B cells have important roles in biology and disease pathogenesis that go beyond their ability to make antibodies. Two reports in this issue of Blood describe the accumulation in aged vertebrates of populations of lymphocytes called age-associated B cells, or ABCs.
In the first article, Hao et al describe a population of ABCs in mice that do not express markers that characterize follicular, marginal zone, B-1, and transitional B cells.1 In the second, Rubtsov et al describe ABCs that express an integrin chain, CD11c (typically a marker of dendritic cells), in aged female mice, and also describe similar cells in women with autoimmunity.2 This female preponderance, while intriguing, remains to be mechanistically explained. TLR7 signaling appears to be required for the generation of these nonself-renewing, BAFF-independent B cells that represent the age-dependent accumulation of lymphocytes that may possess some memory characteristics, explaining their ongoing accumulation. As noted in these reports, these cells are reminiscent of the accumulations of IgD−, CD27− B cells reported previously in aged humans, and in subjects with autoimmune disorders or with common variable immunodeficiency. The resemblance to exhausted B cells described previously in subjects with autoimmunity and chronic HIV disease has also been noted. The accumulations seen in disease may represent the acceleration of a normal, ongoing process that occurs as vertebrates age. ABCs, like exhausted B cells, respond poorly to BCR or CD40 triggering. However, TLR7 or TLR9 ligation, or the combined activation of the BCR and endosomal TLRs, results in ABC activation, enhanced antigen presentation by these B cells, cytokine secretion, the facilitation of Th17 T-cell generation, and antibody secretion. The bias toward Th17 differentiation mediated by ABCs remains to be established in vivo and the underlying basis for this presumed polarizing property is unclear.
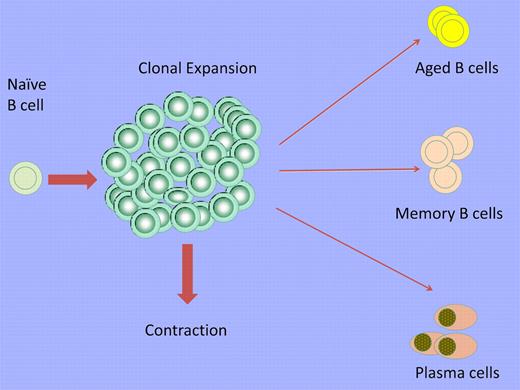
After the activation of a B cell by a typical T-dependent antigen, clonal expansion is followed by a contraction phase in which the majority of the activated B cells are lost but some persist as long-lived memory cells and plasma cells. Some persisting B cells acquire an exhausted phenotype and accumulate with age as ABCs. This expansion is more apparent in aged female subjects and subjects with autoimmunity.
After the activation of a B cell by a typical T-dependent antigen, clonal expansion is followed by a contraction phase in which the majority of the activated B cells are lost but some persist as long-lived memory cells and plasma cells. Some persisting B cells acquire an exhausted phenotype and accumulate with age as ABCs. This expansion is more apparent in aged female subjects and subjects with autoimmunity.
The activation of B cells therefore results in a range of consequences. The majority of activated cells, including short-lived plasma cells, are lost during the process of contraction, but in addition to the survival of memory cells and long-lived plasma cells, exhausted B cells and/or ABCs may also be normally generated (see figure). ABCs accumulate in autoimmune states and probably also in other chronic disorders. The remission of autoimmune disorders such as rheumatoid arthritis, type I diabetes, and multiple sclerosis after human B-cell depletion might reflect the induced loss of B cells that had expanded frequently in response to auto-antigens and then assumed the phenotype of ABCs. It remains to be established whether ABCs represent a stable subset and whether more than 1 phenotypic subset of these aged B cells exist. Their relationship to exhausted B cells needs further clarification. The signals required for ABC differentiation (beyond TLR7) need to be established and it remains to be asked if conventional memory B cells are an obligate developmental intermediate. Finally, it needs to be determined whether ABCs play essential roles in protective responses and/or in the pathogenesis of autoimmunity.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal