How would immune reconstitution gone wrong as a consequence of treatment be detected when the patient received a transplant to treat an autoimmune disorder? The article by Daikeler et al in this issue of Blood demonstrates how complicated this question might be.1
Autoimmune diseases (AIDs) affect about 5% of the population. As systemic therapies are rarely curative for patients with severe, life-threatening forms of AIDs, there is a need for better treatment. During the past 15 years, HSCT has been developed as a potential clinical therapy. Durable, progression-free survivals of approximately 50% after autologous HSCT are now reported.2 Clinical studies of posttransplantation immunologic recovery have demonstrated a renewed T- and B-cell repertoire consistent with “resetting” of the immune system, supporting the fundamental hypothesis for the use of HSCT.3,4 Since 1996, international transplant registries, which capture a large portion of world transplantation activity, have registered patients receiving transplants for AIDs. More than 1300 patients by the European Blood and Marrow Transplant Group (EBMT) and almost 500 by the Center for International Blood and Marrow Transplant Research (CIBMTR) have been reported.5,6 Most of these transplants (∼ 90%) are autologous procedures. The major indications were for multiple sclerosis, systemic sclerosis, or systemic lupus, and less commonly for other indications such as Crohn disease, type I diabetes, and juvenile idiopathic arthritis. Results have demonstrated a significant increase in safety and a decrease in transplant-related mortality over time, largely driven by improved supportive care, better patient selection, and increasing transplant center experience.2 While allogeneic transplantations for these indications remain rare, several randomized trials of autologous HSCT are in progress or being planned in Europe and the United States. The reports of the outcomes for autologous HSCT compared with conventional therapy are eagerly awaited.7 Over the past few years, more biologic therapies for AIDs have been developed, but these may lack the curative potential of transplantation. In addition, the use of biologic therapies raises issues of cumulative costs and morbidity and mortality related to prolonged therapy.8 Thus the prospect of having the therapeutic option of a one-time intervention with the potential for curative or durable, treatment-free survival is very attractive. In fact, this is a reasonable time to ask, “Is autologous HSCT for severe autoimmune disease entering prime time?” The relatively small numbers of such transplants performed, ongoing debates over the short-term risk/benefit ratio in the setting of nonmalignant disease, and the availability of competing nontransplantation therapies dictate that this procedure should be limited to controlled clinical trials.
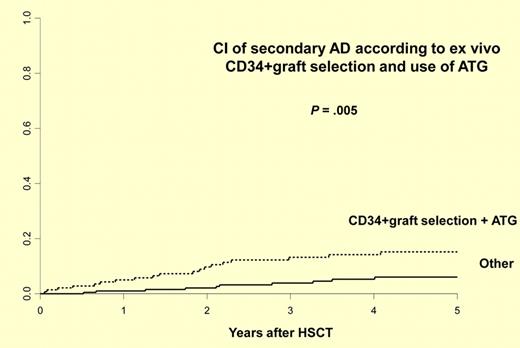
This report by Daikeler et al illustrates the need for careful and well-planned development in this field.1 The authors report the largest study to date describing the incidence and risk factors for new, secondary autoimmune disease after autologous HSCT for AIDs. They retrospectively analyzed 347 patients with AIDs reported to the EBMT from 1995 to 2009 for whom the follow-up information was available. Twenty-nine patients developed at least 1 secondary AID at a median of 21.9 (0.6-49) months after HSCT, with a cumulative incidence of 9.8% at 5 years. Consistent with previous case reports, the majority of these secondary AIDs are organ-specific involving thyroid (n = 15), cytopenias (n = 6), acquired hemophilia (n = 3), and others. Of interest, 3 of 16 patients developed secondary AIDs after allogeneic transplantation. Risk factors for the development of a secondary AID after autologous HSCT included an initial diagnosis of systemic lupus erythematosus (hazard ratio [HR] 3.21), the association of both ex vivo CD34+ cell selection and in vivo T-cell depletion by anti-thymocyte globulin (HR 2.74), and a short interval (< 61 months) from diagnosis to autologous HSCT (HR 2.32; see figure). Two of 29 deaths were because of secondary AIDs. Twenty-four cases received specific therapy for their secondary AIDs and only 5 did not. At last follow-up at a median of 6.2 years, 10 were in remission, 10 required ongoing treatments, and 5 had persistent AIDs without therapy. These data clearly demonstrate the need for careful, long-term monitoring. Many questions remain because follow-up information was obtained for only about 30% of the patients reported to the registry. What is the true incidence of this complication? How does this incidence compare with secondary AIDs reported after autologous transplantations for malignant disease? Are the risk factors the same or different? What is the optimal therapy and monitoring strategy? Providing answers to these questions is essential for understanding the magnitude of the problem, underlying mechanisms, and the development of management strategies. Since transplantations for AIDs are uncommon, the establishment of an effective registry that tracks posttransplantation events is essential. Unfortunately, as is illustrated by this study and by the CIBMTR experience, the current capacities for providing quality, long-term follow-up data after autologous transplantations for AIDs are limited.9 The barriers to registry reporting are somewhat unique in this field because these patients are largely followed by nontransplant specialists, and transplant data managers are not familiar with these diseases. Other barriers to the creation of registries include proprietary concerns, paucity of effective teams composed of both transplant and AID specialists, and the need for additional funding. To address these barriers, CIBMTR is currently increasing efforts focused on creation of a more functional registry for AID transplantations and building new research partnerships between hematologists and other disease specialists. In a collaborative effort between the CIBMTR and EBMT, new retrospective and prospective cohort studies are being implemented to follow patients who are being treated by autologous HSCT for AIDs.5,6 The current study by Daikeler and colleagues creates an exceptionally useful milestone but emphasizes the need for such high-quality tracking of posttransplantation complications in this newly evolving indication for autologous HSCT.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health