Abstract
The triggering receptor expressed on myeloid cells 1 (TREM-1) has been implicated in the production of proinflammatory cytokines and chemokines during bacterial infection and sepsis. For downstream signal transduction, TREM-1 is coupled to the ITAM-containing adaptor DAP12. Here, we demonstrate that Bruton tyrosine kinase (Btk), a member of the Tec kinases, becomes phosphorylated upon TREM-1 triggering. In U937-derived cell lines, in which expression of Btk was diminished by shRNA-mediated knockdown, phosphorylation of Erk1/2 and PLCγ1 and Ca2+ mobilization were reduced after TREM-1 stimulation. Importantly, TREM-1–induced production of the pro-inflammatory cytokines, TNF-α and IL-8, and up-regulation of activation/differentiation cell surface markers were impaired in Btk knockdown cells. Similar results were obtained upon TREM-1 stimulation of BMDCs of Btk−/− mice. The analysis of cells containing Btk mutants revealed that intact membrane localization and a functional kinase domain were required for TREM-1–mediated signaling. Finally, after TREM-1 engagement, TNF-α production by PBMCs was reduced in the majority of patients suffering from X-linked agammaglobulinemia (XLA), a rare hereditary disease caused by mutations in the BTK gene. In conclusion, our data identify Btk as a positive regulator in the ITAM-mediated TREM-1/DAP12 pathway and suggest its implication in inflammatory processes.
Introduction
TREM-1 belongs to the immunoglobulin (Ig)-like superfamily of receptors. In humans, TREM-1 is expressed on neutrophils and CD14high monocytes.1 TREM-1 expression is further up-regulated by TLR ligands. Binding of TREM-1 to endogenous ligand(s) expressed on granulocytes and platelets and present in sera of septic patients,2-4 as well as to exogenous ligands on Marburg and Ebola viruses,5 has been described. The exact nature of TREM-1 ligand(s), however, remains elusive. Engagement of TREM-1 by agonistic monoclonal antibodies (mAbs) results in respiratory burst, degranulation, phagocytosis, secretion of pro-inflammatory cytokines such as TNF-α, of the chemokines, IL-8 and monocyte chemotactic protein-1 (MCP-1), and in the up-regulation of cell surface expressed differentiation/activation markers.1 In animal models of lipopolysacharide (LPS)-induced septic shock and microbial sepsis caused by live Escherichia coli, application of a soluble TREM-1–Ig fusion protein greatly increased survival of experimental animals indicating the importance of TREM-1 in the amplification of inflammation.6
TREM-1 possesses a short intracellular part that lacks intrinsic signaling motifs. Instead, it is coupled to the immunoreceptor tyrosine-based activation motif (ITAM)-containing adaptor protein, DAP12.1 TREM-1 engagement leads to Ca2+ mobilization and phosphorylation of several proteins including DAP12, extracellular-signal regulated kinase (Erk1/2), phospholipase Cγ (PLCγ)1 and the adaptor protein NTAL that further interacts with Grb2.7 Recently, it was reported that the adaptor protein CARD9 is essential for TREM-1-induced secretion of TNF-α, IL-2 and IL-12p40 by mouse bone marrow-derived dendritic cells (BMDCs).8
Bruton tyrosine kinase (Btk), a member of the Tec family of protein tyrosine kinases (PTKs), is involved in signaling via a variety of receptors including the B–cell receptor (BCR), cytokine receptors and integrins.9,10 Loss of Btk function causes X-linked agammaglobulinemia (XLA), a rare primary immunodeficiency disease.11,12 Mutations causing XLA have been described in all Btk domains as well as in noncoding sequences of the gene.13 XLA is manifested by severe defects in early B-cell development, resulting in an almost complete absence of peripheral B cells and Igs of all classes. Affected individuals suffer from recurrent bacterial and enteroviral infections.9 In mice, the R28C point mutation in the pleckstrin homology (PH) domain of Btk leads to X-linked immunodeficiency (Xid).14
In innate immune cells, the role of Btk is less clear. Btk has been implicated in several pathways in myeloid cells.10 Btk associates with certain TLRs and their downstream signaling molecules.15 Contradictory results were obtained from studies of TLR stimulation of monocytes of XLA patients. In one report, relative to healthy controls, monocytes of XLA patients secreted reduced amounts of TNF-α upon LPS stimulation.16 On the other hand, Perez et al17 described that monocytes of XLA patients showed similar TNF-α expression upon TLR4 triggering, compared with healthy individuals. In mouse osteoclasts, Btk mediates signaling in response to RANKL stimulation, and Tec−/−Btk−/− mice show an osteopetrotic phenotype.18 Osteoclasts from XLA patients showed defective resorption activity in vitro but bone density and bone turnover markers were not altered in XLA patients. In serum of XLA patients, increased levels of inflammatory cytokines were detected. Addition of this serum restored the activity of XLA osteoclasts and led to the normalization of bone density in vitro.19 Moreover, Btk is involved in Fcγ receptor-mediated phagocytosis in mouse peritoneal macrophages.20 The role of Btk in DAP12-mediated signaling in monocytes/macrophages is currently unknown.
Here, we demonstrate an important role of Btk in ITAM-mediated TREM-1/DAP12 signaling in myeloid cells. Btk was required for secretion of pro-inflammatory cytokines, Erk1/2 phosphorylation and Ca2+ flux. These data identify Btk as a promising target for the treatment of inflammatory diseases.
Methods
Mice
Tec-deficient mice21 and Btk-deficient mice22 (purchased from The Jackson Laboratory) were intercrossed and maintained in the animal facility of the Medical University of Vienna in concordance with all standards of animal care. Mice were backcrossed onto a C57BL/6 background for at least 8 generations. All animal experiments were approved by the appropriate governmental authorities.
Patients
Eight unrelated male patients diagnosed with XLA, as defined by the World Health Organization (WHO) classification, and 12 healthy individuals were analyzed in this study after informed consent was obtained. This study was approved by the Research Ethics Committee, Medical University of Heidelberg, Germany. All patients received regular Igs substitution therapy and were free of serious infection at the time of blood sampling. All blood samples from XLA patients were taken before Ig treatment. Detailed clinical and molecular information about the XLA patients is depicted in supplemental Table 1 (available on Blood Web site; see the Supplemental Materials link at the top of the online article).
Cell culture and plasmids
All cell lines derived from the human myelomonocytic cell line U937 were cultured in RPMI 1640 supplemented with 10% FCS, 2 mM glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen Life Technologies). Human PBMCs were obtained from peripheral blood by gradient centrifugation using Ficoll-Paque Plus (GE Healthcare; Figure 6) or using Polymorphprep (Axis-Shield; Figure 1C), according to the manufacturer′s instructions, and cultured in X-vivo 20 (Lonza). For a detailed description of BMDCs generation, see supplemental Methods.
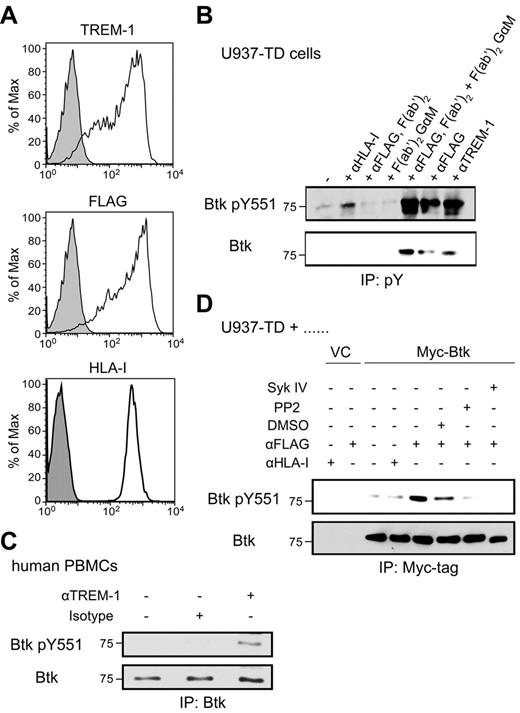
Btk becomes phosphorylated after TREM-1/DAP12 triggering. (A) U937-TD cells were stained with anti–TREM-1, anti-FLAG or anti–HLA-I (open histograms) or isotype-matched control mAbs (filled histograms) followed by staining with goat anti–mouse-PE Abs and analyzed by flow cytometry. (B) U937-TD cells were incubated with the indicated stimuli for 5 minutes at 37°C. pY proteins were immunoprecipitated from postnuclear supernatants using covalently coupled IgG2b-Sepharose followed by anti–pY-Sepharose. Phosphorylation was detected by immunoblotting with anti-Btk pY551 and anti-Btk Abs. (C) Human PBMCs containing 14% TREM-1+ monocytes were incubated with the indicated stimuli for 2 minutes. Btk was immunoprecipitated from postnuclear supernatants using the anti–Btk Ab and Protein A Ultralink Resin. Immunoblotting was performed with anti-Btk pY551 and anti–Btk Abs. (D) U937-TD cells transduced with Myc-tagged Btk were placed in medium alone or in the presence of DMSO or the Src kinase inhibitor, PP2, or the Syk inhibitor IV for 20 minutes at 37°C. Subsequently, cells were incubated with the indicated stimuli for 5 minutes at 37°C. Btk was immunoprecipitated from postnuclear supernatants using the anti–Myc-tag mAb and Protein A Ultralink Resin. Immunoblotting was performed with anti-Btk pY551 and anti-Btk Abs. IP indicates immunoprecipitation; and pY, phosphotyrosine.
Btk becomes phosphorylated after TREM-1/DAP12 triggering. (A) U937-TD cells were stained with anti–TREM-1, anti-FLAG or anti–HLA-I (open histograms) or isotype-matched control mAbs (filled histograms) followed by staining with goat anti–mouse-PE Abs and analyzed by flow cytometry. (B) U937-TD cells were incubated with the indicated stimuli for 5 minutes at 37°C. pY proteins were immunoprecipitated from postnuclear supernatants using covalently coupled IgG2b-Sepharose followed by anti–pY-Sepharose. Phosphorylation was detected by immunoblotting with anti-Btk pY551 and anti-Btk Abs. (C) Human PBMCs containing 14% TREM-1+ monocytes were incubated with the indicated stimuli for 2 minutes. Btk was immunoprecipitated from postnuclear supernatants using the anti–Btk Ab and Protein A Ultralink Resin. Immunoblotting was performed with anti-Btk pY551 and anti–Btk Abs. (D) U937-TD cells transduced with Myc-tagged Btk were placed in medium alone or in the presence of DMSO or the Src kinase inhibitor, PP2, or the Syk inhibitor IV for 20 minutes at 37°C. Subsequently, cells were incubated with the indicated stimuli for 5 minutes at 37°C. Btk was immunoprecipitated from postnuclear supernatants using the anti–Myc-tag mAb and Protein A Ultralink Resin. Immunoblotting was performed with anti-Btk pY551 and anti-Btk Abs. IP indicates immunoprecipitation; and pY, phosphotyrosine.
Vectors, shRNA and cDNA constructs used in this study and methods for the generation of stable cell lines are also described in supplemental Methods.
Stimulation of cells for signal transduction
For cross-linking experiments, cells were incubated with primary mAb (10 μg/mL) for 20 minutes at 4°C, washed twice with warm RPMI 1640 medium and incubated with secondary mAb (10 μg/mL) for 5 minutes at 37°C. For experiments with inhibitors, cells were incubated with PP2, Syk inhibitor IV BAY 61-3606 (both 16 μM; Calbiochem) or DMSO for 20 minutes at 37°C. Primary human PBMCs were incubated for 30 minutes on ice with 2% Venimmun N before stimulation.
Cells (5 × 107/mL) were stimulated with the indicated mAb (10 μg/mL) for 5 minutes at 37°C. Subsequently, cells were washed twice with cold PBS and lysed in 1 mL (per 5 × 107 cells) of LM lysis buffer (1% lauryl-β-D-maltoside [Calbiochem], 20 mM Tris [pH 7.5] 100 mM NaCl, 10% glycerol, 10 mM EDTA [pH 8.0], 50 mM NaF, 1/100 vol/vol of freshly prepared pervanadate, Complete Protease Inhibitor Cocktail [Roche Diagnostics]) for 30 minutes at 4°C. Subsequently, postnuclear supernatants were subjected to immunoprecipitation.
Erk phosphorylation and Ca2+ assay are described in supplemental Methods.
Immunoprecipitation and whole cell lysates
For immunoprecipitation with covalently coupled mAb, postnuclear supernatants were pre-cleared with IgG2b–CNBr-Sepharose 4B beads to eliminate nonspecifically binding proteins. The flow through was enriched for tyrosine-phosphorylated (pY) proteins using anti–pY (clone 4G10)–CNBr-Sepharose 4B beads for 2-3 hours at 4°C. For immunoprecipitation with uncoupled mAb, postnuclear supernatants were precleared with Protein A Ultralink Resin (Thermo Fisher Scientific). The flow through was incubated with the anti–Myc-tag or the anti–Btk Ab for 30 minutes at 4°C, added to 25-40 μL of Protein A Ultralink Resin and samples were incubated for additional 2-3 hours at 4°C. Bound proteins were eluted with 2 × concentrated Laemmli buffer, resolved by SDS-PAGE followed by immunoblotting.
For analysis of whole cell lysates, 5 × 106 cells were lysed for 30 minutes at 4°C in 100 μL of LM lysis buffer. Postnuclear supernatants were mixed at a 1:1 ratio with 2 × concentrated nonreduced Laemmli buffer, heated for 5 minutes at 95°C and reduced with 1% DTT. Proteins were resolved by SDS-PAGE followed by immunoblotting.
Stimulation of cells with immobilized Abs
For cytokine production, human PBMCs from healthy donors or XLA patients, mouse BMDCs and U937-TD–derived cells (5 × 105/mL) were stimulated with the anti–TREM-1, anti-FLAG or the respective isotype control mAb as indicated in the text, immobilized to tissue culture plastic (20 μg/mL). In addition, U937-TD–derived cells were treated with Phorbol 12-Myristate 13-Actetate (PMA; 1 ng/mL; Sigma-Aldrich) and mouse BMDCs were stimulated with PMA (20 ng/mL) and ionomycin (100 ng/mL, Sigma-Aldrich). Supernatants were collected at the indicated time points and the amounts of secreted TNF-α, IL-8 or MIP-2 were analyzed by ELISA (R&D Systems) according to the manufacturer′s instructions.
For the analysis of up-regulation of differentiation/activation markers, cells were harvested, stained with the indicated mAbs and analyzed by flow cytometry using FACSCalibur (BD Biosciences). Subsequent data analyses were performed using the FlowJo Version 9.1 software (TreeStar). Abs used in this study are described in supplemental Methods.
Results
The protein tyrosine kinase Btk is phosphorylated on TREM-1 triggering
To gain insight into signaling events downstream of TREM-1, the U937-TD cell line that ectopically expresses FLAG-tagged TREM-1 (FLAG–TREM-1) and DAP12 was used.7 The parental myelomonocytic cell line, U937, does not express endogenous TREM-1 and has very low levels of endogenous DAP12 (data not shown). U937-TD cells express high levels of FLAG–TREM-1 and human leukocyte antigen class I (HLA-I; Figure 1A). We have shown previously that stimulation of U937-TD cells with anti–TREM-1 or anti-FLAG mAbs led to phosphorylation of several proteins including Erk1/2 kinase and the adaptor protein NTAL, to increased cytokine and chemokine production as well as enhanced Ca2+ flux. Incubation with an isotype-matched anti–HLA-I mAb did not induce these events and was used as a negative control.7
To identify novel regulators in the TREM-1/DAP12 pathway, we focused on proteins known to be involved in Ca2+ mobilization in ITAM-based signaling pathways, such as BCR-mediated signaling in B cells. Accordingly, we selected Btk, a member of the Tec family of PTKs, as a candidate. Indeed, immunoblotting using an anti-Btk pY551 mAb followed by an anti-Btk Ab revealed highly increased levels of Btk pY551 after TREM-1 triggering, compared with incubation with the isotype-matched anti–HLA-I mAb (Figure 1B). In addition, phosphorylation of Btk on Y223 on TREM-1 triggering was observed (data not shown).
To address the possibility that Btk phosphorylation resulted from signaling of Fc receptors, U937-TD cells were stimulated using F(ab′)2 fragments of the anti-FLAG mAb, alone or cross-linked with F(ab′)2-specific goat anti–mouse F(ab′)2 fragments (Figure 1B). F(ab′)2 fragments of the anti-FLAG mAb or the goat anti–mouse F(ab′)2 alone did not induce Btk phosphorylation. However, their cross-linking led to Btk phosphorylation similar to the levels observed during stimulation with anti–TREM-1 or anti-FLAG mAbs (Figure 1B). In the same experiment, we did not observe phosphorylation of the related kinase, Tec, which is also expressed in U937-TD cells (data not shown). Moreover, Btk phosphorylation was observed after TREM-1 stimulation in primary human PBMCs that contained 14% TREM-1+ monocytes (Figure 1C). In PBMCs, only CD14+ monocytes express TREM-1 and thus respond to TREM-1 stimulation (data not shown).1
To determine which kinases are required for Btk phosphorylation upon TREM-1 stimulation, U937-TD cells stably expressing Myc-tagged Btk (Myc-Btk) were stimulated via TREM-1 in the presence or absence of the Src kinase inhibitor, PP2, the Syk kinase inhibitor, Syk IV, or the solvent control, DMSO (Figure 1D). Triggering with the anti-FLAG mAb in the presence of DMSO led to pronounced Btk phosphorylation. In contrast, the presence of Src or Syk kinase inhibitors during the stimulation greatly reduced Btk tyrosine phosphorylation.
Our data indicate that Btk becomes phosphorylated downstream of TREM-1 in a Src and Syk kinase dependent manner, suggesting that Btk might play an important role in the TREM-1/DAP12 signaling pathway.
Btk is a positive regulator of TREM-1 signaling and function
To evaluate the function of Btk in TREM-1 signaling, Btk knockdown cell lines were established. Two different target sequences, one localized in the SH2 domain (B#1 shRNA) of Btk, the other localized in the SH3 domain (B#2 shRNA) of Btk, were selected.23 U937-TD cells were transduced with constructs containing these oligonucleotides or the vector control (VC) resulting in the U937-TD-B#1, U937-TD-B#2 and U937-TD-VC cell lines, respectively. Figure 2A shows that the B#1 shRNA efficiently reduced Btk expression in U937-TD cells, leading to approximately 96% down-regulation of Btk expression relative to the vector control cells. B#2 shRNA impaired Btk expression less efficiently, resulting in 40% down-regulation. Expression of the related kinase, Tec, was not affected by the shRNAs directed against Btk, which indicates the specificity of the selected oligonucleotides (data not shown). Furthermore, all cell lines expressed similar levels of FLAG–TREM-1 on the cell surface (data not shown). For subsequent experiments, U937-TD cells transduced with a construct containing oligonucleotides delivering shRNA directed against GFP (U937-TD-GFP), were used as an additional control.7
TREM-1 signaling is impaired in Btk knockdown cells. (A) Whole cell lysates of U937-TD–derived cells were analyzed by immunoblotting with anti-Btk and anti–β-actin mAbs. LumiImager signals of Btk and β-actin were quantified and the ratio between Btk and β-actin was calculated. (B-C) U937-TD–derived cells were incubated for 16 (B) or 48 (C) hours with either plate-bound anti-FLAG or isotype-matched control mAbs or PMA. (B) Supernatants were analyzed by ELISA for TNF-α and IL-8. Data are displayed as mean ± SD of at least duplicates of ELISA. (C) Cells were analyzed for expression of the differentiation/activation markers, CD11c and CD86, by flow cytometry. (D) U937-TD–derived cells were incubated with medium alone or with isotype-matched anti-HLA-I or with anti-FLAG mAbs for the indicated time periods. Postnuclear supernatants were analyzed by immunoblotting with anti-pErk1/2 and anti-Erk1 Abs. LumiImager signals were quantified, and the ratio between pErk1/2 and Erk1 was calculated. (E) U937-TD cells were incubated with medium, isotype-matched anti–HLA-I or with anti-FLAG mAbs in the presence or absence (w/o) of LY294002 or DMSO for 5 minutes. Postnuclear supernatants were analyzed by immunoblotting with anti–pErk1/2 and anti–β-actin Abs. Signals were quantified, and the ratio between pErk1/2 and β-actin was calculated. (F) Ca2+ mobilization of the Indo-1 AM-labeled U937-TD–derived cells was analyzed by flow cytometry. Cells were stimulated with mAb at the time point indicated by the arrow. (G) U937-TD–derived cells were stimulated with isotype-matched anti–HLA-I or anti-FLAG mAbs for the indicated time periods. Postnuclear supernatants were analyzed by immunoblotting with anti-PLCγ1 pY783 and anti-PLCγ1 Abs. One representative experiment of 2 (G), of 3 (A-F), of 7 (B), of 5 (C) and of 4 (D) independent experiments is shown. n.d. indicates not detectable.
TREM-1 signaling is impaired in Btk knockdown cells. (A) Whole cell lysates of U937-TD–derived cells were analyzed by immunoblotting with anti-Btk and anti–β-actin mAbs. LumiImager signals of Btk and β-actin were quantified and the ratio between Btk and β-actin was calculated. (B-C) U937-TD–derived cells were incubated for 16 (B) or 48 (C) hours with either plate-bound anti-FLAG or isotype-matched control mAbs or PMA. (B) Supernatants were analyzed by ELISA for TNF-α and IL-8. Data are displayed as mean ± SD of at least duplicates of ELISA. (C) Cells were analyzed for expression of the differentiation/activation markers, CD11c and CD86, by flow cytometry. (D) U937-TD–derived cells were incubated with medium alone or with isotype-matched anti-HLA-I or with anti-FLAG mAbs for the indicated time periods. Postnuclear supernatants were analyzed by immunoblotting with anti-pErk1/2 and anti-Erk1 Abs. LumiImager signals were quantified, and the ratio between pErk1/2 and Erk1 was calculated. (E) U937-TD cells were incubated with medium, isotype-matched anti–HLA-I or with anti-FLAG mAbs in the presence or absence (w/o) of LY294002 or DMSO for 5 minutes. Postnuclear supernatants were analyzed by immunoblotting with anti–pErk1/2 and anti–β-actin Abs. Signals were quantified, and the ratio between pErk1/2 and β-actin was calculated. (F) Ca2+ mobilization of the Indo-1 AM-labeled U937-TD–derived cells was analyzed by flow cytometry. Cells were stimulated with mAb at the time point indicated by the arrow. (G) U937-TD–derived cells were stimulated with isotype-matched anti–HLA-I or anti-FLAG mAbs for the indicated time periods. Postnuclear supernatants were analyzed by immunoblotting with anti-PLCγ1 pY783 and anti-PLCγ1 Abs. One representative experiment of 2 (G), of 3 (A-F), of 7 (B), of 5 (C) and of 4 (D) independent experiments is shown. n.d. indicates not detectable.
The stimulation of TREM-1 in U937-TD cells leads to TNF-α and IL-8 production and to up-regulation of the differentiation/activation markers, CD11c and CD86.1,7 To investigate whether Btk affects these processes, U937-TD-VC, U937-TD-B#1, U937-TD-B#2 and U937-TD-GFP were incubated with plate-bound isotype-matched control or anti-FLAG mAbs or with PMA and the production of TNF-α and IL-8 was analyzed. Triggering of TREM-1 for 8, 16, 24 and 48 hours in U937-TD-B#1 led to greatly decreased levels of TNF-α and IL-8 relative to U937-TD-VC (Figure 2B and data not shown). Moreover, lower percentages of CD11c+CD86+ cells were detected after TREM-1 stimulation for 24 and 48 hours of the U937-TD-B#1 cell line (Figure 2C and data not shown). U937-TD-B#2 cells behaved similar to U937-TD-VC cells suggesting that these cells still expressed sufficient levels of Btk for it to perform its function. In addition, cytokine secretion and up-regulation of activation markers in U937-TD-GFP cells was indistinguishable from U937-TD-VC cells. Upon treatment with PMA that bypasses ITAM-based signaling24 no significant differences in cytokine production and up-regulation of CD11c and CD86 were observed. In our previous study,7 TREM-1 stimulation of U937-TD cells in the presence of LPS resulted in highly elevated amounts of TNF-α and IL-8. In Btk knockdown cells (U937-TD-B#1), impaired TNF-α and IL-8 production was observed not only upon TREM-1 stimulation alone but also in combination with LPS (supplemental Figure 1). Stimulation of U937-TD cells by LPS alone led to undetectable or low7 levels of TNF-α. It has been described that Erk1/2 becomes phosphorylated after TREM-1 ligation.1,7 Indeed, Erk1/2 phosphorylation was induced after TREM-1 engagement by the anti-FLAG mAb and on anti-FLAG (Fab′)2 cross-linking (supplemental Figure 2). As depicted in Figure 2D, at 2 minutes after stimulation, levels of Erk1/2 phosphorylation in U937-TD-B#1 cells were similar to U937-TD-VC cells. However, at later time points, they were greatly reduced, suggesting that Btk controls sustained levels of Erk1/2 phosphorylation after TREM-1 triggering. In addition, triggering of TREM-1 in the presence of the PI3K inhibitor, LY294002, led to decreased levels of Erk1/2 phosphorylation (Figure 2E).
Members of the Tec kinase family were shown to be important for Ca2+ mobilization after antigen receptor engagement.25 Indeed, the Btk knockdown cell line, U937-TD-B#1, exhibited a reduced Ca2+ response upon stimulation via TREM-1 compared with control cells (Figure 2F). PLCγ is an important regulator of the initiation of Ca2+ flux.26 Among two PLCγ isoforms, PLCγ1 is the prominent isoform expressed in U937 cells (data not shown). After TREM-1 triggering, phosphorylation of PLCγ1 was reduced but, similar to Ca2+ elevation, was not completely abolished in U937-TD-B#1 cells (Figure 2G).
In summary, our data demonstrate that Btk is a positive regulator of cytokine production, of up-regulation of the differentiation/activation markers CD11c and CD86, of sustained Erk1/2 phosphorylation, of Ca2+ mobilization and PLCγ1 phosphorylation in the TREM-1/DAP12 pathway.
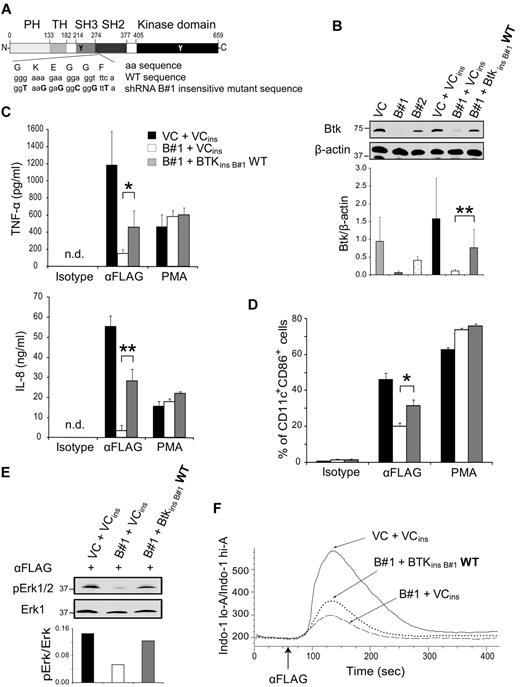
Expression of Btk insensitive to the B#1 shRNA restores the defects in Btk knockdown cells
To confirm that the observed functional defects were because of the down-regulation of Btk expression by RNA silencing, a Btk construct insensitive to the B#1 shRNA was designed. Nucleotides in the target sequence of the B#1 shRNA were mutated by site-directed mutagenesis to disable binding of the B#1 shRNA but preserve the amino acid sequence of Btk (Figure 3A). The U937-TD-B#1 cell line was transduced by either an empty vector or the vector containing Btk insensitive to B#1 shRNA to establish U937-TD-B#1-VCins and U937-TD-B#1-BtkinsB#1WT, respectively. In parallel, U937-TD-VC cells were transduced by an empty vector to produce U937-TD-VC-VCins with endogenous Btk expression. Figure 3B illustrates that Btk expression in the rescue cell line U937-TD-B#1-BtkinsB#1WT was increased to approximately 50% of the amount in U937-TD-VC-VCins cells containing endogenous Btk. Expression of FLAG-TREM-1 by these cell lines was similar to the parental cell lines (data not shown).
Expression of Btk insensitive to the B#1 shRNA restores the defects of the Btk knockdown cell line. (A) A schematic picture of the construct used to prepare the Btk rescue cell line, U937-TD-B#1-BtkinsB#1WT, is depicted. (B) Whole cell lysates of U937-TD–derived cells were analyzed by immunoblotting with anti-Btk and anti–β-actin mAbs. LumiImager signals of the ratio between Btk and β-actin are displayed as mean ± SD from 7 independent experiments. (C, D) U937-TD–derived cells were incubated with either plate-bound anti-FLAG or isotype-matched control mAbs or PMA. (C) ELISA analysis of supernatants collected after 8 (TNF-α) or 16 (IL-8) hours. Data are displayed as mean ± SD from ELISA performed at least in duplicates of duplicate cultures. (D) Expression analysis of CD11c and CD86 after 48 hours by flow cytometry is shown. Data are displayed as mean ± SD from duplicate cultures. (E) U937-TD–derived cells were stimulated with the anti-FLAG mAb for 5 minutes. Postnuclear supernatants were analyzed by immunoblotting with anti–pErk1/2 and anti–Erk1 Abs. LumiImager signals were quantified, and the ratio between pErk1/2 and Erk1 was calculated. (F) Ca2+ mobilization of Indo-1 AM-labeled U937-TD–derived cells was analyzed by flow cytometry. Cells were stimulated with the anti-FLAG mAb at the time point indicated by the arrow. One representative experiment of 7 (B), of 5 (C,F), of 4 (D) and of 3 (E) independent experiments is shown. *P < .05 and **P < .005 (Student t test). ins indicates insensitive; and WT, wild-type.
Expression of Btk insensitive to the B#1 shRNA restores the defects of the Btk knockdown cell line. (A) A schematic picture of the construct used to prepare the Btk rescue cell line, U937-TD-B#1-BtkinsB#1WT, is depicted. (B) Whole cell lysates of U937-TD–derived cells were analyzed by immunoblotting with anti-Btk and anti–β-actin mAbs. LumiImager signals of the ratio between Btk and β-actin are displayed as mean ± SD from 7 independent experiments. (C, D) U937-TD–derived cells were incubated with either plate-bound anti-FLAG or isotype-matched control mAbs or PMA. (C) ELISA analysis of supernatants collected after 8 (TNF-α) or 16 (IL-8) hours. Data are displayed as mean ± SD from ELISA performed at least in duplicates of duplicate cultures. (D) Expression analysis of CD11c and CD86 after 48 hours by flow cytometry is shown. Data are displayed as mean ± SD from duplicate cultures. (E) U937-TD–derived cells were stimulated with the anti-FLAG mAb for 5 minutes. Postnuclear supernatants were analyzed by immunoblotting with anti–pErk1/2 and anti–Erk1 Abs. LumiImager signals were quantified, and the ratio between pErk1/2 and Erk1 was calculated. (F) Ca2+ mobilization of Indo-1 AM-labeled U937-TD–derived cells was analyzed by flow cytometry. Cells were stimulated with the anti-FLAG mAb at the time point indicated by the arrow. One representative experiment of 7 (B), of 5 (C,F), of 4 (D) and of 3 (E) independent experiments is shown. *P < .05 and **P < .005 (Student t test). ins indicates insensitive; and WT, wild-type.
We first investigated whether TREM-1–induced cytokine secretion and up-regulation of CD11c and CD86 were affected upon TREM-1 triggering in the Btk rescue cell line. Indeed, in U937-TD-B#1-BtkinsB#1WT, the levels of TNF-α and IL-8 (Figure 3C) as well as the percentages of CD11c+CD86+ cells (Figure 3D) were significantly increased up to 50% of the amounts produced by cells containing endogenous Btk. The extent of functional responses correlated with the level of Btk expression in U937-TD-B#1-BtkinsB#1WT cells (Figure 3B). Furthermore, no differences in cytokine production or in up-regulation of CD11c and CD86 were observed upon treatment with PMA, demonstrating a similar intrinsic signaling potential of these cells. Likewise, Erk1/2 phosphorylation was improved in U937-TD-B#1-BtkinsB#1WT cells (Figure 3E). After engagement of TREM-1, a modest but consistent increase in Ca2+ mobilization was observed in U937-TD-B#1-BtkinsB#1WT cells compared with that of Btk knockdown cells (Figure 3F).
In summary, transduction of Btk insensitive to B#1 shRNA in U937-TD-B#1 cells led to the recovery of cytokine secretion, up-regulation of CD11c and CD86, and Erk1/2 phosphorylation reaching around 50% of its original levels. The extent of this rescue of functional responses correlated with the relative amounts of Btk expressed in these cells. Our data confirm that the functional defects observed in Btk knockdown cells were because of their decreased levels of Btk.
Intact membrane localization and a functional kinase domain are required for function of Btk in the TREM-1 signaling pathway
We next investigated whether certain domains of Btk were required for TREM-1 signaling and function. To inactivate these domains, point mutations were introduced as illustrated in Figure 4A. The point mutation, E41K, in the PH domain reportedly correlates with increased phosphorylation and translocation to the plasma membrane.27 The R28C mutation in the PH domain impairs Btk binding to PIP3 in the plasma membrane28 and the K430E mutation targets the kinase domain of Btk, rendering it inactive.29
Intact plasma membrane localization and a functional kinase domain of Btk are essential for Btk function after TREM-1 triggering. (A) Schematic illustration of BtkinsB#1 mutants introduced into U937-TD-B#1 cells. (B) Whole cell lysates of U937-TD–derived cells were analyzed by immunoblotting with anti-Btk and anti–β-actin or anti–α-tubulin mAbs. LumiImager signals of the ratio between Btk and the indicated housekeeping protein are displayed as mean ± SD from 7 independent experiments. (C, D) U937-TD–derived cells were incubated with either plate-bound anti–FLAG or isotype-matched control mAbs or PMA. (C) Supernatants were collected after 8 (TNF-α) or 16 (IL-8) hours and cytokine production was analyzed by ELISA. Data are displayed as mean ± SD of at least duplicates of ELISA from duplicate cultures. (D) Expression analysis of CD11c and CD86 after 48 hours by flow cytometry is depicted. Data are displayed as mean ± SD from duplicate cultures. (E) U937-TD derived cells were stimulated with the anti-FLAG mAb for 5 minutes. Postnuclear supernatants were analyzed by immunoblotting with anti–pErk1/2 and anti-Erk1 mAbs. LumiImager signals were quantified, and the ratio between pErk1/2 and Erk1 was calculated. (F) Ca2+ mobilization of Indo-1 AM-labeled U937-TD–derived cells was analyzed by flow cytometry. Cells were stimulated with the anti-FLAG mAb at the time point indicated by the arrow. One representative experiment of 7 (B), of 4 (C,D), of 3 (E) and of 5 (F) independent experiments is shown. *P < .05 and **P < .003 (Student t test).
Intact plasma membrane localization and a functional kinase domain of Btk are essential for Btk function after TREM-1 triggering. (A) Schematic illustration of BtkinsB#1 mutants introduced into U937-TD-B#1 cells. (B) Whole cell lysates of U937-TD–derived cells were analyzed by immunoblotting with anti-Btk and anti–β-actin or anti–α-tubulin mAbs. LumiImager signals of the ratio between Btk and the indicated housekeeping protein are displayed as mean ± SD from 7 independent experiments. (C, D) U937-TD–derived cells were incubated with either plate-bound anti–FLAG or isotype-matched control mAbs or PMA. (C) Supernatants were collected after 8 (TNF-α) or 16 (IL-8) hours and cytokine production was analyzed by ELISA. Data are displayed as mean ± SD of at least duplicates of ELISA from duplicate cultures. (D) Expression analysis of CD11c and CD86 after 48 hours by flow cytometry is depicted. Data are displayed as mean ± SD from duplicate cultures. (E) U937-TD derived cells were stimulated with the anti-FLAG mAb for 5 minutes. Postnuclear supernatants were analyzed by immunoblotting with anti–pErk1/2 and anti-Erk1 mAbs. LumiImager signals were quantified, and the ratio between pErk1/2 and Erk1 was calculated. (F) Ca2+ mobilization of Indo-1 AM-labeled U937-TD–derived cells was analyzed by flow cytometry. Cells were stimulated with the anti-FLAG mAb at the time point indicated by the arrow. One representative experiment of 7 (B), of 4 (C,D), of 3 (E) and of 5 (F) independent experiments is shown. *P < .05 and **P < .003 (Student t test).
U937-TD-B#1 cells were stably transduced with constructs containing Btk WT, E41K, R28C or K430E insensitive to B#1 shRNA or an empty vector. Figure 4B shows expression of Btk in these cell lines. Expression of FLAG–TREM-1 was comparable in all cell lines except for U937-TD-B#1-BtkinsB#1R28C that consistently revealed slightly higher amounts of TREM-1 on the cell surface (data not shown).
First, cytokine production and up-regulation of CD11c and CD86 on TREM-1 triggering were investigated (Figure 4C-D). Cells expressing Btk E41K displayed significantly higher levels of TNF-α and IL-8 relative to U937-TD-B#1-BtkinsB#1WT and U937-TD-B#1-VCins cells. The up-regulation of CD11c and CD86 was consistently increased in cells expressing Btk E41K. In cells carrying the Btk R28C or K430E mutations, greatly reduced cytokine production and percentages of CD11c+CD86+ cells upon TREM-1 triggering were observed.
Then, TREM-1–induced Erk1/2 phosphorylation was analyzed in U937-TD-B#1-VCins, U937-TD-B#1-BtkinsB#1WT and U937-TD-B#1-BtkinsB#1E41K, R28C and K430E cells (Figure 4E). After TREM-1 triggering, cells expressing Btk E41K displayed slightly increased Erk1/2 phosphorylation compared with WT. In contrast, Erk1/2 phosphorylation was lower in cells carrying Btk R28C or K430E.
Finally, we investigated whether Ca2+ flux was affected in the Btk mutant cell lines. Upon TREM-1 engagement, Ca2+ mobilization was enhanced in U937-TD-B#1-BtkinsB#1E41K (Figure 4F). By contrast, in cells expressing Btk R28C or K430E mutants the Ca2+ response was decreased.
Our results indicate that TREM-1–induced cytokine production, up-regulation of activation markers, Erk1/2 phosphorylation and Ca2+ mobilization are dependent on intact plasma membrane localization of Btk and its kinase activity.
TREM-1–induced cytokine secretion is reduced in BMDCs generated from Btk−/− mice
Our recent study revealed that mouse TREM-1 is expressed by subsets of blood monocytes, blood granulocytes and by BMDCs. Upon TREM-1 stimulation, BMDCs, but not other TREM-1–expressing cell populations, produce pro-inflammatory cytokines.30
To investigate whether Btk affects TREM-1 function in mouse primary cells, BMDCs were generated from bone marrow precursors of WT and Btk−/− mice by a 3-day culture in GM-CSF-containing medium. Because the related Tec kinase might compensate for Btk function in Btk−/− mice, we also analyzed BMDCs from Tec−/− and Tec−/−Btk−/− mice.21 On all BMDCs similar expression of TREM-1, CD86, MHC class II or CD40 was observed by flow cytometry (Figure 5A and data not shown).
Btk is required for TREM-1-induced cytokine production in mouse BMDCs. BMDCs were generated from bone marrow cells of WT, Btk−/−, Tec−/− and Tec−/−Btk−/− mice in GM-CSF-containing medium. (A) On day 3, BMDC from WT and Tec−/−Btk−/− mice were stained with isotype-matched (dashed line) or anti–TREM-1 (full line) mAbs and analyzed by flow cytometry. (B) On day 4, cells were incubated for 8 hours with either plate-bound isotype-matched control or plate-bound anti–TREM-1 mAbs or with PMA and ionomycin. Supernatants were analyzed by ELISA for TNF-α and MIP-2. Data are displayed as mean ± SD of at least duplicates of ELISA from duplicate cultures. Figure shows 1 representative result of 3 independent experiments. *P < .01, **P < .006 and ***P < .0001 (Student t test). iono indicates ionomycin.
Btk is required for TREM-1-induced cytokine production in mouse BMDCs. BMDCs were generated from bone marrow cells of WT, Btk−/−, Tec−/− and Tec−/−Btk−/− mice in GM-CSF-containing medium. (A) On day 3, BMDC from WT and Tec−/−Btk−/− mice were stained with isotype-matched (dashed line) or anti–TREM-1 (full line) mAbs and analyzed by flow cytometry. (B) On day 4, cells were incubated for 8 hours with either plate-bound isotype-matched control or plate-bound anti–TREM-1 mAbs or with PMA and ionomycin. Supernatants were analyzed by ELISA for TNF-α and MIP-2. Data are displayed as mean ± SD of at least duplicates of ELISA from duplicate cultures. Figure shows 1 representative result of 3 independent experiments. *P < .01, **P < .006 and ***P < .0001 (Student t test). iono indicates ionomycin.
Upon TREM-1 triggering, production of TNF-α and macrophage inflammatory protein 2 (MIP-2) was significantly reduced by Btk−/− BMDCs, but only slightly impaired by Tec−/− BMDCs. Moreover, in Tec−/−Btk−/− BMDCs, cytokine secretion was almost completely abolished (Figure 5B). All cells produced comparable amounts of cytokines upon treatment with PMA and ionomycin. Similar results were obtained at 8, 24 and 36 hours time points (data not shown). No up-regulation of CD86, CD80 or CD40, on TREM-1 triggering was observed regardless whether BMDCs were derived from WT or knockout mice (data not shown).
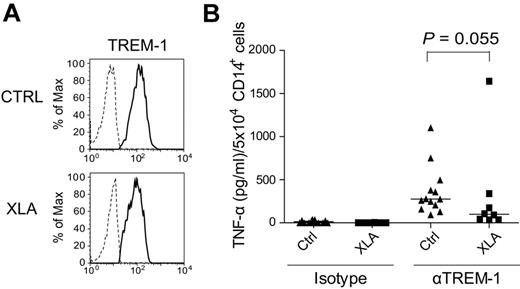
PBMCs from the majority of tested XLA patients secrete decreased levels of TNF-α after TREM-1 stimulation
To study the role of Btk in primary human cells, TREM-1–induced TNF-α production in patients with XLA (supplemental Table 1), a rare hereditary disease caused by mutations in the BTK gene,11,12 was examined. Also within PBMCs of XLA patients, only CD14+ monocytes expressed TREM-1 (data not shown). Similar TREM-1 expression by healthy donors (average MFI = 153.7 ± 87.3) and XLA patients (average MFI = 134.5 ± 37.8) on CD14+ monocytes was observed (Figure 6A). Upon TREM-1 triggering, TNF-α production was greatly impaired in PBMCs of 7 of 8 XLA patients (Figure 6B). The median value of TNF-α production normalized to 5 × 104 of CD14+ cells for healthy controls was 275 pg/mL and for XLA patients 99 pg/mL (P = .055, determined by Mann Whitney test). PBMCs from one patient produced extremely high amounts of TNF-α (1642.5 ± 153 pg/mL) upon TREM-1 stimulation. The reasons for this hyper-responsiveness upon TREM-1 stimulation are currently unknown and will be addressed in future studies. In summary, our data indicate that Btk is a positive regulator of TNF-α production in TREM-1–mediated signaling in primary human PBMCs.
Cytokine production in PBMCs from XLA patients after TREM-1 triggering. (A) Expression of TREM-1 on PBMCs of healthy controls and of XLA patients was analyzed by flow cytometry. Representative histograms show overlays of staining with isotype-matched (dashed line) or anti–TREM-1 (full line) mAbs gated on CD14+ cells. (B) PBMCs were prepared from peripheral blood of XLA patients and healthy individuals by Ficoll-Paque gradient centrifugation. Cells were incubated with plate-bound isotype control or anti–TREM-1 mAbs for 24 hours and TNF-α production was analyzed by ELISA. Because of the lack of B cells in XLA patients, slightly increased frequency of TREM-1+ cells was observed (healthy controls: 21.4 ± 5.4%; XLA: 30.3 ± 8.0%). Thus, levels of TNF-α production were normalized to 5 × 104 of CD14+ cells. Median value of group is depicted in the graph as a line.
Cytokine production in PBMCs from XLA patients after TREM-1 triggering. (A) Expression of TREM-1 on PBMCs of healthy controls and of XLA patients was analyzed by flow cytometry. Representative histograms show overlays of staining with isotype-matched (dashed line) or anti–TREM-1 (full line) mAbs gated on CD14+ cells. (B) PBMCs were prepared from peripheral blood of XLA patients and healthy individuals by Ficoll-Paque gradient centrifugation. Cells were incubated with plate-bound isotype control or anti–TREM-1 mAbs for 24 hours and TNF-α production was analyzed by ELISA. Because of the lack of B cells in XLA patients, slightly increased frequency of TREM-1+ cells was observed (healthy controls: 21.4 ± 5.4%; XLA: 30.3 ± 8.0%). Thus, levels of TNF-α production were normalized to 5 × 104 of CD14+ cells. Median value of group is depicted in the graph as a line.
Discussion
TREM-1 is implicated in the amplification of inflammatory responses and sepsis.6 A better understanding of the TREM-1/DAP12 signaling pathway could help in designing novel therapies to control inflammatory diseases. Here, we demonstrate that after TREM-1 stimulation, Btk, a member of the Tec family of PTKs, becomes phosphorylated at Y551 in a Src and Syk kinase dependent manner. Y551 is located in the activation loop of Btk that was shown to be required for its intact kinase activity.31 Phosphorylation at Y223 in the SH3 domain of Btk results from an internal autokinase activity of Btk and serves as an additional indicator of Btk activation.31 Of note, we observed increased phosphorylation at Y223 of Btk on TREM-1 triggering as well (data not shown). Thus, upon TREM-1 engagement, Btk becomes phosphorylated and activated.
Using several approaches, such as knocking down Btk expression by RNA interference, studying BMDCs from Btk−/− mice and the analysis of PBMCs from patients suffering from XLA immunodeficiency, we demonstrate that Btk is a positive regulator of TNF-α production upon TREM-1 stimulation. Upon LPS stimulation of human monocytes, Btk is involved in the stabilization of mRNA of TNF-α, which was associated with an increase in the phosphorylation of p38 mitogen-activated protein kinase (MAPK).16 We did not observe increased phosphorylation of p38 upon TREM-1 triggering in U937-TD cells (data not shown). Instead, we observed increased Erk1/2 phosphorylation after TREM-1 stimulation. In this context, it has been shown that after LPS stimulation, elevated levels of Erk1/2 phosphorylation correlated with higher production of TNF-α.32 In Btk knockdown cells, Erk1/2 phosphorylation was similar to that of control cells at early time points, but later it was greatly reduced. These data suggest that after TREM-1 engagement, Btk sustains enhanced levels of Erk1/2 phosphorylation. A similar observation was published previously in Btk-deficient DT40 cells after BCR stimulation.33
Upon LPS stimulation, Btk was implicated in the phosphorylation of the p65 subunit of NFκB, which was dependent on the intact kinase activity of Btk.34 Our analysis of cells carrying different mutants of Btk revealed that a functional Btk kinase domain was essential for TREM-1–induced cytokine production. In this context, NFκB was shown to be activated by TREM-1 triggering.35 In addition, weak phosphorylation of IL-1 receptor-associated kinase 1 (IRAK1), a kinase implicated in the activation of NFκB downstream of TLRs, was demonstrated after TREM-1 triggering.36 It is possible that Btk is involved in the phosphorylation of downstream substrates, such as the p65 subunit of NFκB, after TREM-1 engagement, facilitating TNF-α production. Moreover, NFκB is involved in the regulation of transcription of the BTK gene37 which might further amplify an on-going pro-inflammatory immune response.
Our results obtained with BMDCs from knockout mice reveal an important role of Btk in the regulation of TREM-1–induced TNF-α production. TNF-α production upon TREM-1 stimulation was only minimally affected by the absence of just Tec kinase. We did not observe Tec phosphorylation after engagement of the TREM-1 pathway in U937-TD cells (data not shown), suggesting a subordinate role of Tec in TREM-1 signaling.
After BCR engagement, Btk mediates an increase in intracellular Ca2+ required for the activation of transcription factors such as NFAT.25,38 It was shown that Btk phosphorylates PLCγ1 at several tyrosine residues, including Y783, which is necessary for its maximal lipid hydrolase activity.39 We observed greatly impaired Ca2+ flux and PLCγ1 phosphorylation in Btk knockdown cells after TREM-1 triggering which was dependent on intact R28 in the PH of Btk and the functional kinase domain of Btk. Neither Ca2+ flux nor PLCγ1 phosphorylation were completely abolished in Btk knockdown cells. Accordingly, it was shown that Syk kinase is absolutely essential for Ca2+ flux and PLCγ phosphorylation while Btk regulates the magnitude of these processes after stimulation via BCR.38,40
In our study, we used different Btk mutants to dissect the molecular requirements underlying the function of Btk downstream of TREM-1. U937-TD cells carrying the Btk E41K mutation exhibited increased cytokine production, up-regulation of activation markers, Erk1/2 phosphorylation and Ca2+ mobilization compared with control cells. The E41K mutation of Btk was shown to be hyperphosphorylated27 and to exhibit stronger association with membrane phospholipids,41 resulting in higher amounts of Btk at the plasma membrane.27 In concordance with our study, increased Ca2+ mobilization was observed after BCR engagement in Btk−/− DT40 cells reconstituted with Btk E41K.42 However, transgenic mice expressing Btk E41K suffer from similar but more severe B–cell defect compared with Xid mice.43
U937-TD cells carrying Btk R28C displayed greatly impaired responses to TREM-1 stimulation compared with control cells. The R28C mutation disables Btk to bind to PIP3 in the plasma membrane.28 Thus, Btk R28C cannot relocalize from the cytosol to the close proximity of the plasma membrane where activation of PLCγ takes place. Modifications at position 28 are common alterations occurring in XLA patients44 and the reason for the B–cell phenotype observed in Xid mice.14
The Btk K430E mutation targets the kinase domain of Btk, rendering it inactive. We observed that in U937-TD cells expressing Btk K430E cytokine production, up-regulation of activation markers, Erk1/2 phosphorylation and Ca2+ mobilization were impaired compared with U937-TD cells carrying Btk WT. In contrast, in Btk−/− DT40 cells reconstituted with Btk K430E, Ca2+ mobilization is fully restored.45 In this study, it was proposed that besides its kinase activity, Btk might also function as an adaptor protein that facilitates Ca2+ flux. Similarly, Btk‘s tumor suppressor activity is fully restored by the kinase inactive form of Btk.46 Of note, Btk R525Q, another kinase inactive mutant, did not reconstitute BCR-induced Ca2+ flux.38 We conclude that the function of Btk might depend on the cellular context and pathways studied.
Thus, intact localization in the plasma membrane and intact kinase activity of Btk are essential for its function in the TREM-1/DAP12 signaling pathway. Apart from known targets such as PLCγ, Btk might phosphorylate other yet unidentified molecules downstream of TREM-1. Although the comparison of overall tyrosine phosphorylation patterns of Btk knockdown cells did not reveal any apparent differences (data not shown), our future studies will seek to identify the unknown targets of Btk in the TREM-1 pathway. Together with Btk these molecules might represent suitable targets for chemical intervention in patients with inflammatory diseases.
Our experiments revealed greatly reduced TNF-α production by PBMCs from 7 of 8 tested XLA patients compared with healthy controls on TREM-1 stimulation. XLA patients carry mutations in the BTK gene leading to the complete loss or dysfunction of Btk.11,12 Becausethe exact nature of the TREM-1 ligand(s) is currently unknown, the implications of our findings regarding the clinical manifestation of XLA patients, such as their high susceptibility to infection, remain unclear. Elevated levels of inflammatory cytokines including TNF-α were detected in the serum of XLA patients.19 Our data suggest that these high levels of TNF-α arise from TREM-1–unrelated pathways. The stimulation of monocytes of XLA patients with LPS resulted in controversial findings. One study reported defects in TNF-α production by monocytes of XLA patients upon LPS stimulation16 whereas another study observed similar levels of TNF-α expression.17 Thus, the contribution of TLR4 signaling to the high TNF-α serum levels in XLA patients requires further investigation. In one patient, extremely high amounts of TNF-α production (1642.5 ± 153 pg/mL) were detected upon TREM-1 stimulation of PBMCs. The reasons for this increased TNF-α production are currently not known and are under investigation in our laboratory.
In conclusion, Btk is a positive regulator of the TREM-1 signaling pathway that leads to TNF-α production. It is well established that TNF-α is a key mediator of inflammatory responses and sepsis. Therefore, it is tempting to speculate that inhibition of Btk with specific small molecular compounds might be a promising strategy in the treatment of inflammation and sepsis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank G. Superti-Furga (Center for Molecular Medicine, Austrian Academy of Sciences, Vienna, Austria) for providing constructs containing human Btk, O. Witt (Clinical Cooperation Unit Pediatric Oncology, DKFZ, Heidelberg, Germany) for initiating the contacts for the XLA study, S. Ehl (Center for Chronic Immunodeficiency, Research group Pediatric Immunology, Freiburg, Germany), and A. Rölle, M. Kegel, J. Ormsby and P. Willis for critically reading the manuscript.
This work was supported by a Marie Curie Excellence Grant (to A.C.). P.A. and V.H. are supported by project of 1M0506 (MSMT, Czech Republic). T.O. is partially supported by the Faculty of Sciences, Charles University in Prague (Czech Republic).
Authorship
Contribution: T.O. designed and performed the experiments, analyzed the data and wrote the manuscript; E.S. performed experiments with XLA patients; J.F., A.S.T. and P.A. helped with experiments; A.D.K. and W. E. provided knockout mice; K.W., M.B. and I.S. helped with the recruitment of XLA patients; and V.H. and A.C. oversaw the project, designed experiments and wrote the manuscript.
Conflict of interest disclosure: The authors declare no competing financial interests.
Correspondence: Adelheid Cerwenka, German Cancer Research Center (DKFZ/D080), Im Neuenheimer Feld 280, D-69120, Heidelberg, Germany; e-mail: a.cerwenka@dkfz-heidelberg.de.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal