Abstract
Transplantation-associated thrombotic microangiopathy (TA-TMA) is a challenging diagnosis after hematopoietic stem cell transplantation. Although endothelial injury represents the final common pathway of disease, the exact pathophysiology of TA-TMA remains unclear. Potential causes include infections, chemotherapy, radiation, and calcineurin inhibitors. Recent literature addresses the roles of cytokines, graft-versus-host disease, the coagulation cascade, and complement in the pathogenesis of TA-TMA. Current diagnostic criteria are unsatisfactory, because patients who have received a transplant can have multiple other reasons for the laboratory abnormalities currently used to diagnose TA-TMA. Moreover, our lack of understanding of the exact mechanism of disease limits the development and evaluation of potential treatments. Short- and long-term renal complications contribute to TA-TMA's overall poor prognosis. In light of these challenges, future research must validate novel markers of disease to aid in early diagnosis, guide current and future treatments, prevent long-term morbidity, and improve outcomes. We focus on TA-TMA as a distinct complication of hematopoietic stem cell transplantation, emphasizing the central role of the kidney in this disease.
Introduction
Transplantation-associated thrombotic microangiopathy (TA-TMA) is a significant complication of HSCT. TA-TMA belongs to the family of thrombotic microangiopathies, including, among others, hemolytic uremic syndrome (HUS) and thrombotic thrombocytopenic purpura (TTP).1 TA-TMA occurs when endothelial injury in the context of HSCT causes microangiopathic hemolytic anemia and platelet consumption, resulting in thrombosis and fibrin deposition in the microcirculation.2-4 The kidney is most commonly affected, and injury has rarely been reported elsewhere in the body.3,5-9 In its most severe form, mortality rates are often high,7 whereas milder cases have an increased risk of resulting in chronic kidney disease (CKD).2
In part because of its close association with other posttransplantation complications such as GVHD and infections, opinions differ on whether TA-TMA is a separate entity.2,6,9,10 To illustrate this uncertainty, TA-TMA, despite being first recognized > 3 decades ago,6 remains difficult to characterize. For example, TA-TMA has been described as transplantation-associated HUS, posttransplantation TTP, posttransplantaton TTP/HUS, transplantation-associated microangiopathy, or posttransplantation nephropathy.7,9
We believe that there are sufficient data to support the view that TA-TMA is a distinct entity, but this is not universally agreed on. Specifically, we describe here that TA-TMA is defined by characteristic pathologic and clinical findings.2-4,11 Furthermore, TA-TMA can occur in autologous or allogeneic HSC transplant recipients, in the presence or absence of GVHD, and with or without a triggering infection.
Accordingly, any specific and universal cause of small vessel injury in TA-TMA remains unknown. In contrast, several non–HSCT-related thrombotic microangiopathies have been successfully linked to a single cause, such as Shiga toxin in diarrhea-positive HUS or decreased VWF cleaving protease (a disintegrin and metalloprotease with thrombospondin domain 13 or ADAMTS13) activity in TTP. However, given the complexity and heterogeneity of the HSCT population, it is doubtful that a single cause leads to TA-TMA in all affected patients. More probable, TA-TMA is a syndrome representing a “final common pathway” of endothelial injury damaging the kidney and other organs in the setting of HSCT.5 Understanding the mechanisms of endothelial injury, regardless of underlying causes or clinical associations, will eventually lead to improved diagnostic accuracy and better treatment, and may allow consensus to be reached on its place as a distinct posttransplantation complication.
Several excellent reviews, summarizing decades of research and clinical observations, highlight that TA-TMA poses diagnostic and therapeutic challenges and that it is often associated with poor outcomes.5-7,9 We expand on these findings by focusing on the most recent novel insights and by emphasizing the important role of the kidney in the pathophysiology, diagnosis, treatment, and prognosis of TA-TMA. Similar kidney diseases such as atypical HUS (aHUS) and Ab-mediated kidney transplant rejection may serve as models to further our understanding of TA-TMA, because the renal endothelium is central to the pathogenesis of TA-TMA. Greater awareness of its renal manifestations may decrease both short- and long-term morbidity and mortality.
Mechanisms of endothelial damage in TA-TMA
Because of similarities in histology and clinical presentation, TA-TMA was first thought to be TTP. However, because TA-TMA did not respond as well to plasma exchange as TTP, the disorders were subsequently considered distinct.6 TTP is now known to be associated with very low ADAMTS13 activity, either through inherited defects or the acquisition of inhibitory Abs.12 In contrast, TA-TMA is not typically associated with a clinically significant lack of ADAMTS13 activity.5,9
In this section, we review the most recent literature on potential mechanisms of endothelial damage in TA-TMA. As outlined, it is clear there are multiple causative factors that can contribute to the evolution of the “final common pathway” of endothelial injury in TA-TMA.5 Nonetheless, we suggest that TA-TMA can be recognized as a defined entity by its distinct histopathologic characteristics on tissue biopsy (or autopsy) and associated typical clinical and laboratory findings in the context of HSCT.2-4,11
HSCT conditioning regimens
TA-TMA is more common after allogeneic HSCT, but it also remains a significant complication of autologous transplantation.5,13 Both myeloablative and reduced-intensity conditioning regimens are risk factors for TA-TMA, especially with busulfan, fludarabine, platinum-based chemotherapy, and total body irradiation.9,14-17 These individual studies, although limited by variable patient selection, have failed to show a statistical difference in the prevalence of TA-TMA between reduced-intensity and myeloablative conditioning regimens.14,17
Infections
Several infections, most commonly Aspergillus, cytomegalovirus, and adenovirus, have been associated with TA-TMA.7,18,19 A recent publication noted that adenovirus expresses a soluble fms-like tyrosine kinase that binds vascular endothelial growth factor (VEGF), leading to TMA.20 Moreover, elevated levels of thrombomodulin, plasminogen activator inhibitor (PAI-1), and inflammatory cytokines have been observed in patients with viremia.21 Other potential infectious causes include parvovirus B19, human herpes virus-6, and, most recently, BK virus.5,11,19,22 Although BK virus is known to damage renal tubular cells, high levels of viremia (> 10 000 copies/mL) are associated with TA-TMA, which affects the glomerulus.23 It is unclear whether this association indicates that BK virus may also injure endothelial cells.
Calcineurin and mammalian target of rapamycin inhibitors
The calcineurin inhibitors cyclosporine and tacrolimus are often implicated in TA-TMA after both HSCT and solid-organ transplantation.17,24,25 Endothelial injury is related to direct cytotoxic damage, platelet aggregation, elevated VWF and thrombomodulin, altered complement regulator proteins, and decreased production of prostacyclin and nitric oxide (NO).26-28 In recipients of kidney transplants, calcineurin inhibitor-induced TMA has been associated with endothelial damage from tissue ischemia at the time of transplantation.24 It is conceivable that a similar mechanism could lead to TA-TMA in the context of HSCT, whereby endothelial damage could occur secondary to infections, high-dose chemotherapy, or cytokines released during engraftment.
The recent use of both tacrolimus and sirolimus for GVHD prophylaxis in a phase 2 study was shown to increase the risk of TA-TMA, especially in patients receiving busulfan and cyclophosphamide.29 However, others have noted that, although the addition of sirolimus increased the risk for TA-TMA, patients receiving sirolimus had a more favorable renal and survival outcome than patients receiving only calcineurin inhibitors.30 Sirolimus may lead to TA-TMA by preventing repair of injured endothelium and by decreasing local VEGF production.26 Some have cautioned that the combination of calcineurin inhibitors with sirolimus or everolimus necessitates especially close monitoring for TA-TMA.24,31
In light of these findings, there is increased interest in analyzing the role of VEGF and NO in protecting the endothelium. In the kidney, VEGF is produced by podocytes, which induces glomerular endothelial cells to maintain the urinary filtration barrier. The antiangiogenic agent bevacizumab, used in cancer therapy, causes TMA by binding VEGF.20 In patients who received a HSC transplant, higher VEGF levels are associated with less endothelial injury, better survival, and less severe GVHD.32,33 NO prevents cytokine-induced endothelial damage by decreasing the release of P-selectins and VWF.26 In TA-TMA, free hemoglobin from excessive hemolysis may bind NO, perpetuating further endo-thelial injury.27
GVHD and cytokines
Although the literature reports a close association between TA-TMA and GVHD, the relationship is confounded by calcineurin inhibitor use, infections, heterogeneous study populations, and retrospective study designs.2,3,5 A link between GVHD and TA-TMA is not surprising, given that during engraftment, donor T lymphocytes first encounter host endothelial cells.10,17,34 In an autopsy study, Changsirikulchai et al showed that the odds of developing TA-TMA were 4 times higher in patients with acute GVHD than in patients without it.2 The investigators concluded that endothelial injury may result from circulating cytokines, low levels of VEGF with grades III-IV GVHD, activation of the coagulation pathway, or direct endothelial damage from cytotoxic donor T cells.
However, the hypothesis that TA-TMA could be a form of “renal or endothelial GVHD”35,36 remains to be proven, because TA-TMA and GVHD can occur independent of each other and because increasing immunosuppression does not prevent or treat TA-TMA. Indeed, TA-TMA often requires calcineurin inhibitor withdrawal, whereas GVHD necessitates increased immunosuppression.14,18,37 Furthermore, survival decreases when patients with GVHD develop concomitant TA-TMA,38 and renal biopsies in patients with clinical GVHD show pathology other than TA-TMA, such as membranous nephropathy.11,37,39 For these reasons, we believe TA-TMA is a posttransplantation complication distinct from GVHD.
Cytokines may also play a role in the pathophysiology of TA-TMA. Cytokines produced by donor T cells make endothelial cells more susceptible to apoptosis or cell lysis mediated by perforin, granzyme B, or TNF.35 Smaller studies have identified elevated levels of circulating cytokines, including IL-8, IL-12, and thrombomodulin, during TA-TMA.21,40,41 The variable antigenicity of endothelial cells may explain why certain patient populations are more resistant to cytokine-induced vascular injury than others.35
Coagulation cascade and endothelial markers
The coagulation cascade, being regulated by the endothelium, contributes to the development of TA-TMA. PAI-1, an inhibitor of clot breakdown, is increased in other states of endothelial dysfunction such as TTP, veno-occlusive disease of the liver, and sepsis.39 Similarly, heparin cofactor II, a serpin, was found by Takatsuka et al to be increased before HSCT in patients who eventually developed TA-TMA.42 The investigators theorized that this increase was an antithrombotic compensatory response to prior endothelial injury from chemotherapy before HSCT.42
Circulating endothelial cells may serve as both a marker and a mechanism of endothelial cell dysfunction, because of their thrombotic and inflammatory properties.5,34 Erdbruegger et al studied circulating endothelial cells as markers of small blood vessel injury in 15 patients (5 after HSCT) at different periods after a diagnosis of TMA.43 These investigators identified circulating endothelial cells with the use of anti-CD146 Abs and reported that therapeutic plasma exchange (TPE) decreases levels of these cells in patients who achieve a good clinical outcome.43
Complement
TA-TMA is histologically identical to aHUS, in which renal endothelial injury occurs because of identifiable complement dysregulation in many patients.44,45 Specifically, aHUS is caused by mutations in inhibitory or activating proteins of the alternative complement pathway, including factor H (CFH), factor I, membrane cofactor protein, factor B, and C3.1,26,45 Complement has also been theorized to be involved in HUS associated with calcineurin inhibitor therapy after renal transplantation.28 Recently, investigators have identified auto-Abs to CFH which appear to account for ∼ 10% of all cases of aHUS.1 Several studies have shown that mutations and deletions in CFH-related genes (CFHR1, CFHR 3, and CFHR 4) are associated with the development of these auto-Abs, possibly explaining why certain patients are more prone to develop aHUS.46 The prevalence of CFHR1 and CFHR3 deficiency in the healthy white population is ∼ 5%, making them potentially important risk factors for the development of TA-TMA after HSCT.47
Only a few small studies have evaluated the role of complement in TA-TMA. Hale et al retrospectively assessed TA-TMA after pediatric HSCT and reported normal C3, C4, and total complement activity (CH50) in the 11 tested patients with TA-TMA.15 Similarly, we noted no abnormalities of several complement genes by direct sequence analysis in our autologous TA-TMA study.13 However, we identified detectable CFH Abs in a patient with hyperacute TA-TMA that responded to TPE and rituximab.48 Expanding on these results, we found CFH Abs in 3 of 6 patients with TA-TMA tested after allogeneic HSCT (S.J., unpublished data, 2009-2010).
The role of allo-Abs and the complement system in the pathogenesis of TA-TMA requires further study. The involvement of endothelial cells in Ab-mediated kidney transplant rejection may serve as a “counter-model” for TA-TMA. Donor-specific Abs (DSAs) produced by the recipient's immune system drive Ab-mediated rejection and subsequent complement activation after kidney transplantation. These DSAs are typically anti-HLA class I or II Abs directed against the renal graft's endothelium.49 Interestingly, renal transplantation studies have shown that non-HLA DSAs, such as activating Abs of the angiotensin II receptor, are associated with severe hypertension and vascular rejection.50
Recently, the first case that documented TA-TMA secondary to Ab-mediated tissue injury showed by diffuse C4d deposition in the glomerular capillaries was published.40 Because detection of C4d is a reliable marker of Ab deposition and complement activation,49 the investigators concluded that this case of TA-TMA was probably mediated by a humoral immune response. However, no circulating anti-HLA Abs were detected. Nevertheless, it is possible that yet unidentified “host-specific Abs” against Ags expressed by inflamed endothelium lead to direct endothelial damage or complement activation. This seems even more plausible because conditioning agents, especially fludarabine, have the ability to increase the expression of HLA class I Ags.51
C4d, a specific marker of the classic complement pathway,49 is now routinely used to diagnose Ab-mediated kidney transplant rejection. C4d remains covalently bound to the glomerular endothelium, whereas other Abs and complement components are rapidly metabolized, possibly explaining why routine immunofluorescence panels used in prior renal biopsy studies of TA-TMA have failed to show complement staining.40 C4d staining of kidney biopsies from patients with TA-TMA might identify persons who would benefit from complement-directed or Ab-depleting therapies (see “Treatment of TA-TMA”).
In summary, there are numerous identified causative agents for TA-TMA and various potential mechanisms of endothelial injury after HSCT. Potential associations of TA-TMA with GVHD, cytokines, complement, infections, and medications have been recognized, but our ability to detect evolving endothelial damage is hampered by the lack of specific identified and validated markers that can be applied reliably in the clinical setting.35 Although endothelial damage has rarely been reported in other vascular beds, the kidneys are usually most affected in TA-TMA. Several theories explaining the relative tissue specificity of TA-TMA have been advanced, including the fenestrated endothelium of the glomerulus,44 differential expression of proteins regulating the coagulation cascade, and more turbulent blood flow through the renal microvasculature.52
Diagnostic challenges
The reported prevalence of TA-TMA ranges widely (from 0.5% to 76%), reflecting different levels of awareness among institutions, diagnostic uncertainty, and limited prospective data.53 Most large, retrospective studies report a TA-TMA prevalence of 10%-25%, probably reflecting the true burden of disease.2,14,17,19
TA-TMA is a pathologic diagnosis, with renal findings involving the glomerular capillaries and other vessels. The histologic features of TA-TMA in the kidney include thickened capillary walls, fragmented erythrocytes, occluded vascular lumens, and endothelial separation with swelling, fibrin deposition, and necrosis (Figure 1).3,5 Similar pathologic features are also found in TTP, aHUS, and diarrhea-positive HUS, although thrombi may contain relatively different proportions of platelets and fibrin according to diagnosis.5,44 Furthermore, patients with TA-TMA have rarely been reported to have systemic thromboses, in contrast to patients with TTP.3,7
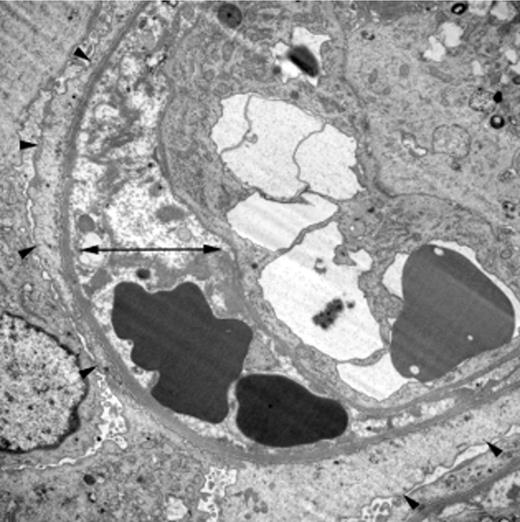
Characteristic renal histology in TA-TMA. Electron microscopy (×8000) from the kidney biopsy of a child who developed TA-TMA after autologous HSCT. It shows irregular electron-lucent expansion of the subendothelial zone (double arrow) and diffuse podocyte foot process fusion, a sign of damage to the glomerular filtration barrier (arrowheads). Reprinted by permission from Macmillan Publishers Ltd.13
Characteristic renal histology in TA-TMA. Electron microscopy (×8000) from the kidney biopsy of a child who developed TA-TMA after autologous HSCT. It shows irregular electron-lucent expansion of the subendothelial zone (double arrow) and diffuse podocyte foot process fusion, a sign of damage to the glomerular filtration barrier (arrowheads). Reprinted by permission from Macmillan Publishers Ltd.13
Although a renal biopsy can aid in the diagnosis of TA-TMA, especially in the presence of clinical uncertainty or significant renal dysfunction, this procedure carries significant risk in the post-HSCT population in whom bleeding complications are common.2,39 Highlighting the difficulty of obtaining renal tissue, large studies report that, despite the high prevalence of renal disease after HSCT, < 2% of patients underwent a renal biopsy.4,54,55 Nevertheless, kidney biopsy, when safe to perform, often provides useful prognostic and treatment information.39
Although TA-TMA almost exclusively affects the kidney,3 recent case reports have identified the involvement of other organs, including the lungs and gastrointestinal tract (Figure 2).8,56 Stressing the importance of a tissue diagnosis, Inamoto et al retrospectively reported that 92% of HSC transplant recipients undergoing colonoscopy for severe diarrhea thought to be secondary to GVHD had histologic evidence of TA-TMA, and only 30% had concomitant histologic GVHD.57
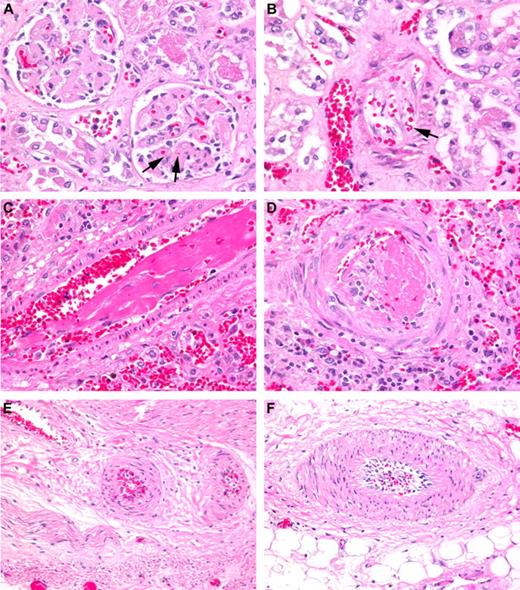
Histologic examples of TA-TMA affecting various organs. (A) Renal cortex with glomeruli showing thickened capillary walls and occluded vessel lumens. Red blood cell fragments can be seen (arrows) trapped in the mesangial matrix (H&E stain; magnification ×200). (B) Renal arteriole with separation of the endothelial cell layer from the arteriole wall with entrapped fragmented red blood cells (arrow). “Floating” endothelial cells (arrows) can be seen occluding the lumen of the vessel (H&E stain; magnification ×200). (C) Lung arteriole showing denuded endothelial layer with large fibrin thrombus trapping red blood cell fragments (H&E stain; magnification ×200). (D) Pulmonary arteriole with a recent thrombus and extravasated red blood cells (H&E stain; magnification ×200). (E-F) Mesenteric arterioles in the small bowel showing endothelial cell separation and red cell extravasation (H&E stain; magnification ×200). Nikon Eclipse 80i, 20×/0.75, Diagnostic Instruments 14.2 color mosaic, Spot software Version 4.6, Gimp 2.6.
Histologic examples of TA-TMA affecting various organs. (A) Renal cortex with glomeruli showing thickened capillary walls and occluded vessel lumens. Red blood cell fragments can be seen (arrows) trapped in the mesangial matrix (H&E stain; magnification ×200). (B) Renal arteriole with separation of the endothelial cell layer from the arteriole wall with entrapped fragmented red blood cells (arrow). “Floating” endothelial cells (arrows) can be seen occluding the lumen of the vessel (H&E stain; magnification ×200). (C) Lung arteriole showing denuded endothelial layer with large fibrin thrombus trapping red blood cell fragments (H&E stain; magnification ×200). (D) Pulmonary arteriole with a recent thrombus and extravasated red blood cells (H&E stain; magnification ×200). (E-F) Mesenteric arterioles in the small bowel showing endothelial cell separation and red cell extravasation (H&E stain; magnification ×200). Nikon Eclipse 80i, 20×/0.75, Diagnostic Instruments 14.2 color mosaic, Spot software Version 4.6, Gimp 2.6.
The limited feasibility of tissue diagnosis after HSCT has led to the development of noninvasive diagnostic criteria for TA-TMA. In fact, before these consensus guidelines,18,58 28 different clinical criteria were available, reflecting the challenges of identifying TA-TMA, when patients are already at independent risk of hematologic and renal abnormalities.7,9 In an attempt to standardize the diagnosis, 2 groups developed separate guidelines.18,58 A validation study was recently published by Cho et al, who noted limitations in the guidelines and therefore expanded on them to include the concept of “probable-TMA,” which does not require renal or neurologic findings53 (Table 1). Uderzo et al supported the concept that requiring neurologic involvement or a doubling of serum creatinine may inadvertently exclude patients with TA-TMA, because ≥ 2-fold increased creatinine and neurologic involvements were present in only 20% and 29% of their patients with TA-TMA, respectively.19
Current diagnostic guidelines for TA-TMA
| Category . | Blood and Marrow Transplant Clinical Trials Network18 . | International Working Group of the European Group for Blood and Marrow Transplantation58 . | Probable TMA as defined by validation study by Cho et al53 . |
|---|---|---|---|
| Schistocytes | ≥ 2 per high-power field in peripheral blood | > 4% in peripheral blood | ≥ 2 per high-power field in peripheral blood |
| LDH | Increased above institutional baseline | Sudden and persistent increase | Increased |
| Renal function | Doubling of serum creatinine or 50% decrease in creatinine clearance from baseline before transplantation | ||
| Platelets | Thrombocytopenia: < 50 × 109/L or a ≥ 50% decrease in platelet count | Thrombocytopenia: < 50 × 109/L or a ≥ 50% decrease in platelet count | |
| Red cells | Decreased hemoglobin or increased red blood cell transfusions | Decreased hemoglobin | |
| CNS | Unexplained neurologic dysfunction | ||
| Coombs test | Negative direct and indirect | Negative | |
| Haptoglobin | Decreased | Decreased | |
| Other | No coagulopathy |
| Category . | Blood and Marrow Transplant Clinical Trials Network18 . | International Working Group of the European Group for Blood and Marrow Transplantation58 . | Probable TMA as defined by validation study by Cho et al53 . |
|---|---|---|---|
| Schistocytes | ≥ 2 per high-power field in peripheral blood | > 4% in peripheral blood | ≥ 2 per high-power field in peripheral blood |
| LDH | Increased above institutional baseline | Sudden and persistent increase | Increased |
| Renal function | Doubling of serum creatinine or 50% decrease in creatinine clearance from baseline before transplantation | ||
| Platelets | Thrombocytopenia: < 50 × 109/L or a ≥ 50% decrease in platelet count | Thrombocytopenia: < 50 × 109/L or a ≥ 50% decrease in platelet count | |
| Red cells | Decreased hemoglobin or increased red blood cell transfusions | Decreased hemoglobin | |
| CNS | Unexplained neurologic dysfunction | ||
| Coombs test | Negative direct and indirect | Negative | |
| Haptoglobin | Decreased | Decreased | |
| Other | No coagulopathy |
Reprinted by permission from Wolters Kluwer Health.53
The limitations of clinical diagnostic criteria were also reported by Changsirikulchai et al in an autopsy study of 314 patients who underwent HSCT, 20% of whom had histologic evidence of TA-TMA in the kidneys.2 The investigators found little correlation between histologic TA-TMA and clinical criteria, and they concluded that TA-TMA is probably underreported. Two other autopsy studies reported renal histologic evidence of TA-TMA in patients who did not meet clinical diagnostic criteria.3,59 Similarly, the intestinal TA-TMA study noted earlier showed that < 15% of patients with biopsy-proven TA-TMA fulfilled clinical consensus criteria for the diagnosis.57
In addition to the difficulty of developing and applying reliable guidelines for the clinical diagnosis of TA-TMA, individual laboratory components of the existing criteria have particular limitations in patients after undergoing HSCT. First, creatinine is a poor marker of kidney function in this population, because it is strongly influenced by muscle mass, and it can remain normal until significant renal dysfunction occurs.2,15,60 Furthermore, after renal injury, serum creatinine can return to normal even when substantial renal pathology persists.61 Our research has also shown the limitations of serum creatinine values in pediatric patients with neuroblastoma developing TA-TMA after autologous HSCT. Kidney function, determined by nuclear glomerular filtration rate (GFR) as the “gold standard,” was noted to decrease by 60% 1 month after transplantation, whereas there was no significant change in serum creatinine.13 Others have also shown that creatinine-based GFR estimation is inferior to nuclear GFR before HSCT.62 Cystatin C, a recently introduced non–muscle-based marker of kidney function measured with a single blood test, may prove to be a useful indicator of GFR in the future, but prospective data validating this approach after HSCT are unavailable.62,63
Second, the inclusion of lactate dehydrogenase (LDH) as a diagnostic criterion presumes that all centers check this parameter on a routine basis.2 An otherwise well-designed study of acute kidney injury (AKI) after HSCT by Hingorani et al was accordingly unable to assess for TA-TMA because LDH was not measured at their center.64 Third, in patients with TA-TMA, fragmented red cells may not be apparent until several days after the manifestation of other clinical symptoms or may be absent altogether.60 Schistocytes remain a nonspecific finding after HSCT and can be inconsistently reported by clinical laboratories.65 Finally, the diagnosis of TA-TMA may be masked by other phenomena occurring after HSCT, such as haptoglobin changes secondary to inflammation or Coombs-positive hemolytic anemia.66
Current guidelines do not include elevation of blood pressure, which is an easily measured and clinically important indicator of renal dysfunction in TA-TMA.60 Although hypertension may be multifactorial after allogeneic HSCT, we identified elevated blood pressure as an early marker of TA-TMA in pediatric patients with neuroblastoma undergoing autologous HSCT, who have a lower risk of hypertension than patients who received allogeneic transplants.13 Importantly, blood pressure elevations were detectable weeks before the appearance of established hematologic or renal abnormalities of microangiopathy (Figure 3). Furthermore, even though allogeneic HSC transplant recipients often need antihypertensive treatment because of calcineurin inhibitor or high-dose steroid exposure or both, our clinical experience has been that patients who have undergone HSCT requiring > 2 blood pressure medications require an evaluation for TA-TMA.13
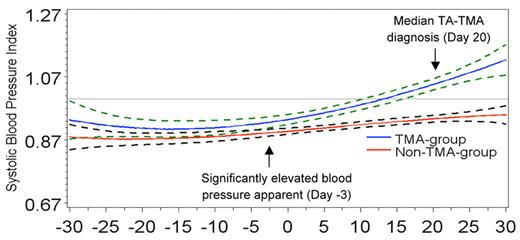
Elevated systolic blood pressure 3 days before stem cell infusion predicted later TA-TMA. A cubic regression model generated systolic blood pressure index plots over time, where day −7 is the start of transplantation high-dose chemotherapy and day 0 indicates stem cell infusion. An index value is the patient's blood pressure divided by their 95th percentile value for age, sex, and height. Therefore, an index ≥ 1 equals hypertension. Average systolic blood pressure indices for the TA-TMA group (blue lines) and the non–TA-TMA group (red lines) are plotted with surrounding 95% confidence intervals (dotted lines). Values above the horizontal line (drawn at a blood pressure index = 1) represent hypertension. Compared with the non–TA-TMA group, average systolic blood pressures in the TA-TMA group were significantly higher on day −3 of high-dose chemotherapy and thus already before stem cell infusion. Systolic hypertension was apparent by day 13 (∼ 1 week before the diagnosis of TA-TMA, which occurred at a median of 20 days after HSCT) and persisted despite aggressive antihypertensive therapy. Reprinted by permission from Macmillan Publishers Ltd.13
Elevated systolic blood pressure 3 days before stem cell infusion predicted later TA-TMA. A cubic regression model generated systolic blood pressure index plots over time, where day −7 is the start of transplantation high-dose chemotherapy and day 0 indicates stem cell infusion. An index value is the patient's blood pressure divided by their 95th percentile value for age, sex, and height. Therefore, an index ≥ 1 equals hypertension. Average systolic blood pressure indices for the TA-TMA group (blue lines) and the non–TA-TMA group (red lines) are plotted with surrounding 95% confidence intervals (dotted lines). Values above the horizontal line (drawn at a blood pressure index = 1) represent hypertension. Compared with the non–TA-TMA group, average systolic blood pressures in the TA-TMA group were significantly higher on day −3 of high-dose chemotherapy and thus already before stem cell infusion. Systolic hypertension was apparent by day 13 (∼ 1 week before the diagnosis of TA-TMA, which occurred at a median of 20 days after HSCT) and persisted despite aggressive antihypertensive therapy. Reprinted by permission from Macmillan Publishers Ltd.13
Finally, proteinuria, usually in the absence of persistent hypoalbuminemia, is an important sign of renal involvement in TA-TMA.60 Albuminuria after HSCT is both an indicator of renal endothelial injury and a predictor of increased mortality.36 Routine urinalyses every 1-2 weeks in the acute posttransplantation period can allow the early identification of hematuria and proteinuria (ie, elevations in first-morning spot protein-to-creatinine ratios, with < 0.2 mg/mg being normal) and can serve as simple markers of an underlying renal disorder when renal biopsy cannot be performed.13
Clearly, the current diagnostic criteria for TA-TMA continue to have limitations, and the bleeding risk of biopsies after HSCT make tissue diagnosis difficult.39 With this in mind, diagnosing TA-TMA requires a high clinical suspicion and close attention to the renal manifestations of disease, such as hypertension and proteinuria, which may aid in earlier and more standardized diagnosis (Figure 4).61 Future research should emphasize the discovery of “early” markers, because the successful treatment of TA-TMA may depend on early initiation of therapy.39,53 Such early markers may be simple “bedside” assessments of blood pressure or urinary findings,60 or they may be novel biochemical tests developed on the laboratory “bench,” akin to the proteomic tools presently revolutionizing the diagnosis of AKI.67
A “renal-centric” approach to detect TA-TMA. Because TA-TMA is unlikely to occur without alterations in renal function or blood pressure, careful routine monitoring of these parameters in the context of HSCT can aid in the differential diagnosis. This includes close attention to creatinine (and its dependence on muscle mass), other potentially more reliable measures of GFR (ie, cystatin C), urinalysis findings, and blood pressure readings. Evidence for proteinuria should be quantified by a first-morning spot urine protein-to-creatinine ratio (> 0.2 mg/mg is elevated). Patients with elevated LDH, abnormal renal findings or elevated blood pressure or a combination should be carefully screened for TA-TMA according to current guidelines and clinical findings. In the presence of diagnostic uncertainty, the benefit of tissue diagnosis, especially renal biopsy, should be carefully weighed against procedural risks. Potential therapeutic interventions to consider for patients with TA-TMA include calcineurin inhibitor withdrawal, plasma exchange, and rituximab. Patients without TA-TMA should be assessed for other causes of renal dysfunction or hypertension, including, but not limited to, BK virus and medication exposure (eg, steroids, calcineurin inhibitors, chemotherapeutic agents, or antimicrobials).
A “renal-centric” approach to detect TA-TMA. Because TA-TMA is unlikely to occur without alterations in renal function or blood pressure, careful routine monitoring of these parameters in the context of HSCT can aid in the differential diagnosis. This includes close attention to creatinine (and its dependence on muscle mass), other potentially more reliable measures of GFR (ie, cystatin C), urinalysis findings, and blood pressure readings. Evidence for proteinuria should be quantified by a first-morning spot urine protein-to-creatinine ratio (> 0.2 mg/mg is elevated). Patients with elevated LDH, abnormal renal findings or elevated blood pressure or a combination should be carefully screened for TA-TMA according to current guidelines and clinical findings. In the presence of diagnostic uncertainty, the benefit of tissue diagnosis, especially renal biopsy, should be carefully weighed against procedural risks. Potential therapeutic interventions to consider for patients with TA-TMA include calcineurin inhibitor withdrawal, plasma exchange, and rituximab. Patients without TA-TMA should be assessed for other causes of renal dysfunction or hypertension, including, but not limited to, BK virus and medication exposure (eg, steroids, calcineurin inhibitors, chemotherapeutic agents, or antimicrobials).
Treatment of TA-TMA
Frequently reported treatment options for TA-TMA include withdrawal of offending causative agents such as calcineurin inhibitors, TPE, rituximab, and defibrotide.53,68,69 In this section, we discuss how these and novel therapeutic methods may improve TA-TMA outcomes, although all have uncertain benefit. In general, patient responses to therapy are variable and may depend on the timing of therapy initiation.5,19,48,69,70 For example, Cho et al recently reported that therapy with TPE, defibrotide, or discontinuation/reduction of calcineurin inhibitors19,71 had the greatest benefit in patients with “probable TA-TMA” and reported outcomes were good.53 This may suggest that these patients had a milder form of disease or that therapy should be considered early, as soon as TA-TMA is suspected, to avoid irreversible organ damage. In the absence of controlled trials to evaluate additional treatment methods, discontinuation of offending agents may be the most promising therapeutic intervention compared with other potential therapies.30
Therapeutic plasma exchange
The effectiveness of TPE in the treatment of TA-TMA is uncertain because of variable outcome measurements and an incomplete understanding of its exact therapeutic mechanism. In their 2005 TA-TMA consensus guidelines, Ho et al reported poor response and high mortality when summarizing 11 studies (from 1991 to 2003) of patients treated with TPE.18 The median response rate was 36.5% (range, 0%-80%) with an associated mortality of 80% (range, 44%-100%). We summarized the most current literature (2003-current) on the use of TPE which demonstrated response rates of 27%-80%, albeit in uncontrolled, heterogeneous populations (Table 2). The success of TPE may be influenced by the timing of clinical interventions, the presence of concomitant acute GVHD, and the disappearance of circulating endothelial cells.16,38,43,70 It is also important to note that TPE, a procedure often requiring central venous access and exposure to blood products, is not without risk. Although some have reported a high rate of serious complications with this procedure,74 others have noted milder adverse events.75
Summary of recent studies (2003-present) assessing outcomes of therapeutic plasma exchange in TA-TMA
| Author, year of publication . | Patients receiving TPE/total patients with TA-TMA (n/n) . | Response to TPE, % . | Mortality, % . | Additional findings and author conclusions . |
|---|---|---|---|---|
| Hahn et al, 200473 | 19/19 | 84 | ||
| Uderzo et al, 200619 | 17/64 | 59 | 50 for all | Outcome influenced by defibrotide |
| Erdbruegger et al, 200643 | 5/5 | 40 | 20 | |
| Worel et al, 200716 | 11/11 | 64 | Treated prospectively with withdrawal of cyclosporine and TPE at TA-TMA diagnosis | |
| Oran et al, 200772 | 63/66 | 64 | 100 for nonresponders 50 for responders | Response was related to GVHD and infection control |
| Cho et al, 200838 | 16/43 | 62 for all | TA-TMA should be treated early before it develops into definite tissue injury | |
| P-TMA | 5/27 | 80 | 48 | |
| D-TMA | 11/16 | 27 | 92 | |
| Willems et al, 201017 | 25/42 | 55 for all | 80 for all | Median survival in responders 218 days versus 27 days in nonresponders |
| Author, year of publication . | Patients receiving TPE/total patients with TA-TMA (n/n) . | Response to TPE, % . | Mortality, % . | Additional findings and author conclusions . |
|---|---|---|---|---|
| Hahn et al, 200473 | 19/19 | 84 | ||
| Uderzo et al, 200619 | 17/64 | 59 | 50 for all | Outcome influenced by defibrotide |
| Erdbruegger et al, 200643 | 5/5 | 40 | 20 | |
| Worel et al, 200716 | 11/11 | 64 | Treated prospectively with withdrawal of cyclosporine and TPE at TA-TMA diagnosis | |
| Oran et al, 200772 | 63/66 | 64 | 100 for nonresponders 50 for responders | Response was related to GVHD and infection control |
| Cho et al, 200838 | 16/43 | 62 for all | TA-TMA should be treated early before it develops into definite tissue injury | |
| P-TMA | 5/27 | 80 | 48 | |
| D-TMA | 11/16 | 27 | 92 | |
| Willems et al, 201017 | 25/42 | 55 for all | 80 for all | Median survival in responders 218 days versus 27 days in nonresponders |
P-TMA indicates probable TA-TMA (see Table 1); and D-TMA, definite TA-TMA.
Because controlled studies do not exist, results are confounded by disease severity, heterogeneous outcome measurements, withdrawal of offending agents, and the use of rituximab and defibrotide. In the only prospective assessment of therapeutic benefit, Worel et al reported responses in 64% of patients treated with withdrawal of cyclosporine and prompt initiation of TPE.16 If future mechanistic studies confirm anecdotal reports on the role of complement, it could be speculated that early initiation of TPE, in appropriately selected patients, may improve outcome by replacing defective complement proteins or by removing inhibitory anti-CFH Abs or inflammatory cytokines.
Manipulation of GVHD prophylaxis
Given the strong clinical association of TA-TMA with GVHD, altering immunosuppressive therapy is not without risk. Furthermore, reducing target trough levels of calcineurin inhibitors is controversial and may not be effective. For example, Changsirikulchai et al showed that there was no correlation between cyclosporine levels and TA-TMA,2 and Oran et al found that patients with supratherapeutic tacrolimus levels at the time of TA-TMA diagnosis had similar outcomes as patients with therapeutic drug levels.72
In contrast to dose reduction, replacing calcineurin inhibitors with other immunosuppressive agents may be more beneficial.18,30,69 Alternative agents for the prophylaxis or treatment of GVHD include mycophenolate mofetil and corticosteroids.76 Daclizumab, an IL-2 receptor antagonist now replaced by basiliximab, has also shown benefit in patients with TA-TMA.69,77
Rituximab
The anti-CD20 monoclonal Ab rituximab has been used with good success in patients with auto-Abs to ADAMTS13 in TTP12 and to prevent recurrence of aHUS in patients with auto-Abs to CFH after kidney transplantation.78 In fact, several case reports have shown benefit with the use of rituximab as monotherapy or in combination with TPE or defibrotide.79-82 Of the 15 reported cases in the literature, 12 showed a positive response to rituximab.48,69,79,83 The exact mechanism of action of rituximab in TA-TMA is not known. Reports of benefit in patients with C4d deposition40 or CFH auto-Abs after HSCT48 suggest possible effects on immune regulation, Ab production, or complement activation.81
Novel therapies
Potential therapies targeting TA-TMA–induced endothelial damage include statins, bosentan, allopurinol, anti-TNF agents, recombinant thrombomodulin, and NO donors.5,26,27,54,82 Complement inhibitors, including eculizumab and others in development, may also become interesting future therapies for TA-TMA, given the potential role of complement and Ab-induced damage in similar diseases such as aHUS.45,84,85
Prevention and treatment of kidney disease
Angiotensin-converting enzyme inhibitors (ACEIs) are well known to control proteinuria and hypertension and, most importantly, to slow the progression of CKD and to decrease cardiovascular risk in patients with primary renal disease.86,87 These agents have other valuable properties, including the ability to diminish inflammation and fibrosis.87 Animal studies have shown that ACEIs have a renoprotective effect in models of HUS secondary to total body irradiation, possibly by decreasing PAI-1.39,61 Although ACEI therapy has not been used in patients with TA-TMA, it seems reasonable to speculate that patients with persistent proteinuria after HSCT could benefit from it, and prospective studies are needed to address this.36
Hale et al noted low erythropoietin levels in their pediatric patients with TA-TMA.15 They accordingly used recombinant erythropoietin to decrease transfusion requirements and to increase hemoglobin in their patients. The investigators theorized that erythropoietin may have aided in endothelial cell recovery, as evidenced by the fact that none of their patients with TA-TMA had severe renal injury.15 Of note, erythropoietin should be used cautiously in patients with malignancy because of the reported increased risk of mortality.88
Renal prognosis after TA-TMA is poor
Despite improvements in overall outcomes, kidney dysfunction after HSCT remains a significant complication.89 Regardless of the cause, up to 8% of patients who underwent HSCT require acute renal replacement therapy,89,90 which is associated with a 6-fold increased odds of death and a mortality rate > 80%.91 For those surviving an acute renal insult, developing CKD markedly increases the risk of cardiovascular disease because of accelerated atherosclerosis from endothelial damage and concomitant hypertension.10,92,93 Furthermore, patients who underwent HSCT and progress to CKD are 16 times more likely to develop end-stage renal disease (ESRD), and those needing chronic dialysis have mortality rates of 90%, much higher than other patients with ESRD.39,94
TA-TMA leads to an even higher risk of AKI, CKD, and the need for long-term dialysis, further amplifying morbidity and mortality.90 A retrospective study of 100 adult patients who underwent allogeneic HSCT found that TA-TMA, diagnosed by biopsy or clinical criteria, was a significant predictor of future renal disease. Compared with patients without TA-TMA, patients with TA-TMA were 4.3 times more likely to develop CKD and 9 times more likely to have hypertension. In patients surviving acute TA-TMA, kidney function was 40% of normal 2 years after HSCT.60
Schwarz et al reported on 101 renal biopsies in patients who received a transplant with liver, lung, heart, or HSCs.54 Compared with other pathologic diagnoses, patients with TA-TMA, regardless of transplant type, had the worst renal survival.54 Hale et al reviewed 293 pediatric recipients of allogeneic HSC transplant and found a 10% prevalence of TA-TMA.15 Moreover, 54% of these patients with TA-TMA required antihypertensive medication for ≥ 6 months. Finally, nuclear GFR decreased by 65% after a diagnosis of TA-TMA.15
The reported mortality rate in patients with TA-TMA may well be high.7 Even though early death after HSCT is often because of other acute comorbidities such as infection or GVHD or both,3 long-term TA-TMA–associated renal complications can lead to significant heart disease, a major cause of morbidity and mortality in childhood survivors of HSCT.95 Diagnosing and managing kidney disease is especially relevant for children, who have a lifetime of increased risk of developing ESRD, with the need for future dialysis and kidney transplantation.95 Accordingly, and considering the tens of thousands of HSCTs performed each year around the world, there is an urgent need for enhanced efforts to decrease transplant-related kidney damage, late CKD, and late cardiac disease in all patients.39
The accurate assessment of renal function is essential to initiating appropriate therapies, although diagnosis of acute and chronic kidney dysfunction can be challenging in patients at risk of TA-TMA. For example, AKI is defined in TA-TMA consensus guidelines as a ≥ 2-fold increase in creatinine.18 However, as mentioned earlier, creatinine-based definitions of kidney injury can underestimate the prevalence of AKI (Table 3) and CKD (Table 4) in this population, necessitating the validation of novel markers of injury and kidney function.62,63,67
Acute kidney injury definitions and staging
| Criteria . | Risk, Injury, Failure, Loss, End-Stage (RIFLE)96 . | Pediatric Risk, Injury, Failure, Loss, End-Stage (pRIFLE)97 . | Acute Kidney Injury Network (AKIN)98 . | |||
|---|---|---|---|---|---|---|
| Creatinine . | Urine . | Creatinine . | Urine . | Creatinine . | Urine . | |
| Risk | 1.5 × ↑ creat or > 25% ↓ GFR* | < 0.5 mL/kg/h × 6 h | 25% ↓ GFR† | < 0.5 mL/kg/h ×8 h | ||
| Injury | 2 × ↑ creat or > 50% ↓ GFR* | < 0.5 mL/kg/h × 12 h | 50% ↓ GFR† | < 0.5 mL/kg/h × 16 h | 1.5 × or ≥ 0.3 mg/dL ↑ creat | < 0.5 mL/kg/h × 6 h |
| Failure | 3 × ↑creat, > 75% ↓ GFR,* or creat > 4 mg/dL | < 0.3 mL/kg/h × 24 h or anuria × 12 h | > 75% ↓ GFR† or GFR† < 35 | < 0.3 mL/kg/h × 24 h or anuria × 12 h | ||
| Loss | Dialysis > 4 wk | Dialysis > 4 wk | ||||
| ESRD | Dialysis > 3 mo | Dialysis > 3 mo | ||||
| Criteria . | Risk, Injury, Failure, Loss, End-Stage (RIFLE)96 . | Pediatric Risk, Injury, Failure, Loss, End-Stage (pRIFLE)97 . | Acute Kidney Injury Network (AKIN)98 . | |||
|---|---|---|---|---|---|---|
| Creatinine . | Urine . | Creatinine . | Urine . | Creatinine . | Urine . | |
| Risk | 1.5 × ↑ creat or > 25% ↓ GFR* | < 0.5 mL/kg/h × 6 h | 25% ↓ GFR† | < 0.5 mL/kg/h ×8 h | ||
| Injury | 2 × ↑ creat or > 50% ↓ GFR* | < 0.5 mL/kg/h × 12 h | 50% ↓ GFR† | < 0.5 mL/kg/h × 16 h | 1.5 × or ≥ 0.3 mg/dL ↑ creat | < 0.5 mL/kg/h × 6 h |
| Failure | 3 × ↑creat, > 75% ↓ GFR,* or creat > 4 mg/dL | < 0.3 mL/kg/h × 24 h or anuria × 12 h | > 75% ↓ GFR† or GFR† < 35 | < 0.3 mL/kg/h × 24 h or anuria × 12 h | ||
| Loss | Dialysis > 4 wk | Dialysis > 4 wk | ||||
| ESRD | Dialysis > 3 mo | Dialysis > 3 mo | ||||
Creat indicates serum creatinine; GFR, glomerular filtration rate (mL/min/1.73 m2); ↑, increased; ↓, decreased; and ESRD, end-stage renal disease.
Based on the Modification of Diet in Renal Disease (MDRD) formula as follows: GFR = [186 × Creat−1.154 × age−0.203 × 0.742 if female × 1.210 if African American].99
Based on the original Schwarz formula as follows: GFR = [k × height in cm/Creat]; where k = 0.33 in premature infants, 0.45 in term infants to 1 year of age, 0.55 in children to 13 years of age, and 0.70 in adolescent males 13-21 years of age. The most recent Schwarz formula uses a k = 0.413 in all children.100
Staging of chronic kidney disease: renal damage or a glomerular filtration rate < 60 mL/min/1.73m2 for ≥ 3 mo
| Stage . | Description . | GFR, mL/min/1.73m2 . |
|---|---|---|
| I | Kidney disease with normal GFR | 90 |
| II | Mild | 60-89 |
| III | Moderate | 30-59 |
| IV | Severe | 15-29 |
| V | End-stage renal disease | < 15 or on renal replacement therapy |
| Stage . | Description . | GFR, mL/min/1.73m2 . |
|---|---|---|
| I | Kidney disease with normal GFR | 90 |
| II | Mild | 60-89 |
| III | Moderate | 30-59 |
| IV | Severe | 15-29 |
| V | End-stage renal disease | < 15 or on renal replacement therapy |
Adapted from Levey et al.99
Conclusion
TA-TMA represents a challenge after HSCT because of diagnostic uncertainties, lack of established treatments, and an overall poor prognosis. Current hematologic and renal parameters associated with TA-TMA have limitations in the post-HSCT population and should be interpreted in the context of coexisting disease.
We believe that the current clinical criteria18,58 for the diagnosis of TA-TMA should be expanded to address the importance of hypertension and proteinuria and the limitations of creatinine-based GFR in the HSCT population (Figure 4).13,60 Requiring a doubling of serum creatinine18 underestimates the prevalence of renal injury in this population, supporting the use of more-sensitive markers of kidney dysfunction. Furthermore, we endorse routine measurement of LDH to determine its validity in the diagnosis of TA-TMA. Severe hypertension, especially refractory to multiple medications, should also trigger an evaluation for TA-TMA. Although nuclear GFR remains the “gold standard” for renal assessment in patients after HSCT, it is expensive, invasive, and requires exposure to radiation, necessitating the evaluation of alternative measures, such as cystatin C-based GFR.62,63 Finally, long-term screening for proteinuria in patients with a history of TA-TMA may identify those at greatest risk of CKD and associated complications and “open the door” for the initiation of ACEI therapy.36,86
It is important to acknowledge that the renal and cardiovascular sequelae of CKD and TA-TMA contribute significantly to short- and long-term morbidity and mortality. Patients requiring acute renal replacement therapy are at the highest risk of poor outcomes, including but not limited to the development of future CKD. Chronic hypertension should be aggressively treated to avoid further renal injury. Close collaboration between BM transplantation, nephrology, and critical care teams is essential for the provision of optimal care.61
Although tissue diagnosis is often helpful in the presence of unexplained renal dysfunction, the risks and benefits of a biopsy must be carefully considered after HSCT, when bleeding complications are common. TA-TMA should be considered in the differential diagnosis in all biopsy samples, including lung biopsies and endoscopies, from patients who have undergone HSCT and have unexplained clinical findings.
Diagnostic and therapeutic uncertainties exist because we do not understand the specific mechanisms of endothelial injury. Current therapy is empiric at best, and future improvement depends on greater knowledge of the pathophysiology of TA-TMA. Emerging scientific advances in the areas of cytokines, GVHD, and specific markers of endothelial damage may eventually lead to better clinical care. Novel markers should be specific enough to differentiate TA-TMA from other complications of HSCT. Such markers may also permit a more objective evaluation of treatment response. Complement activation, either as the primary mode of injury or a secondary insult along a final common pathway of endothelial damage, represents a promising field of research in the diagnosis and management of TA-TMA. Along these lines, identified Abs to CFH may guide targeted Ab-depleting therapy, allowing early intervention before permanent vascular damage occurs.
Acknowledgments
The authors thank Dr David Witte from the Division of Pathology for assisting with the histologic images, Dr Stuart Goldstein from the Center for Acute Care Nephrology for his input on acute kidney injury, and Dr Bradley Dixon from the Division of Nephrology for his help with the figures.
Authorship
Contribution: B.L.L. and S.J. wrote the paper and designed the figures; and J.G. and S.M.D. edited the manuscript and provided vital conceptual insights.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Benjamin L. Laskin, Division of Nephrology and Hypertension, Cincinnati Children's Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: benjamin.laskin@cchmc.org.