Abstract
Dependence on Bcl-2 proteins is a common feature of cancer cells and provides a therapeutic opportunity. ABT-737 is an antagonist of antiapoptotic Bcl-2 proteins and therefore is a good predictor of Bcl-xL/Bcl-2 dependence. Surprisingly, analysis of Mcl-1–dependent multiple myeloma cell lines revealed codependence on Bcl-2/Bcl-xL in half the cells tested. Codependence is not predicted by the expression level of antiapoptotic proteins, rather through interactions with Bim. Consistent with these findings, acquired resistance to ABT-737 results in loss of codependence through redistribution of Bim to Mcl-1. Overall, these results suggest that complex interactions, and not simply expression patterns of Bcl-2 proteins, need to be investigated to understand Bcl-2 dependence and how to better use agents, such as ABT-737.
Introduction
Oncogenic transformation results in up-regulation of apoptotic pathways; therefore, induction of antiapoptotic signals is required for cancer cells to maintain their survival. However, by overcoming the proapoptotic activity of oncogenic transformation, the tumor cells become dependent on the antiapoptotic signals. Thus, the regulation of proapoptotic versus antiapoptotic signals in cancer cells has been the focus of significant research efforts.1-3
Bcl-2 proteins integrate cell death and survival signals to determine whether caspase activation should occur after cellular stress. Caspases are activated after the release of the contents of the mitochondrial intermembrane space, and Bcl-2 proteins regulate the mitochondrial outer membrane permeabilization (MOMP). The effectors of MOMP are Bax and Bak, whose functions are negatively regulated by the antiapoptotic members of the Bcl-2 family (Bcl-2, Bcl-xL, and Mcl-1).1,2,4 The nature of this regulation remains somewhat controversial with one model suggesting that the sequestration of Bax and Bak by the antiapoptotic proteins inhibits MOMP.5 In a second model, Bax and Bak must be activated by the BH3-only proteins, Bim, tBid, and possibly Puma. Sequestration of these BH3-only proteins is the primary function of the antiapoptotic family members in this model, and their release is induced by additional BH3-only proteins.6-9 We and others have previously suggested that these models may not be mutually exclusive.10,11 Regardless of the mechanism tumor cells are generally dependent on antiapoptotic Bcl-2 proteins and would be predicted to be more susceptible to their inhibition.
One of the strategies to antagonize the function of antiapoptotic Bcl-2 proteins is to develop compounds that can mimic BH3-only proteins. One such agent ABT-737 mimics the BH3 domain of BAD and therefore selectively binds to Bcl-2, Bcl-xL, and Bcl-w.12 Preclinical studies demonstrated that ABT-737 and the related orally active compound, ABT-263,13 were active in chronic lymphocytic leukemia, acute lymphocytic leukemia, lymphomas, and small cell lung cancer.12,14-16 Furthermore, ABT-737 also induces apoptosis in multiple myeloma (MM) cells,12,17-19 despite the fact that myeloma cells express Mcl-1,20,21 which does not bind the BH3 domain of BAD and is associated with resistance to ABT-737.22-25 Because ABT-737 is a good predictor of Bcl-2 dependence, we were prompted to investigate how the expression and/or interaction patterns of Bcl-2 family proteins play a role in determining the sensitivity of Mcl-1–expressing MM cells to ABT-737.
Methods
Cell lines
The 6 myeloma cells lines were obtained as previously described.10
Reagents
ABT-737 and its less active enantiomer [(−)ABT] were provided by Abbott Laboratories.
Antibodies
The following primary antibodies were used: rabbit anti-Bak polyclonal antibody (pAb; Upstate Cell Signaling Solution); rabbit anti-Bax pAb (N-20, Santa Cruz Biotechnology); mouse anti-Noxa monoclonal antibody (mAb; Abcam); rabbit anti–Puma pAb (Cell Signaling), rabbit anti-Bim pAb (Chemicon), rabbit anti–Mcl-1 pAb (Stressgen); rabbit anti–Bcl-xL pAb (13.6)26 ; mouse anti–Bcl-2 mAb (sc-509, Santa Cruz Biotechnology), Bcl-w pAb (Chemicon); and the rabbit anti-actin pAb (Sigma-Aldrich). The ECL rabbit IgG, horseradish peroxidase-linked whole Ab (from donkey; GE Healthcare), and the anti–mouse IgG1-horseradish peroxidase conjugate (Roche Applied Science) were used as secondary antibody for Western blot. For coimmunoprecipitation, the following antibodies were used: mouse anti–Mcl-1 mAb (BD Biosciences), mouse anti–Bcl-xL mAb (7B2.5),26 and the mouse anti–Bcl-2 mAb sc-509.
Vectors and stable expression of Bcl-xL
The pcDNA3.1 (Invitrogen), pcDNA3.1-Mcl-1, and pcDNA3.1-Bcl-xL-cDNA vectors were introduced into U266, MM.1s, and 8226/S and KMS11 cells by nucleofection (Amaxa) following the manufacturer's instructions (programs U266:X-005, MM.1s:O-023, 8226/S: G-015, KMS11: G-015). Nucleofected cells were plated in growth medium, and 0.5 μg/mL G418 was used for selection.
Cell death by annexin V–fluorescein isothiocyanate and PI staining
Cell death was measured by annexin V–fluorescein isothiocyanate (BioVision) and propidium iodide (PI) staining, as previously described.10
Western blot analysis
Western blotting was performed using standard techniques as previously described.10
Coimmunoprecipitation studies
Immunoprecipitation experiments were performed using the ExactaCruzTM C Kit (Santa Cruz Biotechnology) following the manufacturer's instructions as previously described.10
siRNAs
Silencing studies using small interfering RNAs (siRNAs) were obtained from Dharmacon RNA Technologies, selecting the ON-TARGETplus SMARTpool duplexes as the RNAi-specific technology platform. siRNA against human Mcl-1, Bak, Bax, Puma, Noxa, and Bim, and the siCONTROL nontargeting siRNA [siRNA(−)] were used. siRNAs were electroporated into cells by nucleofection (Amaxa) following the manufacturer's instructions as previously described.10
ABT-737-resistant cell lines
Myeloma cell lines were cultured with increasing concentrations of ABT-737 beginning with 50nM. Medium was replaced every 2 to 3 days increasing the drug concentration only when cell viability was higher than 50%. Final concentration was 0.5μM ABT-737 for 8226/S-ABTR and KMS18-ABTR and 2μM ABT-737 for U266-ABTR and KMS11-ABTR. Cells grown alongside the resistant cell lines in the absence of drug were used as controls (U266-CR2, 8226/S-CR2, KMS11-CR2, and KMS18-CR2).
Patient sample processing
Bone marrow aspirates from consenting myeloma patients were diluted to 20 mL with 1× PBS, and underlaid with lymphocyte separation medium (Mediatech). All samples were collecting following a University Emory Institutional Review Board–approved protocol. The buffy coat was collected and cells were washed, resuspended in culture medium, and stained with anti–CD38-phycoerythrin, anti–CD45-allophycocyanin-Cy7, and anti-CD138-fluorescein isothiocyanate antibodies (BD Biosciences) for fluorescence-activated cell sorter analysis. The cells were then prepared for plasma cell purification using the MACS Cell Separation MS Columns and CD138 magnetic microbeads per the manufacturer's protocol (Miltenyi Biotec). Once isolated, CD138-positive cells were checked for purity via flow cytometry. A total of 1 million cells (0.25 × 106 cells/mL) were treated with the indicated concentrations of ABT-737 for 24 hours and apoptosis determined as described10 ; 1.5 to 2.0 × 106 cells were used for coimmuoprecipitations and Western blot analysis.
Results
MM cell lines are either Mcl-1–dependent or codependent on Mcl-1 and Bcl-xL/Bcl-2
Previous studies describing dependence on antiapoptotic proteins using BH3 profiling or ABT-737 sensitivity have concluded that cells are typically dependent on a primary antiapoptotic protein. We sought to determine whether cells could display codependence on several proteins, specifically Mcl-1 and Bcl-xL or Bcl-2 as this could provide a therapeutic opportunity to use a Bad-mimetic, such as ABT-737, in tumors that are thought to be Mcl-1–dependent.
MM is a clonal plasma cell neoplasia of the bone marrow. Because myeloma cells are derived from a cell that is destined to be long-lived, it is not surprising that they express several antiapoptotic Bcl-2 proteins. Indeed, if one compares the expression of Mcl-1 from gene expression profiles of normal plasma cells, cells from patients with a premalignant plasma cell dyscrasia known as monoclonal gammopathy of undetermined significance, smoldering myeloma, or newly diagnosed MM, there is no difference in the median expression levels of Mcl-1 (Figure 1A). The only difference is in the top quartile of myeloma patients. This is consistent with a high-risk group of patients that have amplification of chromosome 1q where the Mcl-1 locus resides and is common in many malignancies.27 Thus, nearly all myelomas express Mcl-1 at a level sufficient to predict they would be Mcl-1–dependent, a conclusion that was previously reached when Bcl-2 proteins were silenced with antisense oligonucleotides in 7 MM cell lines.20,21 We confirmed and further expanded the results of others to 4 MM cell lines that were used in the course of these experiments. When Mcl-1 was silenced, all 4 cell lines were found to exhibit high levels of apoptosis consistent with the model that MM cell lines are Mcl-1–dependent (Figure 1B).
All MM cells are dependent on Mcl-1, although they differ in their sensitivity to ABT-737. (A) mRNA levels for Mcl-1 were derived from the analysis of normalized gene expression profile data deposited at GEO from CD138-selected plasma cells from healthy donors: normal PC (n = 22), monoclonal gammopathy of undetermined significance (n = 44), smoldering myeloma (SM, n = 12), and newly diagnosed MM (MM, n = 538). The data are taken from the 214056_at probe on the affymetrix Hu133 2.0 plus array. Similar results were obtained from the 214057_at probe (not shown). (B) Four myeloma cell lines were transfected with siRNA for Mcl-1 and efficiency of knock-down was determined by Western blot (left panel), whereas the apoptosis of each cell line 16 hours after transfection was measured by annexin PI assays. P values compare with si(-), P < .005 (KMS11, MM.1s), P < .001 (8226, KMS18). (C) Six myeloma cell lines were treated with indicated doses of ABT-737 for 24 hours, and apoptosis was measured by annexin V/PI staining. Data are mean ± SD of 4 independent experiments. (D) CD138+ cells purified from bone marrow aspirates of 6 MM patients were incubated with the indicated concentrations of ABT-737 for 24 hours and apoptosis determined by annexin V/PI staining. Each sample was run in parallel with MM.1s (●), which is presented as the mean ± SD of the 6 experiments. The 50% inhibitory concentration values for each sample are as follows (μM): MM.1s, 0.65; MM1-0.3, MM6-0.58, MM7-0.92, MM23-0.1, MM24, and MM26, < 0.1.
All MM cells are dependent on Mcl-1, although they differ in their sensitivity to ABT-737. (A) mRNA levels for Mcl-1 were derived from the analysis of normalized gene expression profile data deposited at GEO from CD138-selected plasma cells from healthy donors: normal PC (n = 22), monoclonal gammopathy of undetermined significance (n = 44), smoldering myeloma (SM, n = 12), and newly diagnosed MM (MM, n = 538). The data are taken from the 214056_at probe on the affymetrix Hu133 2.0 plus array. Similar results were obtained from the 214057_at probe (not shown). (B) Four myeloma cell lines were transfected with siRNA for Mcl-1 and efficiency of knock-down was determined by Western blot (left panel), whereas the apoptosis of each cell line 16 hours after transfection was measured by annexin PI assays. P values compare with si(-), P < .005 (KMS11, MM.1s), P < .001 (8226, KMS18). (C) Six myeloma cell lines were treated with indicated doses of ABT-737 for 24 hours, and apoptosis was measured by annexin V/PI staining. Data are mean ± SD of 4 independent experiments. (D) CD138+ cells purified from bone marrow aspirates of 6 MM patients were incubated with the indicated concentrations of ABT-737 for 24 hours and apoptosis determined by annexin V/PI staining. Each sample was run in parallel with MM.1s (●), which is presented as the mean ± SD of the 6 experiments. The 50% inhibitory concentration values for each sample are as follows (μM): MM.1s, 0.65; MM1-0.3, MM6-0.58, MM7-0.92, MM23-0.1, MM24, and MM26, < 0.1.
Although all MM cell lines appear to be Mcl-1–dependent, it was also previously demonstrated that ABT-737 induces apoptosis in MM cells, suggesting that some of these cell lines may also be Bcl-xL/Bcl-2–dependent.10,17-19 Indeed, when we tested 6 cell lines, apoptosis was detected after ABT-737 treatment, albeit with various levels of sensitivity. Consistent with Mcl-1 dependence, 3 myeloma cell lines (U266, KMS11, and OPM2) were relatively insensitive to ABT-737 with 50% effective concentration values of 2.58, 1.6, and 2.77μM, respectively (Figure 1C). In contrast, 3 additional cell lines (MM.1s, 8226/S, and KMS18) were sensitive to ABT-737 with 50% effective concentration values of 0.58, 0.30, and 0.39μM, respectively (Figure 1C). We also found that CD138+ cells isolated from the bone marrow aspirates of MM patients were sensitive to ABT-737 in a similar manner as the codependent line, MM.1s (Figure 1D). Thus, codependence exists in 50% of the cell lines tested and appears to be relevant in patient-derived samples.
The expression pattern of Bcl-2 family proteins does not predict codependence
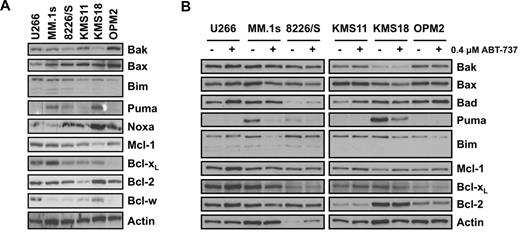
To explain the differences in the sensitivity of MM cell lines to ABT-737 and thereby codependence, we investigated the expression levels of its direct targets, Bcl-2, Bcl-xL, and Bcl-w. As demonstrated in Figure 2A, the sensitive MM.1s and the insensitive U266 cell lines had higher levels of Bcl-xL, whereas the sensitive KMS18 and the insensitive U266 cell lines express greater Bcl-w. Finally, all cell lines tested have similar Bcl-2 levels. Taken together, expression levels of ABT-737 targets do not predict ABT-737 sensitivity. It is also important to note that the sensitivity of MM cell lines to ABT-737 does not correlate with their sensitivity to other agents, including bortezomib28 or arsenic trioxide,10 suggesting that the variation in the sensitivity of MM cell lines to ABT-737 cannot be explained by global differences in their apoptotic threshold.
Sensitivity to ABT-737 does not correlate with the expression pattern of Bcl-2 family proteins. (A) Protein expression of Bcl-2 family proteins was determined by Western blot analysis using lysates from untreated myeloma cell lines. Membranes were probed with specific pAbs against the indicated Bcl-2 family members as described in “Western blot analysis” and “Antibodies.” Actin was used as a loading control. (B) Change in the expression of Bcl-2 proteins after treatment with 0.4μM ABT-737 for 24 hours was determined by Western blot analysis.
Sensitivity to ABT-737 does not correlate with the expression pattern of Bcl-2 family proteins. (A) Protein expression of Bcl-2 family proteins was determined by Western blot analysis using lysates from untreated myeloma cell lines. Membranes were probed with specific pAbs against the indicated Bcl-2 family members as described in “Western blot analysis” and “Antibodies.” Actin was used as a loading control. (B) Change in the expression of Bcl-2 proteins after treatment with 0.4μM ABT-737 for 24 hours was determined by Western blot analysis.
Because ABT-737 mimics the BH3 domain of BAD, it cannot antagonize the antiapoptotic function of Mcl-l. Thus, cells that are dependent on Mcl-1 for survival would be expected to be resistant to the effects of ABT-737. Indeed, several reports demonstrate that increased expression of Mcl-1 correlated with resistance to ABT-737 treatment.22-25 Furthermore, it was also demonstrated that the BH3-only protein, Noxa, which selectively binds and inhibits Mcl-1, renders cells sensitive to ABT-737.29,30 However, when these 2 proteins were analyzed in the 6 MM cell lines, no correlation was detected between the expression of either Mcl-1 or Noxa and ABT-737 sensitivity (Figure 2A). Similarly, the levels of another BH3-only protein, Bim, as well as the main effectors of MOMP, Bax, and Bak, did not predict the sensitivity of MM cells to ABT-737 treatment (Figure 2A). On the other hand, the 3 sensitive cell lines express higher levels of Puma. However, after a 24-hour incubation with 0.4μM ABT-737, there was a marked decrease in Puma expression, suggesting that it does not play a role in ABT-737-induced apoptosis (Figure 2B). Overall, these results demonstrate that the relative levels of Bcl-2-family proteins in myeloma cells do not predict Bcl-2 dependence or ABT-737 sensitivity.
Binding of Bim to Bcl-xL and Bcl-2 correlates with ABT-737 sensitivity
Because the expression profile of the Bcl-2 proteins did not correlate with ABT-737 sensitivity, we next probed the activity of antiapoptotic proteins to determine whether the function of these proteins correlates with ABT-737 activity. The function of these proteins is to bind and sequester proapoptotic proteins; therefore, the interaction between the proapoptotic and antiapoptotic Bcl-2 members was analyzed. Although the initial ex vivo biochemical assays reported that ABT-737 has high affinity toward Bcl-w,12 in addition to Bcl-xL and Bcl-2, these data were recently challenged by a report that demonstrated that Bcl-w is not a target of ABT-737 in vivo.31 Therefore, we focused our studies on investigating the binding of Bcl-2, Bcl-xL, and Mcl-1 to determine which protein interactions may predict ABT-737 sensitivity. Consistent with our previous report as well as the findings of others, we found Bak, but not Bax, to be associated with either Bcl-xL or Mcl-1.10,32 Furthermore, silencing of Bak, but not Bax, inhibited ABT-737–induced apoptosis in 8226/S cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Taken together, these data suggest that, in myeloma cells, the interaction of Bak, but not Bax, is a potential target for ABT-737. A closer look at the association of these proteins revealed that there was a significant amount of Bak bound to Bcl-xL in all 3 sensitive cell lines (MM1.s, 8226/S, and KMS18) and 2 insensitive cell lines (U266 and KMS11, Figure 3A). Only in one insensitive cell line, OPM2, Bak was bound primarily to Mcl-1 (Figure 3A). In addition, if we compare ABT-737–induced release of Bak from Mcl-1 and Bcl-xL in an Mcl-1–dependent line (KMS11) and a codependent line (MM.1s), we observed similar patterns of concentration-dependent release of Bak from Bcl-xL (supplemental Figure 2). Together, these data suggest that, although Bak release is necessary for ABT-737–induced apoptosis, the interaction between Bak and the prosurvival Bcl-2 proteins is not a determinant of ABT-737 sensitivity.
Binding of Bim to Bcl-xL and Bcl-2 in myeloma cell correlates with codependence and sensitivity to ABT-737. (A) Coimmunoprecipitation of proapoptotic proteins with Mcl-1, Bcl-xL, and Bcl-2 was determined by Western blot analysis after lysing all 6 cell lines in 2% 3(3-cholamidopropyl) dimethylammonio-1-propane sulfonate buffer and immunoprecipitating with specific monoclonal antibodies as described in “Antibodies.” and “Coimmunoprecipitation studies.” Lysates and supernatant contain 7%, whereas the immunoprecipitated proteins contain 60% of the input. (B) CD138+ myeloma cells were isolated, and 1 million cells were subjected to lysis and immunoprecipitation with anti–Bcl-x and anti–Mcl-1 antibodies as described in “Antibodies” and “Coimmunoprecipitation studies.” Immunoprecipitates were subjected to SDS-PAGE and Western blot analysis performed for Bcl-xL, Mcl-1, and Bim. Two of 6 patient samples tested are shown, and each experiment was performed in parallel with MM.1s as a reference.
Binding of Bim to Bcl-xL and Bcl-2 in myeloma cell correlates with codependence and sensitivity to ABT-737. (A) Coimmunoprecipitation of proapoptotic proteins with Mcl-1, Bcl-xL, and Bcl-2 was determined by Western blot analysis after lysing all 6 cell lines in 2% 3(3-cholamidopropyl) dimethylammonio-1-propane sulfonate buffer and immunoprecipitating with specific monoclonal antibodies as described in “Antibodies.” and “Coimmunoprecipitation studies.” Lysates and supernatant contain 7%, whereas the immunoprecipitated proteins contain 60% of the input. (B) CD138+ myeloma cells were isolated, and 1 million cells were subjected to lysis and immunoprecipitation with anti–Bcl-x and anti–Mcl-1 antibodies as described in “Antibodies” and “Coimmunoprecipitation studies.” Immunoprecipitates were subjected to SDS-PAGE and Western blot analysis performed for Bcl-xL, Mcl-1, and Bim. Two of 6 patient samples tested are shown, and each experiment was performed in parallel with MM.1s as a reference.
Among the BH3 proteins, we further analyzed Puma and Bim because they can bind to all 3 prosurvival Bcl-2 family proteins. In agreement with the data presented in Figure 2A, Puma was only detected in the 3 sensitive cell lines, and it was primarily associated with Bcl-2 and Bcl-xL (Figure 3A). Furthermore, binding of Bim by either Bcl-xL or Bcl-2 also appeared to correlate with ABT-737 sensitivity. In all 3 insensitive cell lines, Bim was found to primarily interact with Mcl-1; whereas in the 2 sensitive cell lines MM.1s and KMS18, Bim was primarily associated with either Bcl-xL or Bcl-2 (Figure 3A). However, in one sensitive line, 8226/S, Bim was bound primarily to Mcl-1 compared with Bcl-xL and Bcl-2. To determine whether this binding pattern was relevant in patient-derived samples, we performed coimmunoprecipation assays for Mcl-1 and Bcl-xL from CD138+ plasma cells purified from bone marrow aspirates from 6 MM patients. As a reference for each sample, we performed parallel analyses on MM.1s cells. Two representative samples are presented in Figure 3B; and consistent with the sensitivity of these samples to ABT-737 (Figure 1D), Bim was found to be associated with both Bcl-xL and Mcl-1.
To determine whether Puma and/or Bim plays a dominant role in predicting Bcl-xL/Bcl-2 dependence, activity of ABT-737 was measured after silencing of these proteins. Interestingly, silencing of Puma had no protective effect on ABT-737 activity in the 3 lines tested (Figure 4). However, silencing of Bim in 3 MM lines inhibited ABT-737 activity (Figure 4). In addition, Bim is released from Bcl-xL in MM.1s and KMS11 in a concentration-dependent fashion by ABT-737 (supplemental Figure 2). Together, these results suggest that the interaction of Bim with Bcl-xL and Bcl-2 underlies Bcl-2 dependence and the sensitivity to ABT-737. The 8226/S cells appear to be the exception to this conclusion; therefore, we next investigated the possible reasons for codependence in this cell line.
Effect of silencing Bim, Noxa, and Puma on ABT-737–induced apoptosis. Knock-down efficiency was analyzed by Western blotting of each protein in untreated and 0.4μM ABT-737 treated samples (left panels). Activity of ABT-737 was analyzed by annexin/PI staining after knock-down of each protein (right panels). Data are mean ± SD of at least 3 independent experiments. P values: 8226, silencing of all 3 BH3 proteins are significantly different from [si(−)] control (P < .005), with the exception of siPuma at 0.8μM ABT-737 (not significant). KMS11, all values are significantly different from control (P < .005), with the exception of siNoxa at 1.6 and 3.2μM (not significant). MM.1s, siBim significantly different from control (P < .005).
Effect of silencing Bim, Noxa, and Puma on ABT-737–induced apoptosis. Knock-down efficiency was analyzed by Western blotting of each protein in untreated and 0.4μM ABT-737 treated samples (left panels). Activity of ABT-737 was analyzed by annexin/PI staining after knock-down of each protein (right panels). Data are mean ± SD of at least 3 independent experiments. P values: 8226, silencing of all 3 BH3 proteins are significantly different from [si(−)] control (P < .005), with the exception of siPuma at 0.8μM ABT-737 (not significant). KMS11, all values are significantly different from control (P < .005), with the exception of siNoxa at 1.6 and 3.2μM (not significant). MM.1s, siBim significantly different from control (P < .005).
Endogenous expression of Noxa sensitizes 8226/S cells to ABT-737
A possible explanation for why 8226/S cells are sensitive to ABT-737 despite the majority of the Bim being associated with Mcl-1 is that endogenous expression of another BH3-only protein that has high affinity for this prosurvival protein may limit its function, thus rendering these cells to be functionally codependent. One such protein, Noxa, is constitutively expressed in this cell line (Figure 2A). Indeed, previous reports demonstrated that induction of Noxa in various tumor types sensitizes cells to ABT-737–induced apoptosis.29,30 Consistent with this possibility, knock-down of Noxa abrogated the activity of ABT-737 in 8226/S cells, whereas it had no effect in KMS11 or MM.1s cells (Figure 4). Therefore, Noxa and Bim may be cooperating in ABT-737–induced apoptosis in these cells. Immunoprecipitation of Mcl-1 revealed that higher levels of Noxa were bound to Mcl-1 in 8226/S compared with KMS11 and MM.1s cells (Figure 5A).
Noxa expression inhibits Mcl-1 binding to Bim displaced from Bcl-xL and Bcl-2. 8226/S, MM.1s, and KMS11 cell lines were treated with indicated concentrations of ABT-737 for 24 hours and lysates generated as described in “Western blot analysis.” (A) Mcl-1 and (B) Bcl-xL and Bcl-2 were immunoprecipitated using specific monoclonal antibodies. Immunoprecipitates were sequentially probed with pAbs for Bim and Noxa as well as for Bcl-2, Bcl-xL, and Mcl-1. Immunoprecipitates contain 60% of input. (C) The total amount of Bim was assayed by Western blot in lysates from control and treated samples of 8226/S, KMS-11, MM.1S. (D) Twenty-four hours after transfection with si(-) or siNoxa, expression of Noxa and Mcl-1 was determined by Western blot (right panel). The remaining lysate was subjected to immunoprecipitation with Mcl-1, Bcl-xL, or Bcl-2 followed by Western blot analysis of Bim, Mcl-1, Bcl-xL, and Bcl-2 (left panel). (E) The bands from 2 independent experiments were quantified using ImageJ software Version 1.45r, and the graph represents the average ratio of relative intensity of indicated bands.
Noxa expression inhibits Mcl-1 binding to Bim displaced from Bcl-xL and Bcl-2. 8226/S, MM.1s, and KMS11 cell lines were treated with indicated concentrations of ABT-737 for 24 hours and lysates generated as described in “Western blot analysis.” (A) Mcl-1 and (B) Bcl-xL and Bcl-2 were immunoprecipitated using specific monoclonal antibodies. Immunoprecipitates were sequentially probed with pAbs for Bim and Noxa as well as for Bcl-2, Bcl-xL, and Mcl-1. Immunoprecipitates contain 60% of input. (C) The total amount of Bim was assayed by Western blot in lysates from control and treated samples of 8226/S, KMS-11, MM.1S. (D) Twenty-four hours after transfection with si(-) or siNoxa, expression of Noxa and Mcl-1 was determined by Western blot (right panel). The remaining lysate was subjected to immunoprecipitation with Mcl-1, Bcl-xL, or Bcl-2 followed by Western blot analysis of Bim, Mcl-1, Bcl-xL, and Bcl-2 (left panel). (E) The bands from 2 independent experiments were quantified using ImageJ software Version 1.45r, and the graph represents the average ratio of relative intensity of indicated bands.
Because Noxa and Bim are occupying Mcl-1 in 8226/S and should not be released by addition of ABT-737, we next determined the fate of Bim associated with Bcl-xL and Bcl-2 in these cells. Consistent with 8226/S being a codependent line, Bim is easily displaced from Bcl-xL and Bcl-2 by ABT-737 in these cells (Figure 5B). Whereas the released Bim is a minor fraction of the total bound Bim, the Noxa associated with Mcl-1 appears to interfere with Mcl-1's ability to bind drug-released Bim (Figure 5A). This is the probable explanation for the Noxa dependence of ABT-737–induced apoptosis in these cells. Similarly, in the other sensitive cell line tested (MM.1s), concentration-dependent displacement of Bim from Bcl-xL and Bcl-2 is observed. However, because these cells do not express significant levels of Noxa and it is not detected in the Mcl-1 coimmunoprecipitation, marked drug-induced Bim association with Mcl-1 is observed (Figure 5A). However, MM.1s express significantly higher levels of total Bim (Figure 5C) compared with 8226/S and KMS11; therefore, sufficient levels of free Bim may still be available to induce MOMP. In the insensitive KMS11 cells, Bim is released from Bcl-xL and Bcl-2 in concentration-dependent manner; however, this represents a minor faction of the total bound Bim (Figure 5A). Therefore, the concentration of ABT-737 required to release enough Bim to activate Bak appears to be greater in the Mcl-1–dependent cell lines.
To directly test the role of Noxa in these interactions, we silenced Noxa and performed coimmunoprecipitation of the antiapoptotic proteins. Consistent with our model, Noxa silencing resulted in a 61% to 71% increase in the amount of Bim associating with Mcl-1. Interestingly, the Bim appeared to be released from Bcl-2 where a comparable 74% to 83% decrease was observed, whereas no change in the association with Bcl-xL is seen (Figure 5D-E). Furthermore, in the absence of Noxa, ABT-737–released Bim is sequestered by Mcl-1 (supplemental Figure 3). Taken together, these findings further indicate that Noxa expression perturbs the ability of Mcl-1 to bind Bim rendering 8226/S cells sensitive to ABT-737 treatment and thereby codependent.
Enforced expression of Bcl-xL or Mcl-1 is not sufficient to alter dependence on antiapoptotic proteins
We next tested whether forced overexpression of antiapoptotic proteins could change the dependence pattern and thus inhibit ABT-737–induced apoptosis. When Bcl-xL is exogenously overexpressed, the capacity of Bcl-xL–BH3 binding should significantly increase, which would require higher concentrations of ABT-737 to neutralize the antiapoptotic function. Indeed, in the codependent 8226/S and MM.1s cells, stable overexpression of Bcl-xL resulted in diminished sensitivity to ABT-737 compared with their vector control-transfected counterparts (Neo, Figure 6A). However, Bcl-xL did not significantly reduce the activity of ABT-737 in the Mcl-1–dependent lines, U266 and KMS11 (Figure 6A). This is consistent with Bim already being associated with Mcl-1 and the cells maintaining their Mcl-1 dependence. Similar to Bcl-xL, overexpression of Mcl-1 also protected 8226/S and MM.1s, but not U266 cells from ABT-737–induced apoptosis (Figure 6B). Mcl-1 does have a significant effect on ABT-737–induced apoptosis compared with the Neo control; however, they were not different from the parental controls, which were less sensitive than the vector-transfected cells.
Bcl-xL and Mcl-1 overexpression protect codependent cell lines from ABT-737–induced apoptosis. U266, MM.1s, 8226/S, and KMS-11 cell lines were stably transfected with pcDNA3.1 (Neo) and (A) pcDNA–Bcl-xL or (B) pcDNA–Mcl-1 vectors. Cell lines were treated with indicated concentrations of ABT-737 for 24 hours, and viability was determined by annexin V/PI staining. The data presented are the mean ± SD of at least 3 independent experiments. U266–Bcl-xL is significantly different from U266-Neo (P < .05) at 0.1μM ABT-737. MM.1s-Bcl-xL is significantly different from MM.1s-Neo at all concentrations of ABT-737 (0.1μM, P < .05; 0.2μM, P < .001; 0.4-0.8μM, P < .005). 8226-Bcl-xL is significantly different from 8226-Neo at all concentrations of ABT-737 (P < .005). MM.1s-Mcl-1 is significantly different from MM.1s-Neo (P < .005) at 0.4 to 0.8μM ABT-737. KMS11-Mcl-1 is statistically different from KMS11-Neo (P < .05) at all concentrations of ABT-737. 8226-Mcl-1 is significantly different from 8226-Neo (P < .001) at all concentrations of ABT-737. (C) Protein lysates were obtained using 2% 3(3-cholamidopropyl) dimethylammonio-1-propane sulfonate buffer for the indicated transfectants of KMS11, MM.1s, U266. Antiapoptotic proteins Mcl-1 and Bcl-xL were immunoprecipitated using specific monoclonal antibodies as described in “Antibodies” and “Coimmunoprecipitation studies.” The pellets were probed for Bim, Mcl-1, and Bcl-xL. The blots are representative of 2 independent experiments.
Bcl-xL and Mcl-1 overexpression protect codependent cell lines from ABT-737–induced apoptosis. U266, MM.1s, 8226/S, and KMS-11 cell lines were stably transfected with pcDNA3.1 (Neo) and (A) pcDNA–Bcl-xL or (B) pcDNA–Mcl-1 vectors. Cell lines were treated with indicated concentrations of ABT-737 for 24 hours, and viability was determined by annexin V/PI staining. The data presented are the mean ± SD of at least 3 independent experiments. U266–Bcl-xL is significantly different from U266-Neo (P < .05) at 0.1μM ABT-737. MM.1s-Bcl-xL is significantly different from MM.1s-Neo at all concentrations of ABT-737 (0.1μM, P < .05; 0.2μM, P < .001; 0.4-0.8μM, P < .005). 8226-Bcl-xL is significantly different from 8226-Neo at all concentrations of ABT-737 (P < .005). MM.1s-Mcl-1 is significantly different from MM.1s-Neo (P < .005) at 0.4 to 0.8μM ABT-737. KMS11-Mcl-1 is statistically different from KMS11-Neo (P < .05) at all concentrations of ABT-737. 8226-Mcl-1 is significantly different from 8226-Neo (P < .001) at all concentrations of ABT-737. (C) Protein lysates were obtained using 2% 3(3-cholamidopropyl) dimethylammonio-1-propane sulfonate buffer for the indicated transfectants of KMS11, MM.1s, U266. Antiapoptotic proteins Mcl-1 and Bcl-xL were immunoprecipitated using specific monoclonal antibodies as described in “Antibodies” and “Coimmunoprecipitation studies.” The pellets were probed for Bim, Mcl-1, and Bcl-xL. The blots are representative of 2 independent experiments.
To determine whether overexpression of either Mcl-1 or Bcl-xL alters the distribution of Bim bound to the antiapoptotic proteins, the association between these proteins were analyzed in the KMS11, MM.1s, and U266 transfectants. In KMS11 and MM.1s cell lines, stable overexpression did not alter the distribution of Bim (Figure 6C). In U266 cells, a slight increase in Bim–Mcl-1 association was observed in the Mcl-1–transfectants; whereas in the Bcl-xL–transfected cells, Bim occupation of Bcl-xL was increased (Figure 6C). However, these changes had no phenotypic consequences. Thus, forced expression of an antiapoptotic protein does not necessarily alter the distribution of Bim bound to Mcl-1 versus Bcl-xL, suggesting that expression levels of antiapoptotic Bcl-2 family proteins is not the primary determinant of Bim distribution and therefore not adequate to predict the dependence of a cell type on a particular prosurvival protein.
Acquired resistance to ABT-737 results in the loss of Bcl-2/Bcl-xL dependence
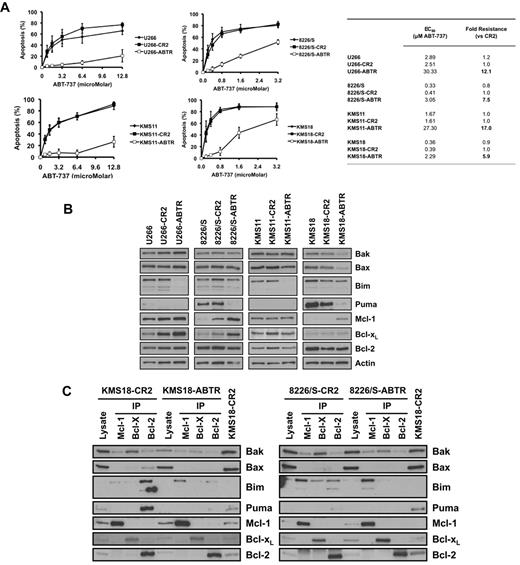
To further investigate the role of the interaction and expression patterns of Bcl-2-family proteins in codependence and ABT-737 sensitivity, ABT-737–resistant 8226/S, KMS18, KMS11, and U266 cell lines were generated. The resistant counterparts of the codependent cell lines, 8226/S and KMS18, displayed 7.5- and 5.9-fold resistance to ABT-737, respectively, compared with parental cells (CR2) that were maintained in culture in parallel to the selected cells to assure that the time in culture during selection was not a contributing factor to resistance (Figure 7A). Acquired resistance of Mcl-1–dependent cell lines, U266 and KMS11, resulted in 12.1- and 17-fold resistance respectively, compared with CR2-cells. (Figure 7A).
Acquired ABT-737 resistance is associated with changes in expression and interactions of Bcl-2 proteins. Four cell lines were rendered resistant to ABT-737 by sequentially increasing the ABT-737 concentration in their growth medium starting from 50nM up to 0.5μM (8226/S, KMS-18) or 2μM (KMS-11, U266). (A) Sensitivity (EC50) of parental, control resistance (CR2), and resistant (ABTR) cell lines to ABT-737 was determined by annexin V/PI staining after 24 hours of treatment. All ABTR cell lines are significantly different from CR2 cells at all concentrations of ABT-737 (P < .001), with the exception of KMS18 at 3.2μM ABT-737 (P < .005). (B) Expression of Bcl-2 family members and (C) interaction between these proteins were determined as described in “Antibodies” and “Coimmunoprecipitation studies.” For all experiments, the cells were removed from the selecting concentration 24 hours before the experiment.
Acquired ABT-737 resistance is associated with changes in expression and interactions of Bcl-2 proteins. Four cell lines were rendered resistant to ABT-737 by sequentially increasing the ABT-737 concentration in their growth medium starting from 50nM up to 0.5μM (8226/S, KMS-18) or 2μM (KMS-11, U266). (A) Sensitivity (EC50) of parental, control resistance (CR2), and resistant (ABTR) cell lines to ABT-737 was determined by annexin V/PI staining after 24 hours of treatment. All ABTR cell lines are significantly different from CR2 cells at all concentrations of ABT-737 (P < .001), with the exception of KMS18 at 3.2μM ABT-737 (P < .005). (B) Expression of Bcl-2 family members and (C) interaction between these proteins were determined as described in “Antibodies” and “Coimmunoprecipitation studies.” For all experiments, the cells were removed from the selecting concentration 24 hours before the experiment.
Analysis of the expression of Bcl-2 family proteins in the resistant cell lines demonstrated that both ABT-737–resistant 8226/S and KMS18 displayed increased expression of Mcl-1 compared with their control counterparts (Figure 7B). Interestingly, there was also a modest reduction in Bak expression in ABT-737–resistant KMS18 cells. Puma expression was lower in both 8226/s and KMS18 (Figure 7B), although the significance of this reduction is unclear. In contrast to 8226/S and KMS18, the expression of Mcl-1 did not change in the resistant counterparts of U266 and KMS11 (Figure 7B). Strikingly, in both of these lines, there was a marked reduction in Bim levels.
We next investigated the effects of acquired resistance on the distribution of Bim and Bak (Figure 7C). Interestingly, when ABT-737 resistance was acquired in the codependent cell lines, Bim is found to be exclusively associated with Mcl-1 (Figure 7C). In contrast, the distribution of Bak did not change in either of the resistant cell lines. As expected, in the ABT-737–resistant U266 and KMS11 cell lines, almost all the remaining Bim was found to be associated with Mcl-1 while there was no apparent change in the interaction of Bak (data not shown). Overall, these data indicate that the total amount of Bim bound to Bcl-xL or Bcl-2 underlies the Bcl-2/Bcl-xL dependence and sensitivity to ABT-737.
Discussion
Recent advances in our understanding of the complex interactions behind the induction of apoptosis revealed that changes that result in an increased apoptotic threshold are not simply limited to overexpression of prosurvival Bcl-2 proteins.33 To determine which Bcl-2 protein interactions are indispensable for a particular tumor cell type, a technique called BH3 profiling has been developed.34 In addition, development of the small molecule BH3-mimetic, ABT-737, provided another effective tool to emulate BH3 profiling in whole cells.34 Goldsmith et al recently demonstrated in neuroblastoma cells that BH3 profiling in isolated mitochondria closely correlates with the sensitivity of these tumors to ABT-737.35 Thus, studies using ABT-737 can shed light on targeting Bcl-2 dependence in tumor cells.
Because ABT-737 mimics Bad, which selectively binds to Bcl-xL, Bcl-2, and Bcl-w, a tumor cell that has a strong Mcl-1 dependence would be expected to be resistant to this agent. Indeed, several reports demonstrate that expression of Mcl-1 as well as its antagonist BH3 protein, Noxa, modulates the sensitivity of acute myeloid leukemia, small cell lung cancer, melanoma, head and neck cancer, and colon cancer cells to ABT-737.22,23,25,29,30,36-38 Similarly, cancers, such as chronic lymphocytic leukemia, in which oncogenic transformation is associated with Bcl-2 up-regulation, are shown to be highly sensitive to ABT-737.14,15 These findings help provide the rationale for phase 1/2 clinical trials with an orally available BH3-mimetic, ABT-263 (Navitoclax), in chronic lymphocytic leukemia, lymphoma, and small cell lung cancer.1
Although MM survival has been known to be Mcl-1–dependent,20,21 data presented here and in other reports17-19 demonstrate the sensitivity of these cells to ABT-737. Three of the 7 MM cell lines that were found to be Mcl-1–dependent in these earlier studies were tested in the current study. Our findings confirm that U266 and OPM-2 are Mcl-1–dependent21 and explain why silencing of Mcl-1 in 8226/S cells would induce apoptosis,20 despite the cells appearing to be Bcl-2/Bcl-xL–dependent. Together, these data suggest that 8226/S is codependent on Mcl-1 and Bcl-2/Bcl-xL. Although all the cell lines tested in this study are Mcl-1-dependent, 50% are codependent on Bcl-xL/Bcl-2. This suggests that expression or even dependence on Mcl-1 does not preclude targeting of Bcl-xL/Bcl-2 in cancer cells. Thus, although it is well documented that overexpression of Mcl-1 confers resistance to ABT-737,25,29,37 our findings here emphasize that interactions, rather than expression levels of Bcl-2 proteins, play a more significant role in determining the sensitivity of a cell to this compound and by extension Bcl-2 dependence.
The analysis of interactions between various Bcl-2 proteins suggests that binding of Bim and Bak to Bcl-xL and Bcl-2 correlates with sensitivity of MM cell lines to ABT-737. However, a closer look at the interactions of these 2 proapoptotic proteins indicate that the distribution of Bim is a better predictor of ABT-737 sensitivity (supplemental Figure 4, codependence models I and II). Bak was equally distributed between Bcl-xL and Mcl-1 in most of the insensitive and sensitive MM cell lines, whereas Bim was highly bound to Mcl-1 in the former cells. Furthermore, cells selected for ABT-737 resistance, either the expression or the interaction of Bim, was found to be altered in all 4 cell lines, whereas Bak levels were slightly reduced in only 1 cell line and its interaction pattern did not change in any of them. Therefore, it appears that the amount of Bim that is displaced from Bcl-xL and Bcl-2 underlies the sensitivity of MM cell lines to ABT-737. These findings suggest that cells that are codependent on Mcl-1 and Bcl-xL/Bcl-2 have levels of Bim priming such that release from any antiapoptotic is sufficient to induce MOMP (supplemental Figure 4). This was not limited to cell lines as we found copriming of Bim in 6 freshly isolated patient samples that displayed sensitivity to ABT-737.
The question of how the distribution of Bim among various antiapoptotic Bcl-2 proteins is determined in each cell line remains elusive. Although phosphorylation of either Bcl-239 or Bcl-xL40 after treatment with various chemotherapeutics has been shown to inhibit their antiapoptotic function, there are no data, to our knowledge, that demonstrate how the interactions between proapoptotic and antiapoptotic proteins are regulated in cells in the absence of external stimuli. A common notion is that expression of an antiapoptotic protein may render a cell dependent on its function for survival. However, findings presented here argue that this is not the only possibility. For example, forced overexpression of either Mcl-1 or Bcl-xL did not result in a shift of Bim binding between these antiapoptotic proteins in 2 of the 3 cell lines tested. Interestingly, in U266 transfectants, overexpression of both Mcl-1 and Bcl-xL leads to greater levels of Bim associated with the transfected antiapoptotic protein, suggesting that in certain cell types Bim distribution may be determined by the expression levels of prosurvival proteins. However, there was no consequence to the change in Bim distribution in these cells; therefore, the relevance of this finding is not completely clear. Nevertheless, taken together, these findings suggest that how the affinity of Bim for each prosurvival Bcl-2 protein is regulated appears to be complex and not solely depend on the expression levels of these proteins. A plausible explanation is that post–translational modifications may alter the structure of the BH3-binding groove in antiapoptotic Bcl-2 proteins modulating the affinity of these proteins for BH3 domains. Alternatively, post–translational modifications of Bim could result in altered affinity for antiapoptotic proteins. Thus, post–translational modification of Bim may play a role in its association with individual antiapoptotic Bcl-2 proteins. Indeed, Bim is reported to contain various serine/threonine phosphorylation sites that regulate its stability and apoptotic activity.41-45 Further research is required to determine whether the phosphorylation of these residues may also affect the affinity of Bim toward each antiapoptotic Bcl-2 protein.
An exception to the model in which Bim association with Bcl-xL/Bcl-2 is shown to correlate with sensitivity to ABT-737 was the 8226/S cell line where Bim is found to be primarily associated with Mcl-1. Interestingly, these cells also highly express another BH3 protein, Noxa, which selectively binds to Mcl-1. Furthermore, knock-down of Noxa significantly protected 8226/S from ABT-737–induced apoptosis. Thus, in the presence of Noxa, Mcl-1 may not be able to adequately sequester the Bim that is displaced from Bcl-xL and Bcl-2; therefore, ABT-737 can effectively kill 8226/S cells (supplemental Figure 4, codependence model II). We have also observed a similar effect in KMS18, suggesting why Mcl-1 does not serve as a sink for Bim in these codependent cells (not shown). Consistent with this idea, when 8226/S and KMS11 cell lines were compared, it was determined that after incubation with ABT-737, the Bim/Mcl-1 association does not change in the former cell line, whereas the association was enhanced in the latter (supplemental Figure 4, Mcl-1–dependent model vs codependent model II). Moreover, in MM.1s cells, in which there is a higher proportion of Bim bound to Bcl-xL and Bcl-2, Bim/Mcl-1 association increases significantly after ABT-737 treatment, indicating that in these cells Mcl-1 cannot neutralize enough of the Bim that is displaced by ABT-737 to prevent MOMP (supplemental Figure 4, codependence model I). Taken together, these data suggest that the ratio of “unprimed” Mcl-1 to the Bim that is bound to Bcl-xL and Bcl-2 predicts sensitivity to ABT-737. This would also explain why increasing Mcl-1 in KMS11 and U266 cells had no effect on ABT-737–induced cell death.
Based on this prediction, there are 2 possible ways to sensitize cells to ABT-737 treatment, either through the up-regulation of Bim bound to Bcl-xL and Bcl-2 or the down-regulation and/or inactivation of Mcl-1. The first possibility is supported by a recent report by Chen et al, which demonstrates that the histone deacetylase inhibitor, suberoyl bis-hydroxamic acid, synergizes with ABT-737 in both leukemia and MM cell lines through induction of Bim, which is sequestered by Bcl-xL and Bcl-2.46 Thus, certain agents not only increase Bim expression but also result in Bcl-2/Bcl-xL dependence, making them excellent choices to combine with ABT-737. In this regard, previous reports have demonstrated either a synergistic or additive activity of dexamethasone,18,19 which is also reported to induce Bim.47,48 The second possibility is supported by the demonstration that agents that induce Noxa, such as bortezomib and arsenic trioxide, synergize with ABT-737.10,19
Overall, our findings indicate that the interactions in conjunction with expression of Bcl-2 proteins determine whether a cell is Bcl-2/Bcl-xL–dependent and can be targeted with ABT-737. These data have several clinical implications, such as selecting the correct subset of patients to treat with ABT-737 as well as searching for agents that may synergize with this BH3-mimetic. Furthermore, although Mcl-1 expression is observed in all MM cells, it appears that some of these cells may still rely on Bcl-xL and Bcl-2 activity; therefore, ABT-737 could have activity in multiple myeloma beyond sensitizing cells to other therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank John Shaughnessy for helpful advice in the analysis of the gene expression profile datasets he deposited in GEO.
This work was supported in part by the National Institutes of Health (grants R01 CA127910 and CA129968) and the T.J. Martell Foundation (L.H.B.) and the Deets Foundation (S.L.). The ABT-737 and its enantiomer were a gift from Abbott Laboratories (Abbott Park, IL). L.H.B. is a Georgia Cancer Coalition Distinguished Scholar.
National Institutes of Health
Authorship
Contribution: A.A.M., M.K., and S.M.M. designed the experiment, executed the experiments, and wrote the manuscript; J.L., D.S., and D.M.G. performed experiments; J.L.K., K.P.L., and S.L. designed the experiment, critically reviewed the data, and prepared the manuscript; and L.H.B. oversaw the project, designed the experiment, interpreted data, and prepared the manuscript.
Conflict-of-interest disclosure: L.H.B. is an author on a patent related to the development of ABT-737 and ABT-263. In the past 3 years, he has received a milestone payment related to the development of ABT-263. The remaining authors declare no competing financial interests.
Correspondence: Lawrence H. Boise, Department of Hematology and Medical Oncology, Emory University School of Medicine, 1365C Clifton Rd, Atlanta, GA 30322; e-mail: lboise@emory.edu.
References
Author notes
A.A.M., M.K., and S.M.M. contributed equally to this study.



![Figure 4. Effect of silencing Bim, Noxa, and Puma on ABT-737–induced apoptosis. Knock-down efficiency was analyzed by Western blotting of each protein in untreated and 0.4μM ABT-737 treated samples (left panels). Activity of ABT-737 was analyzed by annexin/PI staining after knock-down of each protein (right panels). Data are mean ± SD of at least 3 independent experiments. P values: 8226, silencing of all 3 BH3 proteins are significantly different from [si(−)] control (P < .005), with the exception of siPuma at 0.8μM ABT-737 (not significant). KMS11, all values are significantly different from control (P < .005), with the exception of siNoxa at 1.6 and 3.2μM (not significant). MM.1s, siBim significantly different from control (P < .005).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/5/10.1182_blood-2011-01-327197/4/m_zh89991175570004.jpeg?Expires=1765895818&Signature=n0-3uUzul-vh4MuRKNJEDR6swxJ9y7bUhJqNo5lcJkrnQ7OPiLTuD~kb4mBimexGWb8AzUwdijhYL5klOrVeI2PuOOGpCWpENTExNBWqgTgM3blrX6PtMazPokMUVM7MNbpoK6L3neei9CdoYAFw8CJgyyxRqBoTetJav4lwtMGLrkXKb6os3EM4k-wRnP4ETgZureXlR5l8KbUMQw3NxalEGq4hcAeYa4wSJTu~MQsZZWRmbn5Yh45rbq6OBWnX~z6mRhkBV5gmQw0Km44KRUzeVTI5Q5dSrjkxwS3V68GzDIEHFA7Iyur3dJo9YcGmVXabhB8irAX5YXUBVZanMg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal