Abstract
Systemic iron requirements are met predominantly through the recycling of iron from senescent erythrocytes by macrophages, a process in which the iron exporter ferroportin (Fpn1) is considered to be essential. Yet the role of Fpn1 in macrophage iron recycling and whether it influences innate immune responses are poorly understood in vivo. We inactivated Fpn1 in macrophages by crossing Fpn1-floxed animals with macrophage-targeted LysM-Cre or F4/80-Cre transgenic mice. Macrophage Fpn1 deletion mice were overtly normal; however, they displayed a mild anemia and iron accumulation in splenic, hepatic, and bone marrow macrophages when fed a standard diet. Iron loading was exacerbated after the administration of iron dextran or phenylhydrazine. When Fpn1LysM/LysM mice were challenged with an iron-deficient diet, they developed a more severe anemia and strikingly higher splenic iron levels than control mice, indicating significantly impaired iron mobilization from macrophages. Because immune responses can be altered by modulating iron status, we also examined the expression of proinflammatory cytokines. We found that expression levels of TNF-α and IL-6 were significantly enhanced in Fpn1LysM/LysM macrophages lacking Fpn1. These studies demonstrate that Fpn1 plays important roles in macrophage iron release in vivo and in modulating innate immune responses.
Introduction
The adult human has a dietary iron requirement of 1-2 mg/day, but, paradoxically, within the body the erythron alone has a daily requirement for approximately 20 mg of iron.1 This high systemic iron requirement is met predominantly through the recycling of iron from senescent red blood cells by reticuloendothelial macrophages. Thus, macrophages must export a large amount of iron to the plasma so it can be reused for hemoglobin synthesis.2 This export is carried out by the plasma membrane protein ferroportin (Fpn1).
Fpn1 is the only mammalian nonheme iron exporter identified to date3-6 and evidence that it plays a critical role in iron homeostasis has come from a number of studies. Its role as an iron exporter was first demonstrated in zebrafish where embryos carrying mutations in the Fpn1 ortholog showed a defect in iron transfer from the yolk sac to embryo.4 Studies with iron-loaded Xenopus oocytes showed that the expression of Fpn1 was associated with increased iron efflux.5 Furthermore, patients with mutations in FPN1 present with hemochromatosis type IV, an iron overload disorder with progressive iron accumulation in a range of organs and cell types, especially in reticuloendothelial macrophages.7-9 In mice, deletion of Fpn1 is embryonic lethal.10 However, mice carrying a mutation in the Fpn1 promoter or a missense mutation in Fpn1 (the flatiron mouse) are viable and exhibit reticuloendothelial iron overload and reduced plasma iron.11,12 In addition, selective inactivation of Fpn1 in mice with Meox2-Cre, which gives rise to an epiblast-derived tissue expression pattern, led to marked iron retention in enterocytes, hepatocytes, and macrophages.10 These are the tissues in which Fpn1 is most highly expressed, and all are able to export considerable amounts of iron under normal physiologic conditions.5 The strong expression of Fpn1 in macrophages and its increased expression after erythrophagocytosis support the hypothesis that Fpn1 participates in iron recycling from senescent erythrocytes.13
Iron is essential as a cofactor for enzymes that are involved in many basic cellular functions and metabolic pathways, such as oxygen transport, cellular respiration, and DNA synthesis.14 Invading pathogens have similar iron requirements, so partitioning of iron by the host is highly relevant to combating infections. In response to infection, the host has developed a range of countermeasures to limit the availability of iron to pathogens, and those pathogens in turn have evolved strategies to gain access to iron for survival and proliferation.15,16 Iron overload or iron supplementation in the host exacerbates a number of infectious diseases, including salmonellosis, yersiniosis, tuberculosis, and AIDS.17-19 In addition to the direct effects of iron as a microbial virulence factor, iron homeostasis has been shown to play a critical role in host innate immunity,20,21 and disordered iron homeostasis in humans and experimental animal models is associated with alterations in the course of infectious disease.14,22 Moreover, macrophage iron status has been considered to be closely correlated with infection in Hfe−/− mice or primary cultured macrophages.23-25 The mechanisms by which iron influences the innate immune system are complex and remain incompletely understood.
In this study, we used LysM-Cre and F4/80-Cre to delete Fpn1 in the macrophage lineage of mice. These animals were then used as models to investigate the role of Fpn1 in iron homeostasis and the effects of macrophage iron levels on responses to inflammatory stimuli. We found that disruption of Fpn1 in macrophages led to iron accumulation in the spleen and liver and perturbed iron handling in bone marrow-derived macrophages. These mice and their macrophages also showed altered responses to inflammatory stimuli, including enhanced expression of proinflammatory cytokines. These data provide strong evidence that Fpn1 is a critical determinant of macrophage iron recycling and innate immune responses.
Methods
Additional details of materials and methods are provided in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Fpn1-floxed, LysM-Cre, and F4/80-Cre animals were obtained from Dr Nancy Andrews and maintained on the 129/SvEvTac background.10,26,27 The crossing of Fpn1-floxed animals with LysM-Cre and F4/80-Cre animals to generate macrophage Fpn1 deletion mice is described in the supplemental Data. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the Institute for Nutritional Sciences, Shanghai Institutes for Biologic Sciences, and Chinese Academy of Sciences. Iron dextran, phenylhydrazine (PHZ), iron-deficient diet, and lipopolysaccharide (LPS) treatment of animals, measurement of serum iron and hematologic parameters, tissue nonheme iron and heme assays, Southern blotting, Fpn1 immunohistochemistry, tissue iron staining, bone marrow-derived macrophage (BMDM) isolation and culture, measurement of cytokines, RNA extraction, quantitative RT-PCR, Western blot analysis, and statistical analysis were performed as described previously.10,13,28
Microscope: Olympus BX61; Objective lens: UPlanApo 20×/0.70 Japan, UPlanApo 40×/0.85 Japan; Camera: Q Imaging QICAM, Canada, Fast 1394; Imaging software: Q Capture 2.90.1 (Quantitative Imaging). Software used to manipulate images: Adobe Photoshop CS4 11.0. Figure 1D magnification: 40×; and Figures 2D and 3B magnification: 20×.
Results
Efficient LysM-cre mediated deletion of Fpn1 in macrophages
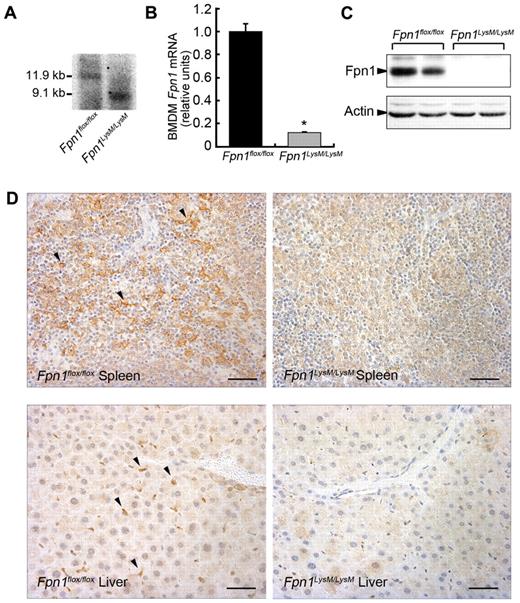
To study the role of Fpn1 in macrophage biology, we generated mice that lacked Fpn1 in the macrophage lineage (Fpn1LysM/LysM) by crossing Fpn1flox/flox mice with Cre-recombinase mice that were under the control of the lysozyme M (LysM) promoter. By this strategy, the floxed allele was deleted in mature macrophages, monocytes, and granulocytes. Southern blotting suggested that the efficiency of LysM-Cre–mediated excision in genomic DNA was very high in BMDMs > 95% (Figure 1A). At the mRNA level, Fpn1 expression was reduced by approximately 90% in BMDMs (Figure 1B) and, importantly, no Fpn1 protein could be detected by Western blotting in these cells (Figure 1C). There was clear Fpn1 staining in splenic macrophages and liver Kupffer cells of Fpn1flox/flox mice, but not in Fpn1LysM/LysM mice (Figure 1D). We also crossed Fpn1flox/flox mice with a transgenic mouse strain that expressed the Cre-recombinase under the control of the macrophage-targeted F4/80 promoter and obtained similar results (data not shown). These data indicate that we successfully deleted Fpn1 in macrophages of the spleen, liver, and bone marrow, and probably other macrophage populations.
Macrophage deletion of murine Fpn1 with LysM-Cre. (A) Southern blot analysis of LysM-cre mediated excision of Fpn1flox/flox genomic DNA in BMDMs. (B) Relative Fpn1 mRNA levels in BMDMs from Fpn1flox/flox or Fpn1LysM/LysM mice were measured by quantitative RT-PCR. Results were normalized to the internal control β-actin and presented as relative expression level calculated by the 2ΔΔCt method and presented as mean plus or minus SEM; n = 4. *P < .05. (C) Fpn1 protein levels in BMDMs from Fpn1flox/flox or Fpn1LysM/LysM mice were assessed by Western blot analysis. (D) Detection of Fpn1 expression (arrowheads). Paraffin sections of tissues from Fpn1flox/flox or Fpn1LysM/LysM mice were stained with a rabbit anti-Fpn1 primary antibody and goat anti–rabbit IgG-horseradish peroxidase secondary antibody. Scale bar represents 50 μm.
Macrophage deletion of murine Fpn1 with LysM-Cre. (A) Southern blot analysis of LysM-cre mediated excision of Fpn1flox/flox genomic DNA in BMDMs. (B) Relative Fpn1 mRNA levels in BMDMs from Fpn1flox/flox or Fpn1LysM/LysM mice were measured by quantitative RT-PCR. Results were normalized to the internal control β-actin and presented as relative expression level calculated by the 2ΔΔCt method and presented as mean plus or minus SEM; n = 4. *P < .05. (C) Fpn1 protein levels in BMDMs from Fpn1flox/flox or Fpn1LysM/LysM mice were assessed by Western blot analysis. (D) Detection of Fpn1 expression (arrowheads). Paraffin sections of tissues from Fpn1flox/flox or Fpn1LysM/LysM mice were stained with a rabbit anti-Fpn1 primary antibody and goat anti–rabbit IgG-horseradish peroxidase secondary antibody. Scale bar represents 50 μm.
Disruption of Fpn1 in mouse macrophages results in iron accumulation of spleen and liver and mild anemia
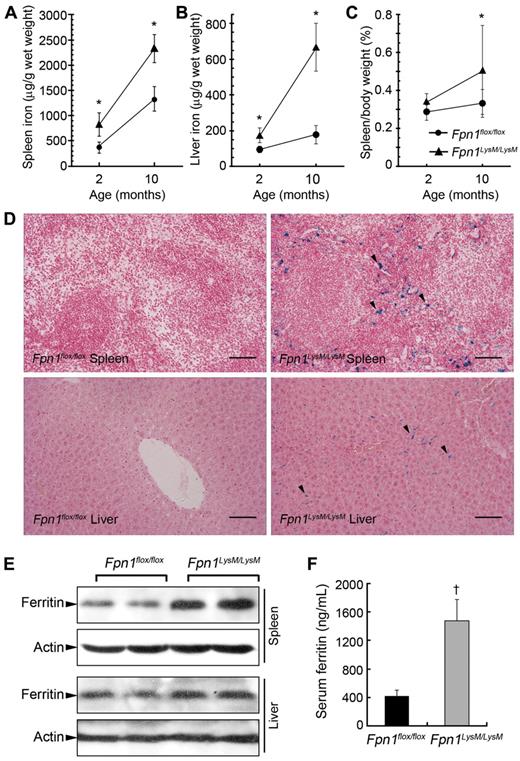
Because Fpn1 functions as an iron exporter in macrophages and other body cells, loss of its activity should result in cellular iron retention. We observed no differences in body weight, postnatal growth rate, or fertility in Fpn1LysM/LysM mice relative to littermate controls when the animals were maintained on a standard diet (data not shown). However, tissue iron loading was observed in Fpn1LysM/LysM mice, with the liver and spleen of 2-month-old animals each having nonheme iron concentrations approximately 2-fold higher than their littermate controls (Figure 2A-B). Iron accumulated in the tissues of both control and Fpn1LysM/LysM mice in a time-dependent manner, and in 10-month-old mice the hepatic and splenic iron content remained significantly higher in the knockouts. Despite increased tissue iron levels, hematologic assessment of the Fpn1LysM/LysM mice revealed decreased red blood cell count, total hemoglobin, mean cell volume, and hematocrit, although these changes were relatively mild (Table 1). However, no difference was found in white blood cell count and red blood cell distribution width. Furthermore, serum iron and transferrin saturation were significantly lower and unsaturated iron-binding capacity (UIBC) was significantly higher in 2- and 10-month-old Fpn1LysM/LysM mice compared with controls (Table 1). The difference was particularly strong in the 10-month-old mice. In accordance with these phenotypes, 2-month-old Fpn1LysM/LysM mice showed mildly enlarged spleens, and this was more evident in 10-month-old animals, consistent with extramedullary erythropoiesis secondary to serum iron restriction (Figure 2C). Tissue sections from the liver and spleen of 2-month-old Fpn1LysM/LysM mice showed pronounced iron accumulation (Perls' Prussian Blue staining) in Kupffer cells and red pulp macrophages (Figure 2D). In Fpn1flox/flox mice, no or only very weak iron staining was observed. Western blotting showed increased expression of ferritin in the spleen and liver of Fpn1LysM/LysM mice, consistent with the iron-loaded phenotype (Figure 2E). Moreover, similar to type IV hemochromatosis patients, Fpn1LysM/LysM mice showed significantly higher serum ferritin levels than Fpn1flox/flox mice (Figure 2F).
Disruption of Fpn1 in mouse macrophages results in tissue iron accumulation and mild anemia. Spleen (A) and liver (B) nonheme iron concentrations were measured in sex-matched 2-month-old (Fpn1flox/flox, 7 male, 7 female; Fpn1LysM/LysM, 7 male, 7 female) and 10-month-old (Fpn1flox/flox, 3 male, 3 female; Fpn1LysM/LysM, 3 male, 3 female) Fpn1flox/flox and Fpn1LysM/LysM mice. (C) Spleen weight/body weight ratio in 2-month-old (Fpn1flox/flox, 6 male, 6 female; Fpn1LysM/LysM, 5 male, 5 female) and 10-month-old (Fpn1flox/flox, 3 male, 3 female; Fpn1LysM/LysM, 3 male, 3 female) mice. (D) Perls' Prussian blue staining in paraffin sections of spleen and liver from Fpn1flox/flox and Fpn1LysM/LysM mice shows iron accumulation in Fpn1LysM/LysM mice (arrowheads) but not in Fpn1flox/flox mice. Scale bar represents 100 μm. (E) Western blot analysis of ferritin expression in liver and spleen of Fpn1flox/flox and Fpn1LysM/LysM mice. (F) Serum ferritin levels in 2-month-old Fpn1flox/flox (3 male, 3 female) and Fpn1LysM/LysM (3 male, 3 female) mice. Data are mean ± SEM. *P < .05. †P < .001.
Disruption of Fpn1 in mouse macrophages results in tissue iron accumulation and mild anemia. Spleen (A) and liver (B) nonheme iron concentrations were measured in sex-matched 2-month-old (Fpn1flox/flox, 7 male, 7 female; Fpn1LysM/LysM, 7 male, 7 female) and 10-month-old (Fpn1flox/flox, 3 male, 3 female; Fpn1LysM/LysM, 3 male, 3 female) Fpn1flox/flox and Fpn1LysM/LysM mice. (C) Spleen weight/body weight ratio in 2-month-old (Fpn1flox/flox, 6 male, 6 female; Fpn1LysM/LysM, 5 male, 5 female) and 10-month-old (Fpn1flox/flox, 3 male, 3 female; Fpn1LysM/LysM, 3 male, 3 female) mice. (D) Perls' Prussian blue staining in paraffin sections of spleen and liver from Fpn1flox/flox and Fpn1LysM/LysM mice shows iron accumulation in Fpn1LysM/LysM mice (arrowheads) but not in Fpn1flox/flox mice. Scale bar represents 100 μm. (E) Western blot analysis of ferritin expression in liver and spleen of Fpn1flox/flox and Fpn1LysM/LysM mice. (F) Serum ferritin levels in 2-month-old Fpn1flox/flox (3 male, 3 female) and Fpn1LysM/LysM (3 male, 3 female) mice. Data are mean ± SEM. *P < .05. †P < .001.
Serum and hematologic parameters of Fpn1flox/flox and Fpn1LysM/LysM mice
| . | 2-month-old mice . | 10-month-old mice . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fpn1flox/flox . | n . | Fpn1LysM/LysM . | n . | P . | Fpn1flox/flox . | n . | Fpn1LysM/LysM . | n . | P . | |
| Serum parameters | ||||||||||
| Serum iron, μg/dL | 238 ± 37 | 12 | 186 ± 34 | 12 | .0016 | 128 ± 4 | 6 | 67 ± 33 | 6 | .0108 |
| UIBC, μg/dL | 192 ± 25 | 12 | 225 ± 54 | 12 | .0680 | 230 ± 35 | 6 | 332 ± 32 | 6 | .0050 |
| TIBC, μg/dL | 430 ± 44 | 12 | 411 ± 49 | 12 | .3225 | 357 ± 36 | 6 | 399 ± 38 | 6 | .1580 |
| TS, % | 55.1 ± 5.1 | 12 | 45.7 ± 9.5 | 12 | .0063 | 36.0 ± 3.6 | 6 | 16.5 ± 7.2 | 6 | .0028 |
| Hematology | ||||||||||
| WBCs, × 109/L | 2.00 ± 0.70 | 10 | 1.99 ± 1.13 | 10 | .9848 | 2.83 ± 1.03 | 6 | 3.15 ± 1.23 | 6 | .6989 |
| RBCs, × 1012/L | 10.3 ± 1.1 | 10 | 8.9 ± 1.2 | 10 | .0380 | 7.8 ± 0.5 | 6 | 7.9 ± 0.4 | 6 | .6753 |
| Hb, g/L | 171 ± 15 | 10 | 143 ± 17 | 10 | .0060 | 126 ± 9 | 6 | 114 ± 9 | 6 | .1008 |
| Hct | 0.54 ± 0.06 | 10 | 0.45 ± 0.06 | 10 | .0120 | 0.41 ± 0.02 | 6 | 0.37 ± 0.1 | 6 | .0798 |
| MCV, fL | 52.9 ± 0.6 | 10 | 51.2 ± 0.9 | 10 | .0010 | 52.3 ± 0.3 | 6 | 46.7 ± 2.4 | 6 | .0038 |
| MCH, pg | 16.7 ± 0.5 | 10 | 16.2 ± 0.54 | 10 | .1110 | 16.2 ± 0.33 | 6 | 14.4 ± 0.98 | 6 | .0112 |
| MCHC, g/L | 315 ± 8.0 | 10 | 316 ± 9.0 | 10 | .7910 | 310 ± 7.0 | 6 | 308 ± 6.0 | 6 | .6635 |
| RDW, % | 14.5 ± 2.0 | 10 | 13.5 ± 1.0 | 10 | .167 | 13.8 ± 0.8 | 6 | 17.5 ± 3.9 | 6 | .045 57 |
| . | 2-month-old mice . | 10-month-old mice . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fpn1flox/flox . | n . | Fpn1LysM/LysM . | n . | P . | Fpn1flox/flox . | n . | Fpn1LysM/LysM . | n . | P . | |
| Serum parameters | ||||||||||
| Serum iron, μg/dL | 238 ± 37 | 12 | 186 ± 34 | 12 | .0016 | 128 ± 4 | 6 | 67 ± 33 | 6 | .0108 |
| UIBC, μg/dL | 192 ± 25 | 12 | 225 ± 54 | 12 | .0680 | 230 ± 35 | 6 | 332 ± 32 | 6 | .0050 |
| TIBC, μg/dL | 430 ± 44 | 12 | 411 ± 49 | 12 | .3225 | 357 ± 36 | 6 | 399 ± 38 | 6 | .1580 |
| TS, % | 55.1 ± 5.1 | 12 | 45.7 ± 9.5 | 12 | .0063 | 36.0 ± 3.6 | 6 | 16.5 ± 7.2 | 6 | .0028 |
| Hematology | ||||||||||
| WBCs, × 109/L | 2.00 ± 0.70 | 10 | 1.99 ± 1.13 | 10 | .9848 | 2.83 ± 1.03 | 6 | 3.15 ± 1.23 | 6 | .6989 |
| RBCs, × 1012/L | 10.3 ± 1.1 | 10 | 8.9 ± 1.2 | 10 | .0380 | 7.8 ± 0.5 | 6 | 7.9 ± 0.4 | 6 | .6753 |
| Hb, g/L | 171 ± 15 | 10 | 143 ± 17 | 10 | .0060 | 126 ± 9 | 6 | 114 ± 9 | 6 | .1008 |
| Hct | 0.54 ± 0.06 | 10 | 0.45 ± 0.06 | 10 | .0120 | 0.41 ± 0.02 | 6 | 0.37 ± 0.1 | 6 | .0798 |
| MCV, fL | 52.9 ± 0.6 | 10 | 51.2 ± 0.9 | 10 | .0010 | 52.3 ± 0.3 | 6 | 46.7 ± 2.4 | 6 | .0038 |
| MCH, pg | 16.7 ± 0.5 | 10 | 16.2 ± 0.54 | 10 | .1110 | 16.2 ± 0.33 | 6 | 14.4 ± 0.98 | 6 | .0112 |
| MCHC, g/L | 315 ± 8.0 | 10 | 316 ± 9.0 | 10 | .7910 | 310 ± 7.0 | 6 | 308 ± 6.0 | 6 | .6635 |
| RDW, % | 14.5 ± 2.0 | 10 | 13.5 ± 1.0 | 10 | .167 | 13.8 ± 0.8 | 6 | 17.5 ± 3.9 | 6 | .045 57 |
Data are mean ± SEM. The sample size (n) is indicated. P represents the value of a Student t test (unpaired, 2-tailed). Serum iron status and hematologic parameters in 2-month-old (Fpn1flox/flox, 6 male, 6 female; Fpn1LysM/LysM, 6 male, 6 female) and 10-month-old (Fpn1flox/flox, 3 male, 3 female; Fpn1LysM/LysM, 3 male, 3 female) mice indicate a mild iron deficiency anemia in Fpn1 knockout animals.
TIBC indicates total iron-binding capacity; TS, transferrin saturation; WBCs, white blood cells; RBCs, red blood cells; Hb, hemoglobin; Hct, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; and RDW, red blood cell distribution width.
To confirm the findings with Fpn1LysM/LysM mice, we also crossed Fpn1flox/flox mice with F4/80-Cre mice, another strain that can drive macrophage-targeted gene deletion. The resulting Fpn1F4/80/F4/80 mice showed iron accumulation in Kupffer cells and splenic macrophages, a similar iron accumulation phenotype to Fpn1LysM/LysM mice (supplemental Figure 1). Taken together, these observations indicate that Fpn1 plays an important role in exporting nonheme iron from macrophages.
Macrophages lacking Fpn1 show enhanced iron retention after iron dextran administration
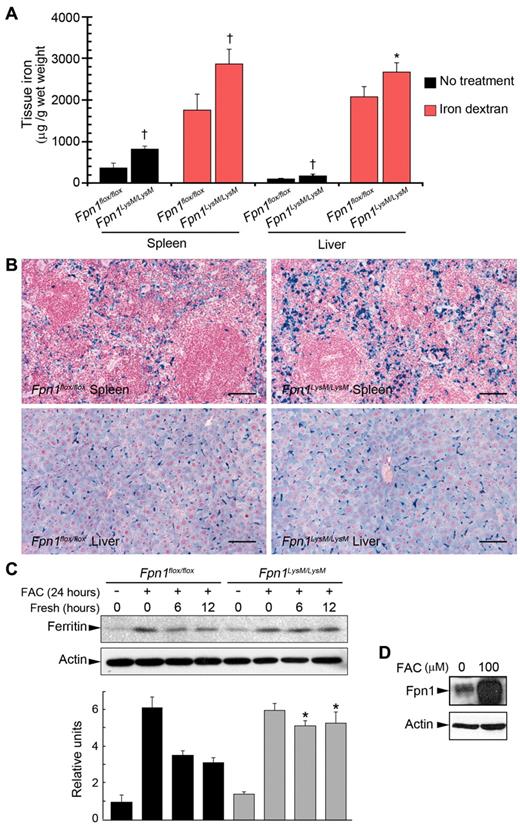
Our data suggest that knockdown of Fpn1 in macrophages impairs iron export into the plasma. A prediction from these findings is that the ability of macrophages to recycle an acute iron load would also be impaired. To investigate this, 2-month-old Fpn1LysM/LysM mice received a single intraperitoneal injection of iron dextran (250 μg per gram of body weight) and were examined one week later. Although iron dextran treatment led to some improvement in the hematologic parameters of Fpn1LysM/LysM mice, these animals still had a significantly lower serum iron level and transferrin saturation, and higher UIBC than control mice (supplemental Table 1). Quantitative measurement of tissue iron and histologic staining for iron (Figure 3A-B) demonstrated that both Fpn1flox/flox and Fpn1LysM/LysM macrophages were equally effective in accumulating iron from iron dextran, suggesting that phagocytosis was not impaired in cells lacking Fpn1. This was confirmed by phagocytosis assays using BMDMs (supplemental Figure 2). However, after iron dextran injection, significantly more iron was retained in the spleen and liver of Fpn1LysM/LysM mice than that of controls (Figure 3A-B), consistent with impaired release of the iron dextran-derived iron.
Iron accumulation in spleen and liver and correction of anemia with iron dextran in mice lacking Fpn1 in their macrophages. Sex-matched 2-month-old Fpn1flox/flox (3 male, 3 female) and Fpn1LysM/LysM (3 male, 3 female) mice were injected intraperitoneally with iron dextran at 250 μg/g body weight. The same volume of PBS was delivered by intraperitoneal injection in the control group (Fpn1flox/flox, 4 male, 4 female; Fpn1LysM/LysM, 4 male, 4 female). One week after injection, mice were killed and tissues and blood were harvested for analysis. (A) Spleen and liver nonheme iron concentrations in Fpn1flox/flox and Fpn1LysM/LysM mice treated with iron dextran. Fpn1LysM/LysM mice accumulated more iron than Fpn1flox/flox controls in both tissues. (B) Iron dextran treatment led to iron accumulation in the spleen and liver as indicated by Perls' Prussian blue staining. Fpn1LysM/LysM mice accumulated more iron, and this effect was particularly prominent in the spleen. Scale bar represents 100 μm. (C) BMDMs from Fpn1flox/flox and Fpn1LysM/LysM mice were loaded with iron by incubation with FAC (100μM) overnight. After removing the medium, cells were washed and then incubated in fresh medium for 0, 6, or 12 hours. Ferritin levels, an indicator of cellular iron stores, were measured by Western blot analysis, and ferritin/actin ratios were also quantitated by densitometry for 3 independent experiments. (D) Western blot analysis of Fpn1 expression in BMDMs from Fpn1flox/flox mice incubated with FAC. Data are mean ± SEM. *P < .05. †P < .001.
Iron accumulation in spleen and liver and correction of anemia with iron dextran in mice lacking Fpn1 in their macrophages. Sex-matched 2-month-old Fpn1flox/flox (3 male, 3 female) and Fpn1LysM/LysM (3 male, 3 female) mice were injected intraperitoneally with iron dextran at 250 μg/g body weight. The same volume of PBS was delivered by intraperitoneal injection in the control group (Fpn1flox/flox, 4 male, 4 female; Fpn1LysM/LysM, 4 male, 4 female). One week after injection, mice were killed and tissues and blood were harvested for analysis. (A) Spleen and liver nonheme iron concentrations in Fpn1flox/flox and Fpn1LysM/LysM mice treated with iron dextran. Fpn1LysM/LysM mice accumulated more iron than Fpn1flox/flox controls in both tissues. (B) Iron dextran treatment led to iron accumulation in the spleen and liver as indicated by Perls' Prussian blue staining. Fpn1LysM/LysM mice accumulated more iron, and this effect was particularly prominent in the spleen. Scale bar represents 100 μm. (C) BMDMs from Fpn1flox/flox and Fpn1LysM/LysM mice were loaded with iron by incubation with FAC (100μM) overnight. After removing the medium, cells were washed and then incubated in fresh medium for 0, 6, or 12 hours. Ferritin levels, an indicator of cellular iron stores, were measured by Western blot analysis, and ferritin/actin ratios were also quantitated by densitometry for 3 independent experiments. (D) Western blot analysis of Fpn1 expression in BMDMs from Fpn1flox/flox mice incubated with FAC. Data are mean ± SEM. *P < .05. †P < .001.
In associated experiments, we treated BMDMs with ferric ammonium citrate (FAC) for 24 hours, and then continued the incubation in fresh culture medium without added iron for a further 12 hours. Both Fpn1flox/flox and Fpn1LysM/LysM BMDMs acquired iron equally well, but Fpn1LysM/LysM cells failed to divest themselves of iron effectively as indicated by a continued high level of ferritin protein, even after 12 hours (Figure 3C). Fpn1 protein expression, which is known to increase in response to elevated iron in macrophages, was analyzed as positive control (Figure 3D). These data further demonstrated that Fpn1 played an important role in nonheme iron efflux from macrophages.
Macrophage iron recycling is impaired in Fpn1LysM/LysM mice after PHZ-induced hemolysis
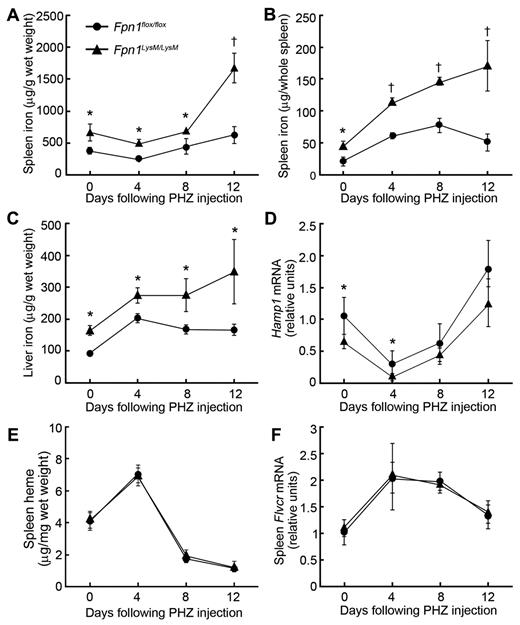
To further examine the mechanism of iron trafficking in macrophage Fpn1 null mice, we induced hemolytic anemia with PHZ. After PHZ treatment, damaged red cells and hemoglobin released from lysed erythrocytes will be cleared by macrophages. Under normal circumstances, the red cell/hemoglobin-derived iron would be recycled to the plasma to provide iron for the enhanced erythropoiesis that follows hemolysis. Administration of PHZ resulted in anemia in both knockout and control mice, with statistically significant decreases in red blood cell number, hemoglobin concentration, hematocrit (supplemental Table 2), and significant increases in the volume of spleen (data not shown). Although most parameters were similar in the 2 strains, tissue iron levels declined after a peak at 8 days in control mice but continued to rise in Fpn1LysM/LysM animals. By 12 days after treatment, Fpn1LysM/LysM mice retained significantly more iron in their livers and spleens than Fpn1flox/flox controls (Figure 4A-C). Iron accumulation in these tissues was confirmed with Perls' Prussian blue staining (supplemental Figure 3). Hepatic Hamp1 mRNA (which encodes hepcidin) also transiently declined after PHZ treatment (reaching its lowest level after 4 days) in both mouse strains, but at all time points, Hamp1 levels in Fpn1LysM/LysM mice were lower than those of control animals (Figure 4D). These data again are consistent with impaired macrophage iron export in Fpn1LysM/LysM animals.
PHZ-induced hemolysis results in iron accumulation in spleen and liver of mice lacking Fpn1 in their macrophages. Sex-matched 2-month-old Fpn1flox/flox and Fpn1LysM/LysM mice were injected intraperitoneally with PHZ at 40 μg per gram of body weight on each of 2 consecutive days. Animals were killed on days 4, 8, or 12, and tissue and blood were harvested for analysis. The first day of PHZ injection was designated as day 0. (A) Spleen nonheme iron concentrations. (B) Total nonheme iron content of the spleen. (C) Liver nonheme iron concentrations. (D) Relative Hamp1 mRNA levels in liver. (E) Heme concentrations in the spleen. (F) Relative Flvcr mRNA levels in the spleen of Fpn1flox/flox and Fpn1LysM/LysM mice were measured by quantitative RT-PCR. Results were normalized to the internal control β-actin and presented as relative expression level calculated by the 2ΔΔCt method; data are mean ± SEM; n = 6 per group (3 male, 3 female). *P < .05. †P < .001.
PHZ-induced hemolysis results in iron accumulation in spleen and liver of mice lacking Fpn1 in their macrophages. Sex-matched 2-month-old Fpn1flox/flox and Fpn1LysM/LysM mice were injected intraperitoneally with PHZ at 40 μg per gram of body weight on each of 2 consecutive days. Animals were killed on days 4, 8, or 12, and tissue and blood were harvested for analysis. The first day of PHZ injection was designated as day 0. (A) Spleen nonheme iron concentrations. (B) Total nonheme iron content of the spleen. (C) Liver nonheme iron concentrations. (D) Relative Hamp1 mRNA levels in liver. (E) Heme concentrations in the spleen. (F) Relative Flvcr mRNA levels in the spleen of Fpn1flox/flox and Fpn1LysM/LysM mice were measured by quantitative RT-PCR. Results were normalized to the internal control β-actin and presented as relative expression level calculated by the 2ΔΔCt method; data are mean ± SEM; n = 6 per group (3 male, 3 female). *P < .05. †P < .001.
In this same series of experiments, we also investigated the possibility that mechanisms other than Fpn1-mediated efflux could be used by macrophages to export iron. FLVCR is a protein that has been shown to mediate heme export from macrophages after erythrophagocytosis. Four days after PHZ treatment, we found that the spleen of both Fpn1LysM/LysM and Fpn1flox/flox mice had an increased concentration of heme (Figure 4E). Consistent with this, splenic Flvcr mRNA expression was approximately 2-fold higher in PHZ-treated mice than in PBS-treated controls, irrespective of the genotype (Figure 4F). These data indicate that heme export provides a potential mechanism for the release of iron from macrophages. However, no difference was found in Flvcr expression between Fpn1flox/flox and Fpn1LysM/LysM mice. The ability of macrophages to export heme could be one factor explaining why the anemia in macrophage-Fpn1 knockout mice was relatively mild, but other factors are probably involved.
Dietary iron restriction leads to more severe anemia and iron accumulation in Fpn1LysM/LysM mice
One possible explanation for the relatively mild anemia of Fpn1LysM/LysM mice is that enhanced iron absorption was partially compensating for the reduction in plasma iron associated with Fpn1 depletion in macrophages. To address this, 3-week-old Fpn1floxflox and Fpn1LysM/LysM mice were fed a low iron diet (0.9 mg/kg iron) for 5 weeks. Both mouse strains developed iron deficiency anemia, but the anemia was more severe in Fpn1LysM/LysM mice (supplemental Table 3). Relative to Fpn1floxflox mice, iron mobilization in Fpn1LysM/LysM mice from both spleen and liver was greatly impaired (Figure 5). Thus, restricting dietary iron supply led to a significant enhancement of the macrophage Fpn1 knockout phenotype. The spleen iron concentration in Fpn1LysM/LysM mice was approximately 22-fold higher than that of Fpn1flox/flox mice on the iron-deficient diet, but on the iron-replete normal diet the difference was only approximately 2- to 3-fold (Figure 5A). The corresponding values for the liver iron content were 4-fold (low iron diet) and 2-fold (control diet; Figure 5C). These data indicate the importance of iron absorption in ameliorating the phenotype of the knockouts and further indicate that Fpn1 is a main iron exporter in macrophages.
Macrophage iron release was greatly impaired in Fpn1LysM/LysM mice fed an iron-deficient diet. Three-week-old Fpn1flox/flox (3 male, 3 female) and Fpn1LysM/LysM (3 male, 3 female) weanling mice were fed with AIN76A iron-deficient diet for 5 weeks. (A) Spleen nonheme iron concentrations, (B) total nonheme iron content in the spleen, and (C) liver nonheme iron concentrations were measured in these mice. The spleen and liver nonheme iron concentrations of 2-month-old mice (Fpn1flox/flox, 7 male, 7 female; Fpn1LysM/LysM, 7 male, 7 female) that were fed with normal chow diet are also presented for comparison. Data are mean ± SEM. *P < .05. †P < .001.
Macrophage iron release was greatly impaired in Fpn1LysM/LysM mice fed an iron-deficient diet. Three-week-old Fpn1flox/flox (3 male, 3 female) and Fpn1LysM/LysM (3 male, 3 female) weanling mice were fed with AIN76A iron-deficient diet for 5 weeks. (A) Spleen nonheme iron concentrations, (B) total nonheme iron content in the spleen, and (C) liver nonheme iron concentrations were measured in these mice. The spleen and liver nonheme iron concentrations of 2-month-old mice (Fpn1flox/flox, 7 male, 7 female; Fpn1LysM/LysM, 7 male, 7 female) that were fed with normal chow diet are also presented for comparison. Data are mean ± SEM. *P < .05. †P < .001.
Abnormal inflammatory cytokine expression in Fpn1LysM/LysM macrophages is associated with altered cellular iron status
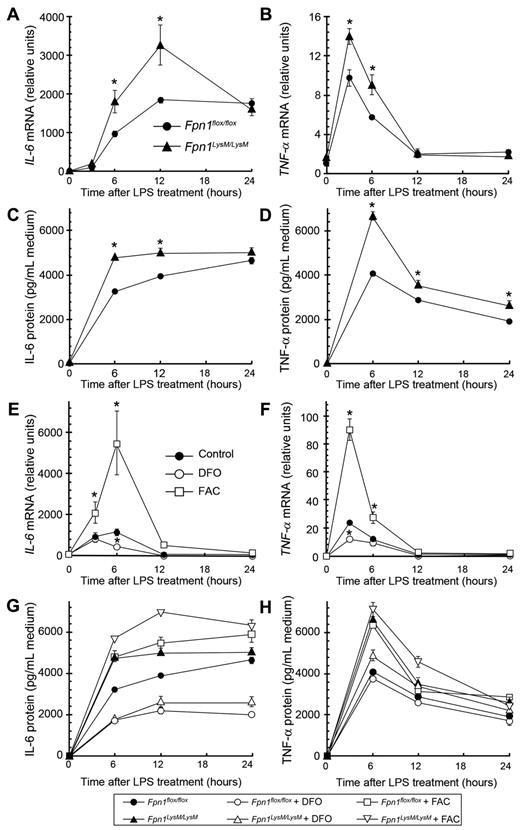
Because macrophages play an important role in the innate immune response, we investigated whether loss of Fpn1 altered the capacity of macrophages to produce cytokines in a defined in vitro setting. BMDMs were stimulated with LPS and, at various times thereafter up to 24 hours, both mRNA and protein levels of IL-6 and TNF-α were measured (Figure 6A-D). In Fpn1LysM/LysM macrophages, cytokine expression was significantly higher than in control cells. Because iron status has previously been shown to alter cytokine levels,25,29,30 we investigated whether the higher iron levels associated with the knockdown of Fpn1 could explain why IL-6 and TNF-α expression was increased in Fpn1LysM/LysM macrophages. We treated wild-type BMDMs overnight with either the iron chelator desferrioscamine (DFO) or the iron supplement FAC to change the intracellular pool of free iron. As predicted, reducing iron levels had a clear inhibitory effect on LPS-induced IL-6 and TNF-α mRNA (Figure 6E-F) and protein (Figure 6G-H) expression, whereas iron overload had the opposite effect. These findings indicate that the increased iron content of Fpn1 null macrophages contributes to the alterations in cytokine production.
Intracellular iron increases IL-6 and TNF-α expression and secretion in BMDMs. (A-D) BMDMs were isolated from Fpn1flox/flox and Fpn1LysM/LysM mice and incubated with 100 ng/mL LPS for the times indicated. Relative IL-6 (A) and TNF-α (B) mRNA levels, and IL-6 (C) and TNF-α (D) protein concentrations in the cell culture medium. (E-F) Relative IL-6 (E) and TNF-α mRNA (F) levels in BMDMs from wild-type mice. Cells were incubated overnight with FAC (100μM) or DFO (50μM) and then treated with 100 ng/mL LPS for the times indicated. (G-H) BMDMs from Fpn1flox/flox and Fpn1LysM/LysM mice were incubated overnight with FAC (100μM) or DFO (50μM) and then treated with 100 ng/mL LPS for the times indicated. IL-6 (G) and TNF-α (H) protein concentration in the cell culture medium. Data are representative of 3 independent experiments with similar results. For quantitative RT-PCR analysis, results were normalized to the internal control β-actin and presented as relative expression level calculated by the 2ΔΔCt method. Data are mean ± SEM. *P < .05.
Intracellular iron increases IL-6 and TNF-α expression and secretion in BMDMs. (A-D) BMDMs were isolated from Fpn1flox/flox and Fpn1LysM/LysM mice and incubated with 100 ng/mL LPS for the times indicated. Relative IL-6 (A) and TNF-α (B) mRNA levels, and IL-6 (C) and TNF-α (D) protein concentrations in the cell culture medium. (E-F) Relative IL-6 (E) and TNF-α mRNA (F) levels in BMDMs from wild-type mice. Cells were incubated overnight with FAC (100μM) or DFO (50μM) and then treated with 100 ng/mL LPS for the times indicated. (G-H) BMDMs from Fpn1flox/flox and Fpn1LysM/LysM mice were incubated overnight with FAC (100μM) or DFO (50μM) and then treated with 100 ng/mL LPS for the times indicated. IL-6 (G) and TNF-α (H) protein concentration in the cell culture medium. Data are representative of 3 independent experiments with similar results. For quantitative RT-PCR analysis, results were normalized to the internal control β-actin and presented as relative expression level calculated by the 2ΔΔCt method. Data are mean ± SEM. *P < .05.
Response of Fpn1LysM/LysM mice to an inflammatory stimulus in vivo
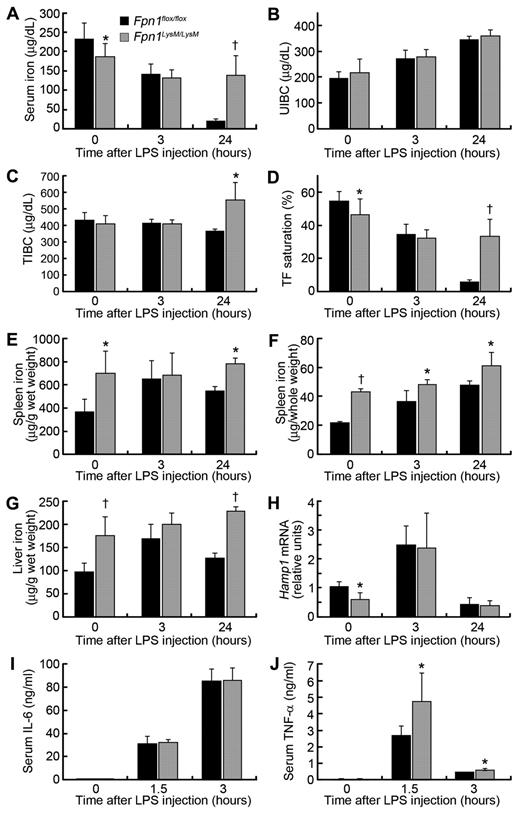
Fpn1flox/flox and Fpn1LysM/LysM mice were injected intraperitoneally with LPS to elicit an acute phase response. As expected, Fpn1flox/flox control mice developed hypoferremia after LPS treatment as indicated by low serum iron and transferrin saturation and increased UIBC at 3 and 24 hours (Figure 7A-D). In Fpn1LysM/LysM mice, serum iron levels were lower than in Fpn1flox/flox mice before LPS treatment and declined further after LPS administration, as expected (Figure 7A-D). Interestingly, after 24 hours the serum iron level was significantly higher in Fpn1LysM/LysM mice than in the controls (Figure 7A,D). The reasons for this are unclear, but it could reflect a more rapid recovery from the transient hyopferremia driven by enhanced iron absorption. Total splenic iron increased progressively with time in both Fpn1flox/flox and Fpn1LysM/LysM mice but was significantly higher in Fpn1LysM/LysM mice at all time points (Figure 7E-F). The hepatic iron concentration was also higher in Fpn1LysM/LysM mice and increased progressively with time, but in control animals it was only increased at 3 hours and returned to a near-normal level by 24 hours (Figure 7G). Liver Hamp1 mRNA level was up-regulated at 3 hours and had returned to normal at 24 hours, but there were no differences between Fpn1LysM/LysM and control mice, except before treatment where Hamp1 was significantly lower in the knockouts (Figure 7H), presumably in response to reduced plasma iron and enhanced erythropoiesis.
Lack of macrophage Fpn1 diminishes the hypoferremic response to LPS. Sex-matched 2-month-old Fpn1flox/flox and Fpn1LysM/LysM mice were injected intraperitoneally with LPS at 5 μg per gram of body weight. After LPS injection, the animals were killed at the times indicated, and tissue and blood were harvested for analysis. (A-D) Serum iron (A), UIBC (B), total iron-binding capacity (TIBC; C), and transferrin saturation (D) were measured at 0, 3, and 24 hours after injection with LPS. (E) Spleen nonheme iron concentrations. (F) Total nonheme iron content in the spleen (n = 6 per group). (G) Liver nonheme iron concentrations. (H) Relative Hamp1 mRNA levels in the liver of Fpn1flox/flox and Fpn1LysM/LysM mice were measured by quantitative RT-PCR. Results were normalized to the internal control β-actin and presented as relative expression level calculated by the 2ΔΔCt method. Data are mean ± SEM. (I) Serum IL-6 concentrations measured 0, 1.5, and 3 hours after LPS treatment. (J) Serum TNF-α concentrations measured 0, 1.5, and 3 hours after LPS treatment. Data are mean ± SEM; n = 6 per group (3 male, 3 female). *P < .05. †P < .001.
Lack of macrophage Fpn1 diminishes the hypoferremic response to LPS. Sex-matched 2-month-old Fpn1flox/flox and Fpn1LysM/LysM mice were injected intraperitoneally with LPS at 5 μg per gram of body weight. After LPS injection, the animals were killed at the times indicated, and tissue and blood were harvested for analysis. (A-D) Serum iron (A), UIBC (B), total iron-binding capacity (TIBC; C), and transferrin saturation (D) were measured at 0, 3, and 24 hours after injection with LPS. (E) Spleen nonheme iron concentrations. (F) Total nonheme iron content in the spleen (n = 6 per group). (G) Liver nonheme iron concentrations. (H) Relative Hamp1 mRNA levels in the liver of Fpn1flox/flox and Fpn1LysM/LysM mice were measured by quantitative RT-PCR. Results were normalized to the internal control β-actin and presented as relative expression level calculated by the 2ΔΔCt method. Data are mean ± SEM. (I) Serum IL-6 concentrations measured 0, 1.5, and 3 hours after LPS treatment. (J) Serum TNF-α concentrations measured 0, 1.5, and 3 hours after LPS treatment. Data are mean ± SEM; n = 6 per group (3 male, 3 female). *P < .05. †P < .001.
It has previously been demonstrated that iron exerts a stimulatory effect on cytokine production, and our studies with isolated BMDMs were in agreement with this. To investigate whether altered macrophage iron status could influence proinflammatory cytokine production in vivo, we treated Fpn1LysM/LysM and Fpn1flox/flox mice with LPS and examined the expression of IL-6 and TNF-α. LPS treatment led to strong increases in the serum concentrations of the proinflammatory cytokines IL-6 and TNF-α in both mouse strains. In the case of IL-6, the response in both Fpn1flox/flox and Fpn1LysM/LysM mice was similar; however, serum TNF-α was significantly higher in LPS treated Fpn1LysM/LysM mice than in controls (Figure 7I-J). These data suggest that increased macrophage iron may contribute to the enhanced TNF-α response. To examine this further, we injected mice with iron dextran before the administration of LPS. Serum TNF-α was strikingly up-regulated in both mouse strains before prior iron loading, but levels in Fpn1LysM/LysM mice remained significantly higher than in control mice (data not shown). With the same treatments, however, serum IL-6 levels remained relatively unaffected. The serum levels of IL-6 and TNF-α represent the combined secretion from multiple cell types, not just macrophages, and this may explain the discrepancy between TNF-α and IL-6 levels. These data provide further evidence that the iron status of macrophages is an important determinant of TNF-α expression.
Discussion
Most of the iron for daily use within the body is derived from macrophages through the recycling of iron from senescent erythrocytes. Because this process was defective in Fpn1LysM/LysM mice, we predicted that iron retention in the macrophages of these animals would deprive erythroid precursors of iron for hemoglobin production, with the consequence that the mice would develop a severe anemia. The Fpn1LysM/LysM mice did show an iron deficiency anemia with low serum iron and decreased hemoglobin, but surprisingly, the anemia was relatively mild (Table 1). Severe iron deficiency anemia is usually associated with impaired growth in mice, but we observed no differences in either body weight or growth rate between Fpn1LysM/LysM and wild-type mice. To ensure that the unexpectedly mild phenotype was not related to the use of the LysM promoter, we also generated macrophage Fpn1 knockout mice using F4/80-Cre. The resulting Fpn1F4/80/F4/80 mice also exhibited only a mild anemia and were phenotypically similar to Fpn1LysM/LysM mice. The demonstration that plasma iron levels are reduced in Fpn1LysM/LysM animals is consistent with findings in the flatiron mouse where a missense mutation in the Fpn1 gene leads to a protein with reduced iron export activity.12
To further investigate the role of Fpn1 in macrophage nonheme iron transport, we challenged mice with iron dextran, which must be processed by macrophages before its iron can be exported into the circulation. After iron dextran treatment, more iron accumulated in hepatic Kupffer cells and splenic macrophages in Fpn1LysM/LysM mice than in controls, consistent with Fpn1 playing an important role in nonheme iron efflux. In accordance with this interpretation was the demonstration that iron release from FAC-treated Fpn1LysM/LysM macrophages was impaired compared with control macrophages (Figure 3C). We also used PHZ to induce an acute hemolytic anemia in mice. The prolonged retention of iron in the liver and spleen after PHZ administration in Fpn1LysM/LysM mice provided further evidence that nonheme iron release from macrophages was impaired.
The iron regulatory hormone hepcidin is predominantly expressed and secreted by the liver. It can directly bind to Fpn1, inducing its internalization and degradation, thereby regulating iron donation to the plasma.6,31 Hepcidin expression is normally decreased in response to reduced body iron stores or stimulated erythropoiesis. In Fpn1LysM/LysM mice, liver hepcidin mRNA levels were approximately 50% lower than those of wild-type mice, reflecting the plasma iron deficiency and mild anemia associated with the deletion of Fpn1 from macrophages. This feedback loop would normally stimulate intestinal iron absorption and iron release from macrophages and storage sites. When erythropoiesis was enhanced after PHZ treatment, hepcidin expression declined in both Fpn1LysM/LysM and control mice but at all time points levels remained lower in the knockout animals. This provided further evidence that the stimulus to reduce hepcidin expression is strong in the absence of macrophage Fpn1.
One possibility to explain why Fpn1LysM/LysM mice developed only a mild anemia is that they have increased dietary iron absorption. The low hepcidin levels in the knockout mice would be consistent with this, and an increased influx of dietary iron into the plasma could at least partially compensate for the loss of macrophage Fpn1 and supply much needed iron for erythropoiesis. To investigate this hypothesis, we fed Fpn1LysM/LysM and control mice an iron-deficient diet for 5 weeks. Under these conditions, Fpn1LysM/LysM mice did indeed show a more severe anemia than animals on an iron-replete diet, confirming that dietary iron could ameliorate the anemia. Furthermore, splenic and hepatic iron was able to be mobilized very effectively in Fpn1flox/flox mice on the iron-deficient diet, but Fpn1LysM/LysM mice mobilized their iron far less efficiently. As a result, the nonheme iron content in the spleen of Fpn1LysM/LysM mice was 22-fold that of control mice, whereas on an iron-replete diet the difference was only 2- to 3-fold (Figure 5A). These data further validate the critical role Fpn1 plays in macrophage iron export and highlight the important contribution of intestinal iron absorption in compensating for Fpn1 loss.
Although we have strong evidence that enhanced iron absorption can ameliorate the macrophage Fpn1 knockout phenotype, it remains possible that macrophages are exporting iron through a pathway that does not involve Fpn1.32,33 Indeed, Keel et al32 showed that the heme export protein FLVCR was able to mediate a considerable fraction of the iron export from macrophages after the ingestion of erythrocytes. We compared Flvcr expression in both BMDMs and the spleen of Fpn1flox/flox and Fpn1LysM/LysM mice, but no differences were found. In addition, we studied Flvcr expression after erythrophagocytosis by BMDMs and in whole spleen after PHZ treatment. In each case, we found that Flvcr expression and heme content were up-regulated, but again there was no difference between the Fpn1 deletion and control groups. Nevertheless, these results do not exclude a role for Flvcr in macrophage iron metabolism. Combined deletion of Fpn1 and Flvcr in macrophages could provide important insights into the mechanisms of macrophage iron release. There are also some data to suggest that efflux of the iron storage protein ferritin is a potential pathway for the export of macrophage iron.34-36 Although we did not directly examine ferritin release in this study, the finding that ferritin levels did not decline over time in BMDMs from Fpn1LysM/LysM mice that had been preloaded with iron (Figure 3C) suggests that ferritin release was not playing a significant role. In vivo, Fpn1LysM/LysM mice had significantly higher serum ferritin levels than that of control mice, suggesting a more complex situation that probably reflects ferritin release from a range of cell types.36
Iron plays important roles in both pathogen virulence and host antimicrobial responses14 ; consequently, disturbances of iron homeostasis can alter the body's susceptibility to infectious disease. As Fpn1 deletion in macrophages disturbed iron homeostasis, we examined whether Fpn1 deficiency in macrophages had effects on innate immunity. In response to LPS stimulation, BMDMs from Fpn1LysM/LysM mice expressed higher IL-6 and TNF-α mRNA and protein than wild-type macrophages (Figure 6A-D). This altered cytokine biosynthesis appeared to be related to elevated intracellular iron levels in macrophages that resulted from the loss of Fpn1. In associated studies, we found that cellular iron levels in both wild-type and Fpn1 knockout macrophages positively correlated with proinflammatory cytokine production. These data are consistent with earlier studies that have demonstrated that increased iron can enhance signaling through the NF-κB pathway.37 Furthermore, 2 recent studies38,39 have provided evidence that the binding of hepcidin to Fpn1 leads to a dampening of TLR4 and cytokine signaling through the activation of SOCS3. When hepcidin levels are low, this dampening effect is relieved and the response to LPS is more robust. In our studies, hepcidin-Fpn1 signaling is abrogated as Fpn1 is missing in macrophages. This should mimic the low hepcidin situation and the lead to enhanced TLR4 signaling, which we have observed. Thus, reduced SOCS3 expression could also contribute to the increased expression of proinflammatory cytokines by macrophages lacking Fpn1.
Although the effect of an inflammatory stimulus in vitro appears clear, the situation is more complicated in vivo. When Fpn1LysM/LysM mice were injected intraperitoneally with LPS, serum TNF-α was significantly higher compared with control mice (Figure 7J). Administration of iron dextran increased TNF-α expression in both genotypes of mice, but TNF-α expression remained higher in Fpn1LysM/LysM mice. These data were consistent with the in vitro results. However, for IL-6, no differences were found between Fpn1LysM/LysM and control mice in vivo (Figure 7I). As IL-6 and TNF-α are secreted not only by macrophages but also by other cell types, it is difficult to isolate the effects of elevated macrophage iron content alone on whole body cytokine levels. Macrophage IL-6 secretion may be elevated in Fpn1LysM/LysM mice, but lower expression from other cell types (perhaps in response to serum iron depletion) may compensate, resulting in no net change in circulating IL-6.
In both Fpn1flox/flox and Fpn1LysM/LysM mice, there was a decline in serum iron after LPS treatment. This effect is mediated by hepcidin. Hepcidin levels increase after an inflammatory stimulus and act on various cells types, including macrophages, to remove Fpn1 from the cell surface and limit iron release into the plasma.40,41 Thus, a strong inflammatory stimulus will, in effect, approach the Fpn1 knockout situation with respect to serum iron. As expected, untreated Fpn1LysM/LysM mice had lower serum iron than Fpn1flox/flox mice, and this declined further 3 hours after LPS treatment. The probable reason for the additional decrease in Fpn1LysM/LysM mice is that iron export from cells other than macrophages is also depressed in response to inflammation. However, it is also possible that other mechanisms are operating. For example, expression of the iron import protein Zip14 is increased in response to LPS, and this could contribute to lowered serum iron.42 Interestingly, 24 hours after LPS treatment, Fpn1LysM/LysM animals had a higher serum iron than control mice (Figure 7A). The hypoferremia that follows a single dose of LPS is only transient, so these data suggest that the macrophage Fpn1 knockout animals are recovering more rapidly that their wild-type counterparts. It is possible that this reflects enhanced intestinal iron absorption or iron release from hepatocytes and other cells, but a more detailed analysis of the time course of the LPS response and the changes in tissues iron levels will be required to answer this question.
In conclusion, Fpn1 plays an important role in macrophage iron recycling in vivo, and that it is the predominant protein involved in this process. Furthermore, we have presented evidence that alterations in macrophage iron content can have a significant influence in modulating innate immune responses. Fpn1 is one of a number of proteins of iron homeostasis that influence the function of the immune system. These studies highlight the complexity of the links between iron metabolism and immunity in vivo and emphasize the need for further investigation of the molecules and pathways involved using novel murine model systems.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Nancy C. Andrews, Adriana Donovan, and Cindy N. Roy for providing Fpn1 flox, LysM-Cre, and F4/80-Cre mice and BMDM technique support and other members of the F.W. laboratory for encouragement and helpful comments. E coli-GFP was a generous gift from Dr Bao-Xue Ge at the Institute of Health Sciences, Chinese Academy of Sciences.
This work was supported by the Chinese Academy of Sciences (grant KSCX2-YW-R-141, F.W.), National Natural Science Foundation of China (grants 10979071, 30970665, and 31030039, F.W.; grant 81000358, Y.S.; and grants 30901193 and 2010KIP309, Y.Y.), Ministry of Science and Technology of China (grants 2009CB941400 and 2011CB966200, F.W.), Science & Technology Commission of Shanghai Municipality (grant 10JC1416800, F.W.), and Clinical Center of Shanghai Xuhui District Central Hospital (grant CRC2010012, F.W.). G.J.A. holds a Senior Research Fellowship from the National Health and Medical Research Council of Australia. F.W. is a scholar of the Hundred Talents Program of the Chinese Academy of Sciences.
Authorship
Contribution: Z.Z. designed research, performed most experiments, analyzed data, and wrote the paper; F.Z., P.A., X.G., Y.S., Y.T., Q.W., Y.Z., and Y.Y. performed experiments; B.N. and G.N. analyzed tissue heme; M.D.K. provided Fpn1 antiserum and helpful comments on manuscript; G.J.A. provided scientific advice and helpful comments on paper writing; and F.W. oversaw the study, designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fudi Wang, Mineral Molecular Nutrition Laboratory, Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, 294 Taiyuan Road, Shanghai 200031, PR China; e-mail: wangfd@sibs.ac.cn or fudiwang.lab@gmail.com.