is still feasible that both experimentally identified targets collaborate in the control of leukemic and normal hematopoietic precursor expansion.
Interestingly, a multivariable cox regression analysis on data from 85 cytogenetically normal cases of AML, accounting for known confounders (age of the patient, white blood cell count, mutations in genes coding for NPM1, CEBPA and FLT3) indicated that high miR-106 expression significantly correlates with adverse survival (P = .018, hazard ratio = 1.69, Table 1). We found that SQSTM1 protein levels were markedly down-regulated in normal karyotype AML samples exhibiting high expression of miR-106. Recent data indicate both oncogenic and tumor suppressor functions for SQSTM1 in various types of cancer.52-54 Our patient data suggest that AAAGUGC seed-containing miRNAs play a role in leukemogenesis by deregulation of SQSTM1-controlled mechanisms. Additional studies are needed to further test this hypothesis and to determine whether miR-106 could be a potential biomarker for diagnosis of this particular group of AML patients. In conclusion, AAAGUGC-seed containing miRNAs in hematopoietic cells control cell expansion, replating capacity and MAP-kinase signaling, in part by the interference with SQSTM1-regulated pathways.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank P. van Geel and Dr E. Rombouts for sorting cell populations and E. Simons for assistance with the preparation of figures. We thank Dr T. Jevdovic, O. Roovers, C. Verwijmeren, S. Hoefnagels, L. Schulte, M.A. Sanders, and V. Rockova for technical and bioinformatics assistance. We thank Drs M. von Lindern, E. Bindels, J. van Bergen, F. Ossendorp for critical reading of the manuscript and discussions.
This work was supported by grants from the Netherlands Organization for Scientific Research (NWO-VENI) and the Dutch Cancer Society (KWF). This work was also supported by US Public Health Service grants RO1-GM34277 from the National Institutes of Health, PO1-CA42063 from the National Cancer Institute to P.A.S., and partially by Cancer Center Support (core) grant P30-CA14051 from the National Cancer Institute.
National Institutes of Health
Authorship
Contribution: A.M., P.A.v.V., and S.J.E. designed research, analyzed data, and wrote paper; I.P.T. and P.A.S. designed research and discussed data; H.d.L., N.v.B., and I.J.v.d.B. performed cellular and in vivo research; P.S. designed and developed the bar-coded retroviral miRNA expression system; A.H.d.R., A.J.v.A., and J.D. performed quantitative proteomics and analyzed the data; S.M.S. and E.T. performed miRNA expression profiling and bio-informatic analyses; and B.L. and M.J.-L. provided and analyzed clinical patient data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefan J. Erkeland, PhD, Department of Hematology, Erasmus MC, Dr Molewaterplein 50, 3015 GE Rotterdam, The Netherlands; e-mail: s.erkeland@erasmusmc.nl.
References
Author notes
A.M. and P.A.v.V. contributed equally to this work.

![Figure 2. MiR-17/20/93/106 expression levels during mouse and human neutrophil differentiation. (A) 32D-CSF3R cells were switched from IL-3 to CSF3-containing medium on day t = 0. Micrographs show the morphology of 32D-CSF3R cells on indicated time points of CSF3 treatment. Bars indicate 10 μm. Total RNA was isolated at indicated time points. Expression of indicated mature miRNAs was measured in triplicate with quantitative RT-PCR. The miRNA expressions relative to SnoRNA-234 were measured from immature (steady state, IL-3) samples and set to 1. The average fold expression (n = 3) at indicated time points relative to the IL-3 condition are shown. Error bars represent SD. Significance was calculated by comparing the samples of steady state and the different time points of CSF3 treatment with the Mann-Whitney test (asymptotic significance [2-tailed]). *P < .05. (B) Indicated myeloid cell fractions from human cord blood were stained with cell type specific antibodies and isolated using FACS sort. Micrographs show morphology of sorted cell fractions. Bars indicate 10 μm. MiRNA levels and significance were measured and calculated as described in panel A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/4/10.1182_blood-2011-02-336487/4/m_zh89991174920002.jpeg?Expires=1769377128&Signature=3j1FjcLtkOGPFIb1BDAjrZOwGeAj3M3D91oTq67L90aHtP9pGSEoW-mGZRlTuTK50Eugft43PJPiL-U3VMnS1RpwPfbWKImeGkufouLW-ccCYqCIwDYkc-H43qnWhj-r8rf7nwOSo8tjhWSxdIB0Ln3Fo8lXC5RM-gbp3EPu86WV1m00NvVgtzvThg~oZmc4jxo-qAH7NWsEEEoubZc-MCfJg40XSDVqI~Mmk3Juoeyr81elwiMJ86ZrGGtGN2mtcEwXwxWIFOb~m3cCqn3RGAG~Q6KPDhVH~tsoUwtUeiuTSYjpT1SDxJrOtr9lh4YWHmmEZSbNBiqMhwKp3J9zOw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Functional analysis of miR-17/20/93/106 in primary mouse Lin− BM cells. (A) CFU-G assay of mouse lin− BM progenitors infected with MSCV–miR-17, -miR-20, -miR-93, miR-106 or empty vector control virus. Cells were plated in triplicate at densities of 1 × 104 cells per dish in 1 mL methylcellulose medium containing CSF3 (100 ng/mL). Colonies consisting of more than 50 cells were counted after 7 days of growth. Micrographs show size of CFU-CSF3 on day 7 after plating. Bar indicates 100 μm. Significance was calculated by comparing the samples with EV control and the different miRNAs with the Mann-Whitney test (asymptotic significance [2-tailed]). *P < .05. (B) Replating assay of mouse lin− BM progenitors infected with MSCV–miR-17, -miR-20, -miR-93, miR-106 or empty vector control virus. Cells were plated in triplicate at densities of 1 × 104 cells per dish in 1 mL methylcellulose medium containing IL-6 (10 ng/mL), IL-3 (supernatant 1/1000), SCF (10 ng/mL), CSF2 (10 ng/mL). Cells were isolated from dishes, counted and replated under the same conditions. Colonies from the second plating were counted after 7 days of growth. Micrographs show size of CFU's on day 7 after plating. Bar indicates 100 μm. Significance was calculated as described in panel A. (C) Mouse lineage negative progenitor cells isolated from the BM of C57BL/6 mice were infected with MSCV–miR-17, -miR-20, -miR-93, miR-106 or empty vector control virus. Recipient C57BL/6 mice were irradiated (8.5 Gy) and reconstituted with ∼ 20% GFP positive cells and ∼ 80% WT cells. Six weeks after transplantation, mice were killed and BM cells were isolated. Lin− cells were stained for flow cytometry analysis. The fold induction of the percentage GFP+ control (n = 4), miR-17 (n = 6), miR-20 (n = 4), miR-93 (n = 4), miR-106 (n = 6) Lin−; Sca-I+; c-Kit+/− cells in the BM compared with the input are shown. Significance was calculated by comparing the samples of mice transplanted with EV control and the different miRNAs with the Mann-Whitney test (asymptotic significance [2-tailed]). *P < .05 and **P = .057. (D) Ba/F3-CSF3R cells were infected with MSCV–miR-17, miR-20, miR-93, miR-106 or EV control viruses. Cells were factor deprived for 4 hours (t = 0) followed by CSF3 (100 ng/mL) stimulation for 10 minutes. Cells were washed 2 times with PBS and incubated in RPMI medium for 60 or 120 minutes. Samples for cell lysates were taken at indicated time points and analyzed by Western blotting using total and phospho-specific antibodies against ERK.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/4/10.1182_blood-2011-02-336487/4/m_zh89991174920003.jpeg?Expires=1769377128&Signature=s2jzkDBRMrDzxVcgJb-s~gknzgnlG19TpQMu-joLd0g5zEuvpbOwMb8yya9sGVmkV6bFIpWrNnpFHXX3EQ44wjzkLlJlKF~qRqr4XZ6zqYKjlriXixY6A3DdXmqLQ7KHEak4SJpzpc7BOdkwi7GPtnjNuYUsGwA~y~SWf~EdqFvAHbfgSgFxZw-opEve0YB~lelOBEiaEFEJ1Ue2YyBH1JYYy3QOjhUOEwunVCgIEy1qcwmKsieXQgSW3ULKeC9GwOA8IME5-Quwv4p32qSCPK0l9gJdfenvu1Nr0THHg7Ljp~Z~d5aADlCcKzayWcxIs4VLQxaNXG4rwkrXuHdiEw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Identification of SQSTM1 as a target for AAAGUGC seed-containing miRNAs. The myeloid cell line 32D was infected with miR-93 or empty vector (EV) control viruses. The EV control cells were grown in RPMI media containing 13C-labeled Lys and Arg (heavy) and 32D–miR-93 cells in medium containing regular Lys and Arg (light). Cells were counted and miR-93 containing cells were either mixed with EV control cells in a 1:1 ratio for proteomics analysis or subjected for mRNA isolation. (A) Samples were prepared and analyzed by quantitative proteomics. The ratios of protein expression in steady state (ss) conditions and at 4 days of CSF3 treatment are plotted. The correlation of the average log (L/H) values (n = 2 independent measurements) of a biologic duplicate (rep1 = x-axis and rep2 = y-axis) of all the identified proteins are indicated by the black dots. Single identifications from one experiment are indicated by the green dots. SQSTM1 is indicated by the red arrow and dot. (B) The average SQSTM1 protein levels (n = 2 independent measurements) of a biologic duplicate and the average mRNA abundances relative to GAPDH and EV control cells in steady state (ss) conditions and at 4 days of CSF3 treatment are shown (n = 3). Error bars represent SD (mRNA). Significance was calculated by comparing the EV control with the steady state and CSF3 condition with the Mann-Whitney test (asymptotic significance [2-tailed]). *P < .05. (C) Luciferase reporter plasmids containing the full-length 3′UTR sequence of Sqstm1 with a wt or mutated miRNA binding site were generated and transfected with the Firefly control vector into HEK293 cells. Two days after transfection, cells were lysed and assayed for Luciferase activity. Luciferase values were normalized against Firefly activity. Normalized values for wt 3′UTR were put to 100%. The Luciferase activity values of the mutant 3′UTR relative to the wt is shown. Error bars represent SD of 3 experiments. (D) The Luciferase reporter plasmids containing the full-length 3′UTR sequence of Sqstm1 were cotransfected with indicated MSCV-miRs. The Luciferase activity values of the miRNA expressing cells relative to the Empty vector control are shown. Error bars represent SD of 3 experiments. (E) The Luciferase reporter plasmids containing the mutant 3′UTR sequence of Sqstm1 were cotransfected with indicated MSCV-miRs. (F) 32D-CSF3R cells were switched from IL-3 to CSF3-containing medium on t = 0 days (steady state, SS). Total RNA was isolated at indicated time points. Sqstm1 levels were measured by qPCR in triplicate. Sqstm1 expression relative to Gapdh and to SS condition is shown. (G) Western blot analysis of normal karyotype AML samples exhibiting low and high miR-106 with monoclonal anti-SQSTM1 antibodies. Actin was stained for loading control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/4/10.1182_blood-2011-02-336487/4/m_zh89991174920004.jpeg?Expires=1769377128&Signature=QXWOx-e7kwuvzdidLl~hBXk1LFABxqUD4q0VS5O~7bxheprHNOctOZpNXsc02wJOXkM-EnHKHcqi7iC7~VHE-sBoZfT~Btl1cyqAWiBKgziUd-IqTFsqPAv8-SVxpSq1Zs-rxbqmcmpmf-5ZntErAAjr7h-KiQDMKsof5jPNENv0TxfOq8HO3pubpPA5BcmQUQvdWoMdXN080HuGzfokg3DHlGyz-FFvDOFtH2DA9Xsa-tFqSyWVxrpStctypClW4aGJ9hHwYguu1Vw6Zh-5WpFQkv5lCxZZkzp~hqso~COckfnEUSzzoB8rn-F~UWJqcn4bP1BFp9euwa6IVR4Q4A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. ShRNA-mediated silencing of SQSTM1 phenocopies miRNA overexpression. (A) Replating assay of mouse lin− BM progenitors infected with 2 different hairpin vectors against SQSTM1, pSM2C-SH2024 and pSM2C-SH-2219, or empty vector control virus. Cells were plated in triplicate at densities of 1 × 104 cells per dish in 1 mL methylcellulose medium containing IL-6 (10 ng/mL), IL-3 (supernatant 1/1000), SCF (10 ng/mL), CSF2 (10 ng/mL). Cells were grown for a week, isolated and replated in a secondary CFU-assay (104 cells per plate). Colonies from the second plating were counted after 7 days of growth. Micrographs show size of CFUs on day 7 after plating. Black bar indicates 100 μm, red bars indicate 200 μm. Significance was calculated by comparing the samples with EV control and the different shRNAs with the Mann-Whitney test (asymoptotic significance [2-tailed]). *P < .05. (B) Mouse lineage negative progenitor cells isolated from the BM of C57BL/6 mice were infected with pSM2C-sh-2024 and pSM2C-sh-2219, empty vector control virus or with GFP containing control virus. Cells were selected in PURO 1.5 (μg/mL) containing expansion medium for 2 days. Recipient C57BL/6 mice were lethally irradiated (8.5 Gy) and reconstituted with 45% GFP positive cells mixed with shRNA or EV control infected cells. Six weeks after transplantation, mice were killed and BM cells were isolated. Lin− cells were stained for flow cytometry analysis. The fold induction of the percentage of GFP− Lin−; Sca-I+; c-Kit+ cells in the BM of pSM2C-EV (n = 4), sh-2024 (n = 5) and sh-2219 (n = 4) relative to the input are shown. Significance was calculated by comparing the samples of mice transplanted with EV control and the different shRNAs with the Mann-Whitney test (asymptotic significance [2-tailed]). *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/4/10.1182_blood-2011-02-336487/4/m_zh89991174920005.jpeg?Expires=1769377128&Signature=sq6aMjZlKhAPTsh13tD3lrNwXczuzPA6VbVRAJu1HbzVXRe1fusgKFPvGM4j4Jlv8csf8RrzvD~9uOQHWBGdnoHkHDsouGaLEpwfcU2m7N-gUIClKp8jeTRvnMHoLmt7hmVohriZnMI9J~55pTSeEdCGaobHymk5RSFQbaLuBNJjb7O8lK2tKsihCq-JvMWJnDHs60PNSaOfhinTQOgBFqWqcclffaFiFl0FwDjF5qa9m-JDvUSKHJBdRjv6HAfiBM-gd0A~s~2iBgGnqX7BPDIAXr8lJdHKeEzV8iPY0mQR6YT4ZqG7DyhvcIRnD2xqUX7C6J0ktoLxJ9tdDK61Nw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Role of SQSTM1 in regulation of ligand-induced CSF3R routing, stability and signaling. (A) HeLa-CSF3R cells were transfected with siRNAs against Sqstm1 or control siRNAs. Western blotting showed a ∼ 75% knock-down (KD) in cells transfected with Sqstm1 siRNAs compared with cells transfected with control siRNAs. (B) Transfected cells were stimulated with Bio-CSF3 or left unstimulated for 10 minutes at RT (t = 0), washed with RPMI and transferred to 37°C for indicated time points. CSF3R proteins were pulled down and analyzed as described in panel A. The ratio of detected CSFR protein levels siSQSTM1/siControl samples (n = 3) was calculated for 15 and 120 minutes CSF3 treatment and is depicted. The significance of the difference between the calculated ratios of time point 120 minutes and 15 minutes was calculated with the Mann-Whitney test (asymptotic significance [2-tailed]; P < .10). (C) HeLa-CSF3R cells were transfected with siRNAs against Sqstm1 (+) or control siRNAs (−). Western blotting showed a > 95% knockdown (KD) in cells transfected with siRNAs targeting Sqstm1 transcripts compared with cells transfected with control siRNAs. Cells were serum starved for 24 hours followed by CSF3 (100 ng/mL) treatment as described in panel A. Samples for cell lysates were taken at indicated time points and analyzed by Western blotting with total and/or phospho-specific antibodies against ERK.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/4/10.1182_blood-2011-02-336487/4/m_zh89991174920007.jpeg?Expires=1769377128&Signature=4jhuktAMLofkfsZenAcmnbHtbYfdeq5mNYdwA24tZ6ji0~OEKQI~UUav2EDAJGeFSc-B0x8Q~nN-53LjhiFE69tIc4AVyVImBH0TKvle-xlUXjizto4YQnfXTvFGktEn7DJ2sJxf5kyggeqkLHldwS6r-iiTIpqhygSh7E7VG2zI03X7rADym73yr-XiT5CvQ~rJd1lU3ERG~ORjcZaNI0AtjQxladC56JPbF1KYUOuSJamVlFOFNpLw3jhcEEnJi4Y9Xs7~px09Z1TVy5~6T1L14G~Y7oWEo0gRiJONYmpW9ydRtBFubiJDnUPweFedvVsMMm550Af5oylcC7gVjg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)