In this issue of Blood, Kusakabe et al make a compelling case that the transcription factor c-Maf is critical for erythroblastic island formation and fetal erythropoiesis.1 The cell adhesion molecule VCAM-1 is a potential mediator of c-Maf activity.
The erythroblastic island was ultrastructurally identified as a common organizational unit of bone marrow more than 50 years ago.2 Erythroblastic islands are composed of erythroblasts, at various stages of maturation, and a central macrophage. The central macrophage supports erythroid cell survival and proliferation through intercellular signaling, and facilitates enucleation.3 Along this line, several molecules have been identified that mediate intercellular attachments and macrophage-erythroblast interactions within the island.4-6 Still, despite these advances the role of the central macrophage in erythroid development remains somewhat obscure. Kusakabe et al now show that the basic region-leucine zipper transcription factor c-Maf is critical for erythroblastic island formation.1,6 The macrophage is the site of c-Maf activity, and c-Maf deficiency is associated with severe embryonic anemia and lethality. This study opens a new avenue into the investigation of erythroblastic islands.
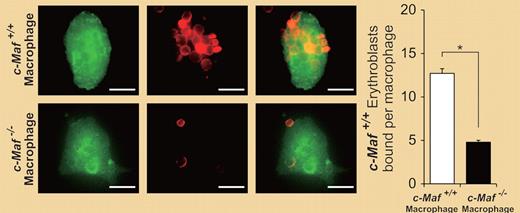
Erythroblastic islands reconstituted with c-Maf+/+ erythroblasts were immunostained. The number of c-Maf+/+ erythroblasts surrounding each c-Maf+/+ or c-Maf−/− macrophage is shown. c-Maf+/+ erythroblasts surrounding c-Maf−/− macrophages were significantly reduced as compared with those seen for c-Maf+/+ macrophages. See the entire figure in the article by Kusakabe et al on page 1374.
Erythroblastic islands reconstituted with c-Maf+/+ erythroblasts were immunostained. The number of c-Maf+/+ erythroblasts surrounding each c-Maf+/+ or c-Maf−/− macrophage is shown. c-Maf+/+ erythroblasts surrounding c-Maf−/− macrophages were significantly reduced as compared with those seen for c-Maf+/+ macrophages. See the entire figure in the article by Kusakabe et al on page 1374.
Establishing the cell type responsible for a phenotypic effect is challenging and critical in any study of heterotypic intercellular interactions. Kusakabe et al performed mixing experiments with c-Maf–deficient erythroblasts and macrophages that convincingly show that defects in erythroblastic island formation, caused by the loss of c-Maf, reside with the macrophage.1 However, can the same be said of the embryonic anemia and lethality, or does c-Maf deficiency also have an erythroid cell autonomous effect on development? One approach to address this question is to examine the effect of c-Maf deficiency on erythropoiesis, when it is induced specifically in the macrophage and erythroid lineages; however, this requires a conditional Maf-mutant mouse strain. In lieu of this, 2 other lines of evidence suggest that c-Maf function in macrophages and erythroblastic island formation are required for efficient erythropoiesis. First, c-Maf is expressed in macrophages but not erythroblasts in the fetal liver, which suggests that macrophages are the primary site of c-Maf activity. Second, adult mice transplanted with c-Maf–deficient fetal liver cells do not develop anemia. This experiment shows that there is no cell autonomous requirement for c-Maf in erythroid development, and further implies that erythroblastic island function is most important during fetal development. Alternatively, there may be a fetal-specific requirement for c-Maf in erythroblastic island formation. Finally, consistent with the proposed role of the central macrophage, Kusakabe et al show that survival of mature c-Maf–deficient erythroblasts is defective in vivo.1 Thus, c-Maf deficiency causes defective macrophage development and impairs erythroid cell survival and development through its effect on the erythroblastic island.
Given that c-Maf is the first transcription factor identified that is important for erythroblastic island function, it raises the question of the relevant targets of c-Maf in macrophages. Here, Kusakabe et al provide evidence that VCAM-1 is one such target. VCAM-1 has a proven role in erythroblastic island formation,4 which makes it an excellent candidate for a mediator of c-Maf activity; however, because VCAM-1 is not essential for erythropoiesis, there are likely to be other relevant c-Maf targets.7 Known regulators of erythroblastic island formation, such as retinoblastoma and erythroblast-macrophage protein, have been excluded; thus, additional targets of c-Maf are likely to be novel and their identification an important future objective. Beyond the role of macrophages in erythroid development, erythroblastic islands also serve as a paradigm for interactions between hematopoietic cells and their microenvironment. In this regard, the present identification of a transcriptional regulator of erythroblastic island formation may lead to broader insights into the regulation of hematopoiesis.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal