Hyperhomocysteinemia (HHcy), a potent risk factor for cardiovascular disease, is associated with impaired endothelium-dependent vasodilation. In this issue of Blood, Cheng et al report that severe HHcy causes oxidation and tyrosine nitration of small and intermediate conductance Ca2+-activated potassium channels, resulting in impaired endothelium-derived hyperpolarizing factor (EDHF)–mediated relaxation of resistance arterioles.1
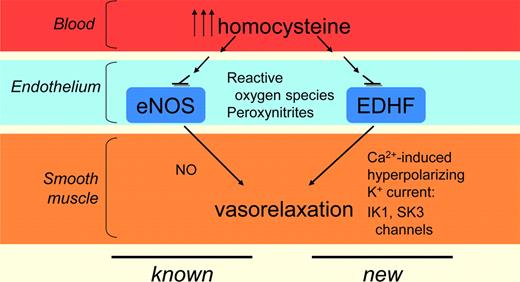
When functioning normally, the endothelium regulates vasomotor tone and local hemostasis by releasing vasodilator substances (nitric oxide, prostacyclin, or EDHF mediators) and vasoconstrictor substances (endothelin-1, angiotensin II, thromboxane A2, or free radicals).2 Linking HHcy to specific potassium channel dysfunction and impaired EDHF-mediated relaxation points to new therapeutic targets with potentially broad relevance in HHcy-related atherothrombotic disease (see figure).
Summary of known and novel adverse effects of hyperhomocysteinemia on vascular cells.
Summary of known and novel adverse effects of hyperhomocysteinemia on vascular cells.
Loss of endothelial responsiveness, an important aspect of overall endothelial dysfunction, is an early event in the pathogenesis of atherosclerosis, and maintaining endothelial integrity is critical for the preservation of blood flow and the prevention of thrombosis. Smooth muscle cell hyperpolarization mediated through endothelial cell potassium channel opening is a fundamental mechanism for vasodilation; it is particularly important at the arteriolar level for control of tissue perfusion, and often compensates for loss of other dilator mechanisms in disease settings.3
Although HHcy has previously been linked to endothelial dysfunction via effects on both endothelial nitric oxide synthase (eNOS) and prostacyclin production, Cheng et al focus on the effect of severe HHcy on EDHF-mediated relaxation. It is now recognized that EDHF-mediated responses, characterized first in the 1980s as alternative to the cyclooxygenase and the NO synthase pathways, can be mediated by a variety of agents, not just the single diffusible factor implied by the name. Regardless of triggering agent, the small and intermediate conductance potassium channels, SK3 and IK1, are key components of EDHF-mediated relaxation.4,5
Cheng et al performed ex vivo studies of small mesenteric arterioles (150-200 μm in diameter) derived from control mice, or transgenic mice with severe HHcy, to characterize vascular relaxation in response to a battery of agonists and inhibitors, and also to provide material for biochemical and immunohistologic studies. The central finding is that EDHF-mediated relaxation is compromised in the mice with severe HHcy; interestingly, despite robust increases in arterial SK3 and IK1 expression, the net function of these channels is significantly impaired in the setting of severe HHcy. This pathologic condition corresponds to increased superoxide and nitrotyrosine in the vascular wall, and Cheng et al show also that superoxide scavengers, peroxynitrite inhibitors, or the K+ channel opener NS309, which robustly increases channel Ca2+ sensitivity,6 restore most of the EDHF-mediated relaxation.
While rescue of relaxation by these interventions is intriguing, the target(s) of these untoward reactive species within the arterial wall is not entirely clear. Cheng et al studies of cultured endothelial cells show that homocysteine-mediated stress increases expression of IK1 and tyrosine nitration of both SK3 and IK1, but how these modifications interfere with channel function remains to be determined. Large conductance Ca2+-gated potassium channels (BKCa) become more active with exposure to superoxide and hydrogen peroxide, but less so with peroxynitrite7 ; little information is available on how these compounds affect SK or IK channel function.
Likewise, the increase in channel expression coincident with decreased function is also of interest. In one possible scenario, the HHcy-induced modifications that impair channel function might also stabilize the channels by interrupting their normal processing. Alternatively, a more complicated feedback loop may increase channel expression in response to decreased function, but be unable to compensate in full. Further detailed studies of direct channel modifications, with assessment of corresponding function and stability, as well as gene activation could yield insight on these points.
The findings of Cheng et al also bear on clinical HHcy. Pharmacologic rescue of EDHF-mediated vasorelaxation in severe HHcy, commonly defined by a serum Hcy concentration of > 100μM, has clear potential therapeutic value for children with homocystinuria, a highly morbid autosomal recessive syndrome affecting 1 in 200 000 live births in the United States8 and accompanied by atherothrombosis of medium and small arterial vessels, in addition to optic lens dislocation, premature osteoporosis, and mental retardation. Going forward, it will also be important to assess how EDHF-mediated mechanisms fare in the setting of the less extreme elevations of Hcy that are far more prevalent in our society; such increased levels of Hcy can occur as a result of other, less severe genetic mutations, inadequate dietary intake of folate and vitamins B6 and B12, or as a consequence of some medications including methotrexate. It has been suggested that cardiovascular disease risk increases nearly 20% for each 5μM increase in serum homocysteine level,9 so if the threshold at which HHcy can impair EDHF-mediation relaxation falls in the range of moderate or intermediate HHcy, this newly identified mechanism may contribute much more broadly to the pathologic consequences of HTN, diabetic vascular disease, endothelial dysfunction in cardiovascular interventions, and small vessel disease known to cause end-organ damage.
Finally, the insights offered by Cheng et al may inform new strategies for more effective manipulation of HHcy. Recent clinical trials of combined folic acid and B-vitamin therapy have achieved biochemical goals by lowering Hcy levels, but failed to show clinical benefit in terms of cardiovascular disease outcomes. This lack of clinical effect may reflect the complexities of folate and other B-vitamin biochemistry: folic acid supplementation not only decreases Hcy, but also indirectly impairs eNOS function, in effect nullifying the vascular protective effect of Hcy lowering.8 Potentially, more nuanced understanding of the pathogenic mechanisms provoked by HHcy may point toward therapeutic approaches that can discretely interrupt its deleterious effects.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal