The connection between inflammation and thrombosis has progressed from purely separate entities into a delicate network that often wavers precariously between the normal and diseased state. In this issue of Blood, Semeraro and colleagues develop another pathway in the network.1
By linking known players of thrombosis, specifically, platelet activation and thrombin generation with inflammation via circulating histones, Semeraro et al add a new mechanism that bridges immunity, occlusive vascular disease, and inflammation. The data presented also support the concept that platelets are sentinels in our circulation, detecting foreign bodies, damaged tissue, and immune cells. Have our platelets retained more of their evolutionary predecessors, the hemocytes, than once thought?
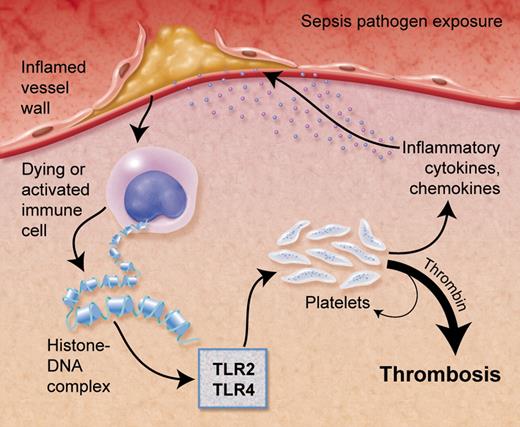
In the setting of sepsis or localized inflammation, such as with a ruptured atherosclerotic plaque, histone-DNA complexes are released from cells leading to platelet activation and thrombin generation via toll-like receptors (TLR2/TLR4). Professional illustration by Alice Y. Chen.
In the setting of sepsis or localized inflammation, such as with a ruptured atherosclerotic plaque, histone-DNA complexes are released from cells leading to platelet activation and thrombin generation via toll-like receptors (TLR2/TLR4). Professional illustration by Alice Y. Chen.
Hemostasis has been the primary function ascribed to platelets. With exposure of the underlining tissue factor–bearing cells beneath the vascular endothelium, both the coagulation pathway and platelets become activated to maintain hemostasis and facilitate repair of the endothelial cell layer. Conversion of prothrombin to thrombin contributes to the activation of platelets, which adhere and aggregate to form a platelet-fibrin plug. However, even our understanding of the well-established thrombin/platelet interaction is still evolving.
More recently, a role for platelets in inflammation has been demonstrated. Platelets have been shown to interact with bacteria2 and they are known to release inflammatory factors including interleukin (IL)–1β, P-selectin, and CD40 ligand (CD40L). Platelets also express immune-related receptors, such as toll-like receptor (TLRs). TLR2 and TLR4 are pathogen-associated molecular pattern (PAMP)–recognizing receptors. With coreceptors, they are able to recognize a wide variety of PAMPs and activate the NFκB pathway in immune cells to release other inflammatory chemokines and cytokines, such as IL6 and monocyte chemotactic protein-1 (MCP-1). On platelets, TLR23 and TLR44 can activate or prime these cells to not only aggregate and adhere, but also form heterotypic aggregates with other immune cells3 and release proinflammatory factors from their granules.5 Platelet TLR4 also promotes the formation of platelet-neutrophil aggregates leading to the release of DNA strands coated with histones and antimicrobial proteins,6 called neutrophil extracellular traps (NETs). These structures bind to platelets and red blood cells and form thrombi,7 suggesting another pathway by which inflammation activates thrombus formation. However, questions remain, such as how are platelets binding to DNA and becoming activated?
Histones, normally found bound with DNA in the nucleus, are released from dying cells and activated immune cells, including neutrophils (see figure). Mice injected with histones die from multiple microvascular thrombi similar to that seen in sepsis.1 How histones contribute to thrombosis is examined in this work by Semeraro and colleagues.1 Their interesting study shows that histones, particularly histone H4 and, in part, H3, stimulate thrombin generation, independent of the contact system. Importantly, histones are not activating proteases or inhibiting regulators of the coagulation pathway. Instead, histone-stimulated thrombin generation was dependent on activating platelets, as inhibitors to P2Y12 and glycoprotein IIbIIIa reduced the effects of the histones. Platelets aggregate and are activated in a dose-dependent manner in the presence of histones. In addition, polyphosphate (PolyP), is released by platelets and activates the coagulation pathway. To determine the specific inflammatory link, they showed that histones were interacting with platelets through TLR2 and TLR4. How TLR2 and TLR4 are interacting with histones and if there is a coreceptor, such as TLR1, 6, or 9, is unknown. In addition, TLRs typically signal through the NFκB pathway in nucleated cells, but what pathway is being used in anucleated platelets (ie, PI3K/Akt) remains unknown. Finally, Semeraro and et al put these findings into context and show that histones bound to DNA, as would be the case if released by dying cells or in NETs, enhance the thrombin generation further compared with histones alone. Treatment with activated protein C or heparin, used to treat sepsis and coagulopathies, respectively, inhibited the histone-induced thrombin generation.1
The study by Semeraro and colleagues increases our understanding of the connection between inflammation and thrombosis. It is possible that in the setting of generalized or localized inflammation such as sepsis or an atherosclerotic plaque, respectively, histones bound to DNA are released by dying and/or immune cells (see figure). These complexes bind to platelets through TLR2 and/or TLR4 leading to aggregation and heterotypic aggregate formation as well as activation of the coagulation pathway to form thrombi. This reaction could lead to a myriad of pathophysiologic events depending on the specific clinical setting, including unstable coronary syndromes or sepsis-induced vascular occlusion. The next step will be demonstrating the presence and importance of these pathways in vivo, particularly in clinical settings. Understanding the specific clinical role of the complex interaction between histones, platelets, and immune receptors will continue to add to our understanding of thrombotic disease and the interaction of inflammation and platelets.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal