Abstract
Despite increasing use of prothrombin complex concentrate (PCC) to treat hemorrhage-associated coagulopathy, few studies have investigated PCC in trauma, and there is a particular lack of safety data. This study was performed to evaluate PCC therapy in a porcine model of coagulopathy with blunt liver injury. Coagulopathy was induced in 27 anesthetized pigs by replacing approximately 70% blood volume with hydroxyethyl starch 130/0.4 and Ringer's lactate solution; erythrocytes were collected and retransfused. Ten minutes after trauma, animals randomly received PCC (35 or 50 IU/kg) or saline. Coagulation parameters including thromboelastometry, thrombin generation, and blood loss were monitored for 2 hours. Internal organs were examined macroscopically and histologically to determine the presence of emboli and assess liver injury. Total blood loss was significantly lower and survival was higher in both PCC groups versus the control group (P < .05). These outcomes appeared to be dose-independent. Thromboembolism was found in all animals treated with 50 IU/kg PCC; 44% also showed signs of disseminated intravascular coagulation. Liver injury was similar in all animals. In conclusion, 35 IU/kg PCC safely improved coagulation and attenuated blood loss. However, the higher dose of PCC (50 IU/kg) appeared to increase the risk of thromboembolism and disseminated intravascular coagulation.
Introduction
The complexity of trauma-related coagulopathy requires an early and targeted strategy to protect traumatized patients from exsanguination.1 Most trauma centers use fresh frozen plasma (FFP) as part of their massive transfusion protocol.2 To avoid the well-known complications of treatment with FFP and red blood cells (eg, volume overload and transfusion-related lung injury),3,4 the use of coagulation factor concentrates has been investigated in animal models.5-10
Prothrombin complex concentrates (PCCs) are derived from human plasma. Most of these products contain the vitamin K–dependent coagulation factors II, VII, IX, and X. PCCs with low levels of factor VII are available (3-factor PCCs), but products with higher levels of factor VII (4-factor PCCs) are used more commonly. PCCs are standardized and administered according to the quantity of factor IX, and there are considerable differences between PCCs in the quantities of coagulation factors II, VII, and X, relative to factor IX.11 PCCs may contain coagulation inhibitors (protein C, protein S, protein Z, and antithrombin), but the levels of these constituents differ among PCCs.11 The primary safety concerns with PCCs are thromboembolic complications.12-17 The propensity of PCCs to increase thrombin generation is the key to their efficacy, but it may also be the key to the risk of thromboembolic events. Excessive concentrations of prothrombin (factor II) in relation to levels of antithrombin have been suggested as the cause of thromboembolic complications.18,19 The quantity of factor II administered varies among PCCs, and it is not usually monitored. Moreover, the ratio of antithrombin to thrombin is low in all preparations (range 0-1:50),11 meaning that there is always a risk of factor II overload.
Currently, there are 2 major indications for PCCs: rapid reversal of oral anticoagulation (vitamin K antagonists) and deficiency of vitamin K–dependent coagulation factors in life-threatening bleeding.20,21 PCCs have been well studied for the first of these indications, and their safety in that setting seems to be generally acceptable.22 However, no prospective studies have been performed to investigate the use of PCC as first-line monosubstance therapy for trauma-related coagulopathy. All evidence supporting the use of PCCs in trauma-related coagulopathy is from observational and animal studies.5-7,23-25 Although these data show the potential for PCCs to be effective in correcting coagulopathy associated with bleeding,26 there is no clinical evidence demonstrating their safety in this setting. The potential risk is illustrated by a recent animal study, which reported a fatal thromboembolic complication after treatment of dilutional coagulopathy with fibrinogen concentrate and PCC.27
Using a pig model of blunt liver injury, we attempted to investigate the safety and efficacy of 2 concentrations of PCC in trauma-related coagulopathy. The primary endpoint of the study was blood loss; secondary endpoints including thrombin generation were chosen to show the impact of PCC therapy on overall coagulation status.
Methods
Ethics and anesthesia
This study was performed at the RWTH Aachen University Hospital, Aachen, Germany. All experiments were performed in accordance with German legislation governing animal studies, which follows the Guide for the Care and Use of Laboratory Animals.28 Official permission was granted from the appropriate government office for animal care and use (Landesamt für Natur, Umwelt und Verbraucherschutz, Recklinghausen; No. 8.87-50.10.35.09.019). Before surgery, pigs were housed in ventilated rooms and allowed to acclimatize to their surroundings for a minimum of 5 days. Animals were fasted overnight before the surgical procedure; water was provided.
Twenty-seven male German landrace pigs (body weight 30-36 kg) received an intramuscular injection of 4 mg/kg azaperone (Stresnil; Janssen Pharmaceuticals) and 0.1 mg/kg IM atropine (Atropinsulfate; B. Braun) as premedication. Anesthesia was induced by intravenous injection of 3 mg/kg propofol (Disoprivan; AstraZeneca) followed by orotracheal intubation. The animals were ventilated in a closed circuit (Physioflex; Draeger) with 16-20 breaths/min and a tidal volume of 10 mL/kg to keep the end-tidal partial pressure of carbon dioxide between 36 and 42 mmHg. Anesthesia was maintained with isoflurane (Forane; Abbott Laboratories) at end-tidal concentrations of 1% and a continuous infusion of fentanyl (Janssen Pharmaceuticals) at 3-4 μg/kg/h at an inspiratory oxygen fraction of 1.0. Ringer's lactate solution (RL) was infused, initially at 4 mL/kg/h at first and increased to 10 mL/kg/h after laparotomy until infliction of trauma. Temperature was maintained at 36.5-37.0°C throughout the experiment by using a warming blanket.
The animals were monitored by electrocardiogram, tail pulse oximetry, temperature, and arterial as well as central venous pressure by femorally introduced catheters connected to a standard anesthesia monitor (AS/3; Datex Ohmeda).
Surgical preparation and hemodilution
Two 8.5-French catheters were surgically implanted in the right and left jugular veins for volume substitution and insertion of a pulmonary artery catheter. After line placement, a midline laparotomy with splenectomy and cystostomy was performed. Intravascular volume was diluted by replacing approximately 70% of the estimated blood volume29 with hydroxyethylstarch 130/0.4 (Voluven; Fresenius) and RL in a ratio of 1:1. Blood was withdrawn at a rate of 100 mL/min. Volume was substituted as soon as the blood pressure had dropped to 40%-50% of the baseline value and continued until it had returned to 80% of the baseline value. The collected blood was processed (Cell Saver 5; Haemonetics), and the red cells were retransfused (20 mL/kg) over 10 minutes to avoid early death from anemia.
Liver injury and PCC substitution
A reproducible grade 3 blunt liver injury30 was induced with a force of 219-289N, using a custom-made instrument described elsewhere.31 Five minutes after injury, all animals received RL as a fluid bolus of 35 mL/kg, followed by continuous infusion at 40 mL/kg/h until the end of the experiment. Using field envelopes, the 27 animals were randomized 1:1:1 to receive normal saline solution (controls), 35 IU/kg PCC (Cofact; Sanquin; PCC35), or 50 IU/kg PCC (PCC50). PCC was prepared according to the manufacturer's instructions and infused at a rate of 4 mL/min. Based on a biochemical analysis of 10 consecutive lots performed by the manufacturer, reconstituted Cofact contains a mean of 26.8 IU/mL factor II, 12.1 IU/mL factor VII, 25.4 IU/mL factor IX, and 26.2 IU/mL factor X. In the same biochemical analysis, Cofact was found to contain the following mean levels of anticoagulants: 24.3 IU/mL protein C and 3.1 IU/mL protein S. Conversely, Cofact contains only a low level of antithrombin (≤ 0.6 IU/mL) and no heparin. The doses of PCC were chosen according to the results of studies published previously.5,8,32,33
The observation period ended 120 minutes after injury. Animals surviving for the whole of this period were euthanized with fentanyl, propofol, and potassium chloride. Immediately after death, the abdomen was reopened, the vena cava was clamped cranial to the liver, and the intraperitoneal blood was collected to determine the total blood loss postinjury. The lungs, heart, liver, and kidneys were removed and prepared for histologic examination.
Blood sampling and analytical methods
Blood was collected and arterial blood gas analysis was performed after splenectomy (“baseline”), at the end of hemodilution (“hemodilution”), and 30, 60, and 120 minutes after liver injury. For animals dying before 120 minutes postinjury, the last assessment was performed immediately after death. Hemoglobin concentration, pH, partial pressure of oxygen, and partial pressure of carbon dioxide were measured with a blood gas analyzer (ABL500; Radiometer). Prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen concentration, D-dimers, and antithrombin were determined by standard laboratory methods using the appropriate tests from Dade Behring on an MC 4 plus steel-ball coagulometer (Merlin Medical); fibrinogen concentration was measured using the Clauss assay. Thrombin–antithrombin (TAT) complexes were quantified by ELISAs (Enzygnost; Dade Behring).
Thromboelastometry and thrombin generation
A coagulation analyzer (ROTEM; Tem International GmbH) was used to perform the EXTEM assay. The following parameters were measured: clotting time (CT; seconds), clot formation time (CFT; seconds), and maximum clot firmness (MCF; millimeters).
Thrombin generation in plasma was measured using the Calibrated Automated Thrombogram (CAT; Thrombinoscope BV) as described previously, using final concentrations of 1pM tissue factor and 4μM phospholipids.34 To correct for inner filter effects and substrate consumption, each thrombin generation analysis was calibrated against the fluorescence curve obtained in the same plasma with a fixed amount of calibrator (Thrombin Calibrator; Thrombinoscope BV). Fluorescence was read in an Ascent Reader (Thermolabsystems OY) equipped with a 390/460 nm filter set, and thrombin generation curves were calculated using Thrombinoscope Version 4 software (Thrombinoscope BV). All measurements were performed in duplicate.
Pathologic examination
After death, internal organs (heart, lungs, liver, and kidneys) were removed immediately and fixed in 10% buffered formalin. Injured parts of the liver were cut into 3-mm-thick slices and examined macroscopically and microscopically by a pathologist blinded to therapy to assess the degree of injury. In addition, representative tissue sections of all 4 organs were processed to determine the occurrence of thromboembolic events. All samples were embedded in paraffin and stained, both by H&E and by a standard elastica van Gieson protocol, for histologic examination under light microscopy (Microscope: Nikon, Eclipse 50i; Camera: Nikon, DS-2Mv; Hardware-Camera-Adaptor with Software: Nikon, DS-L1 [Software-version; Firmware Ver3.22], recording on CF card; Mode: TIF, 2 MP; Objectives: Nikon, Plan Achromat, 2×, NA 0.06 [used for images with 20× magnification]; Nikon, Plan Achromat 4×, NA 0.1 [used for images with 40× magnification]; and Nikon, Plan Fluor 40×, NA 0.75 [used for images with 400× magnification]). Both staining methods were applied to sections from all of the tissues. Sections of lung and liver tissue from regions with a high likelihood of thrombus formation were immunostained to test for fibrinogen and von Willebrand factor (both antibodies and detection kit from Dako) as described elsewhere.9,10
Statistics
All statistical analyses were conducted using SAS (Version 9.1.3; SAS Institute Inc). For graphical purposes, GraphPad Prism (Version 5.01; GraphPad Software Inc) was used. Continuous variables were calculated as mean values ± SD.
Initially, the experiment was designed to compare 3 groups (control, PCC35, and PCC50). Because of the occurrence of disseminated intravascular coagulation (DIC), the PCC50 group was divided into 2 groups: those who developed DIC (PCC50DIC+) and those who did not (PCC50DIC−). Determination of whether DIC had occurred in each animal was based on clinical judgment, with due consideration of the International Society on Thrombosis and Hemostasis criteria.35 Differences in total blood loss among groups (control, PCC35, PCC50DIC+, and PCC50DIC−) were analyzed using analysis of variance, with Tukey post hoc adjustment. Pairwise log-rank tests were used for survival analysis. For coagulation parameters, blood cell count, and hemodynamic variables, a repeated-measures model was conducted with group and time as factors; the interaction between time and group was included. Post hoc tests for subgroup analysis were calculated; because of a statistically significant interaction term for each parameter analyzed, these results (except for those appearing in the main text) are provided in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Because of the exponential distribution of TAT and D-dimers, these data were log-transformed for the model. Statistical tests were performed 2-tailed, and P < .05 was considered a significant result.
Results
Baseline conditions
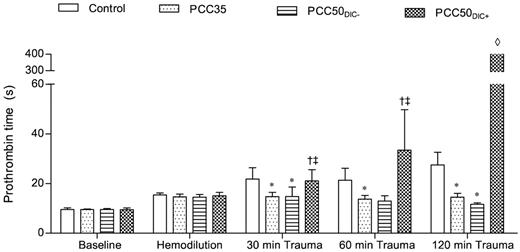
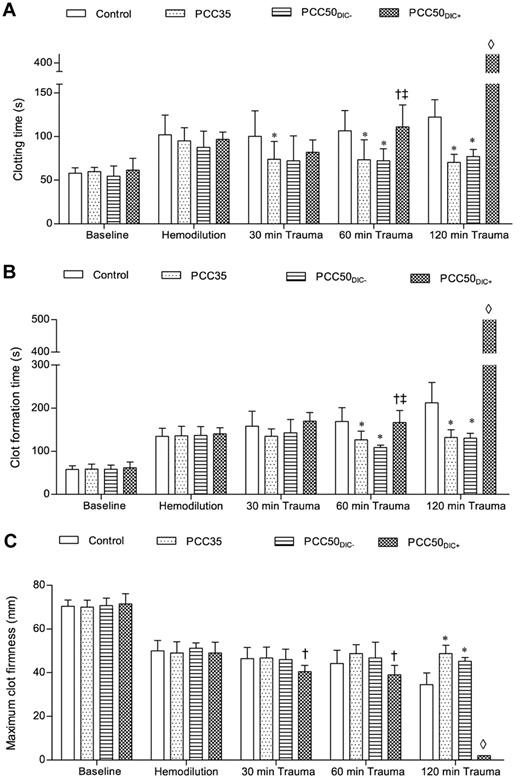
At baseline and after hemodilution, there were no clinically significant differences among the study groups (Tables 1 and 2). The hemodilution resulted in significant prolongation of PT and aPTT (Figure 1; Table 1). The concentrations of fibrinogen and antithrombin, as well as the platelet count, decreased significantly as expected (Table 1). All parameters of ROTEM assessment using the EXTEM assay indicated coagulopathy (Figure 2), although TAT complexes and D-dimers were not yet elevated (Figure 3). The hemodilution decreased maximum thrombin generation (peak height decreased from 95 ± 9 to 52 ± 4nM; P < .001), whereas overall thrombin generation (endogenous thrombin potential [ETP]) remained constant (521 ± 48nM · min before hemodilution vs 532 ± 44nM · min after hemodilution; Figure 4).
Laboratory parameters
| . | Baseline . | Hemodilution . | 30 min after trauma . | 60 min after trauma . | 120 min after trauma . |
|---|---|---|---|---|---|
| Hemoglobin, g/L | |||||
| Control | 8.0 ± 0.5 | 7.5 ± 0.3* | 4.6 ± 1.3 | 4.0 ± 0.9 | 2.5 ± 0.3 |
| PCC35 | 8.0 ± 0.4 | 7.4 ± 0.4* | 5.8 ± 0.7† | 5.9 ± 0.6† | 5.5 ± 0.8† |
| PCC50DIC− | 8.2 ± 0.4 | 7.5 ± 0.2* | 5.5 ± 0.7 | 5.5 ± 0.5† | 5.6 ± 0.6† |
| PCC50DIC+ | 8.3 ± 0.5 | 7.3 ± 0.4* | 3.6 ± 0.4‡§ | 3.5 ± 1.1‡§ | 2.8 ± 0.9‡§ |
| Platelet count, × 103/μL | |||||
| Control | 304 ± 32 | 110 ± 18* | 100 ± 25 | 87 ± 28 | 71 ± 21 |
| PCC35 | 280 ± 33 | 119 ± 10* | 118 ± 19 | 114 ± 11† | 106 ± 11† |
| PCC50DIC− | 290 ± 36 | 120 ± 8* | 99 ± 14 | 86 ± 29‡ | 97 ± 22† |
| PCC50DIC+ | 325 ± 44‡ | 115 ± 20* | 81 ± 18‡ | 75 ± 23‡ | 40 ± 8†‡§ |
| Fibrinogen, mg/dL | |||||
| Control | 322 ± 29 | 106 ± 15* | 61 ± 24 | 55 ± 18 | 38 ± 18 |
| PCC35 | 308 ± 32 | 110 ± 14* | 87 ± 13† | 87 ± 12† | 79 ± 10† |
| PCC50DIC− | 323 ± 25 | 120 ± 22* | 98 ± 17† | 88 ± 15† | 92 ± 12† |
| PCC50DIC+ | 343 ± 24 | 114 ± 19* | 43 ± 23‡§ | 32 ± 13‡§ | < 20, ND |
| aPTT, s | |||||
| Control | 12 ± 1 | 19 ± 2* | 23 ± 4 | 21 ± 3 | 25 ± 4 |
| PCC35 | 12 ± 1 | 19 ± 2* | 22 ± 2 | 21 ± 1 | 22 ± 2 |
| PCC50DIC− | 11 ± 1 | 18 ± 1* | 21 ± 2 | 21 ± 2 | 24 ± 2 |
| PCC50DIC+ | 11 ± 1 | 20 ± 2* | 26 ± 2‡§ | 43 ± 14†‡§ | > 300, ND |
| Antithrombin, % | |||||
| Control | 79 ± 6 | 36 ± 5* | 29 ± 9 | 33 ± 6 | 25 ± 8 |
| PCC35 | 88 ± 9† | 39 ± 7* | 38 ± 3† | 39 ± 4† | 41 ± 6† |
| PCC50DIC− | 79 ± 5 | 34 ± 3* | 32 ± 6 | 38 ± 5† | 40 ± 6† |
| PCC50DIC+ | 93 ± 16†§ | 41 ± 8* | 23 ± 7‡§ | 19 ± 4†‡§ | 21 ± 12‡§ |
| . | Baseline . | Hemodilution . | 30 min after trauma . | 60 min after trauma . | 120 min after trauma . |
|---|---|---|---|---|---|
| Hemoglobin, g/L | |||||
| Control | 8.0 ± 0.5 | 7.5 ± 0.3* | 4.6 ± 1.3 | 4.0 ± 0.9 | 2.5 ± 0.3 |
| PCC35 | 8.0 ± 0.4 | 7.4 ± 0.4* | 5.8 ± 0.7† | 5.9 ± 0.6† | 5.5 ± 0.8† |
| PCC50DIC− | 8.2 ± 0.4 | 7.5 ± 0.2* | 5.5 ± 0.7 | 5.5 ± 0.5† | 5.6 ± 0.6† |
| PCC50DIC+ | 8.3 ± 0.5 | 7.3 ± 0.4* | 3.6 ± 0.4‡§ | 3.5 ± 1.1‡§ | 2.8 ± 0.9‡§ |
| Platelet count, × 103/μL | |||||
| Control | 304 ± 32 | 110 ± 18* | 100 ± 25 | 87 ± 28 | 71 ± 21 |
| PCC35 | 280 ± 33 | 119 ± 10* | 118 ± 19 | 114 ± 11† | 106 ± 11† |
| PCC50DIC− | 290 ± 36 | 120 ± 8* | 99 ± 14 | 86 ± 29‡ | 97 ± 22† |
| PCC50DIC+ | 325 ± 44‡ | 115 ± 20* | 81 ± 18‡ | 75 ± 23‡ | 40 ± 8†‡§ |
| Fibrinogen, mg/dL | |||||
| Control | 322 ± 29 | 106 ± 15* | 61 ± 24 | 55 ± 18 | 38 ± 18 |
| PCC35 | 308 ± 32 | 110 ± 14* | 87 ± 13† | 87 ± 12† | 79 ± 10† |
| PCC50DIC− | 323 ± 25 | 120 ± 22* | 98 ± 17† | 88 ± 15† | 92 ± 12† |
| PCC50DIC+ | 343 ± 24 | 114 ± 19* | 43 ± 23‡§ | 32 ± 13‡§ | < 20, ND |
| aPTT, s | |||||
| Control | 12 ± 1 | 19 ± 2* | 23 ± 4 | 21 ± 3 | 25 ± 4 |
| PCC35 | 12 ± 1 | 19 ± 2* | 22 ± 2 | 21 ± 1 | 22 ± 2 |
| PCC50DIC− | 11 ± 1 | 18 ± 1* | 21 ± 2 | 21 ± 2 | 24 ± 2 |
| PCC50DIC+ | 11 ± 1 | 20 ± 2* | 26 ± 2‡§ | 43 ± 14†‡§ | > 300, ND |
| Antithrombin, % | |||||
| Control | 79 ± 6 | 36 ± 5* | 29 ± 9 | 33 ± 6 | 25 ± 8 |
| PCC35 | 88 ± 9† | 39 ± 7* | 38 ± 3† | 39 ± 4† | 41 ± 6† |
| PCC50DIC− | 79 ± 5 | 34 ± 3* | 32 ± 6 | 38 ± 5† | 40 ± 6† |
| PCC50DIC+ | 93 ± 16†§ | 41 ± 8* | 23 ± 7‡§ | 19 ± 4†‡§ | 21 ± 12‡§ |
Data are mean ± SD.
ND indicates not detectable.
P < .05 versus each group over time.
P < .05 versus control.
P < .05 versus PCC35.
P < .05 versus PCC50DIC−.
Hemodynamic variables
| . | Baseline . | Hemodilution . | 30 min after trauma . | 60 min after trauma . | 120 min after trauma . |
|---|---|---|---|---|---|
| HR, beats/min | |||||
| Control | 86 ± 8 | 80 ± 6* | 120 ± 12 | 140 ± 23 | 155 ± 26 |
| PCC35 | 82 ± 11 | 83 ± 8 | 94 ± 10† | 98 ± 10† | 93 ± 13† |
| PCC50DIC− | 91 ± 6 | 85 ± 5 | 99 ± 17† | 94 ± 19† | 108 ± 18† |
| PCC50DIC+ | 85 ± 9 | 76 ± 4*§ | 133 ± 22‡§ | 132 ± 24‡§ | 165 ± 21‡§ |
| MAP, mmHg | |||||
| Control | 96 ± 11 | 86 ± 7* | 44 ± 13 | 40 ± 11 | 30 ± 8 |
| PCC35 | 94 ± 10 | 87 ± 10* | 56 ± 18 | 66 ± 13† | 65 ± 12† |
| PCC50DIC− | 88 ± 9 | 84 ± 5 | 61 ± 9† | 69 ± 9† | 68 ± 5† |
| PCC50DIC+ | 87 ± 11 | 78 ± 4§ | 35 ± 10‡§ | 31 ± 14‡§ | 26 ± 13‡§ |
| CVP, mmHg | |||||
| Control | 7 ± 1 | 7 ± 1 | 4 ± 1 | 3 ± 1 | 2 ± 1 |
| PCC35 | 7 ± 2 | 8 ± 1 | 4 ± 1 | 4 ± 2 | 5 ± 1† |
| PCC50DIC− | 6 ± 2 | 8 ± 1 | 5 ± 1† | 5 ± 1† | 6 ± 1† |
| PCC50DIC+ | 6 ± 1 | 8 ± 1* | 4 ± 1 | 5 ± 1 | 5 ± 1† |
| MPAP, mmHg | |||||
| Control | 18 ± 2 | 18 ± 2 | 11 ± 3 | 10 ± 2 | 9 ± 2 |
| PCC35 | 17 ± 3 | 20 ± 3 | 12 ± 4 | 13 ± 3 | 13 ± 5† |
| PCC50DIC− | 17 ± 2 | 18 ± 2* | 15 ± 4 | 15 ± 1† | 16 ± 2† |
| PCC50DIC+ | 18 ± 5 | 17 ± 2 | 13 ± 4 | 11 ± 2§ | 11 ± 1§ |
| CO, L/min | |||||
| Control | 4.2 ± 0.5 | 4.3 ± 0.4 | 3.0 ± 1.2 | 2.4 ± 1.3 | 1.3 ± 0.5 |
| PCC35 | 4.3 ± 0.4 | 4.4 ± 0.5 | 3.5 ± 0.4 | 3.3 ± 0.4† | 3.3 ± 0.5† |
| PCC50DIC− | 3.9 ± 0.4 | 4.3 ± 0.6 | 3.7 ± 0.7 | 3.7 ± 0.6† | 3.2 ± 0.6† |
| PCC50DIC+ | 4.1 ± 0.5 | 3.9 ± 0.2 | 2.6 ± 0.9 | 2.4 ± 0.9 | 1.7 ± 0.2‡§ |
| . | Baseline . | Hemodilution . | 30 min after trauma . | 60 min after trauma . | 120 min after trauma . |
|---|---|---|---|---|---|
| HR, beats/min | |||||
| Control | 86 ± 8 | 80 ± 6* | 120 ± 12 | 140 ± 23 | 155 ± 26 |
| PCC35 | 82 ± 11 | 83 ± 8 | 94 ± 10† | 98 ± 10† | 93 ± 13† |
| PCC50DIC− | 91 ± 6 | 85 ± 5 | 99 ± 17† | 94 ± 19† | 108 ± 18† |
| PCC50DIC+ | 85 ± 9 | 76 ± 4*§ | 133 ± 22‡§ | 132 ± 24‡§ | 165 ± 21‡§ |
| MAP, mmHg | |||||
| Control | 96 ± 11 | 86 ± 7* | 44 ± 13 | 40 ± 11 | 30 ± 8 |
| PCC35 | 94 ± 10 | 87 ± 10* | 56 ± 18 | 66 ± 13† | 65 ± 12† |
| PCC50DIC− | 88 ± 9 | 84 ± 5 | 61 ± 9† | 69 ± 9† | 68 ± 5† |
| PCC50DIC+ | 87 ± 11 | 78 ± 4§ | 35 ± 10‡§ | 31 ± 14‡§ | 26 ± 13‡§ |
| CVP, mmHg | |||||
| Control | 7 ± 1 | 7 ± 1 | 4 ± 1 | 3 ± 1 | 2 ± 1 |
| PCC35 | 7 ± 2 | 8 ± 1 | 4 ± 1 | 4 ± 2 | 5 ± 1† |
| PCC50DIC− | 6 ± 2 | 8 ± 1 | 5 ± 1† | 5 ± 1† | 6 ± 1† |
| PCC50DIC+ | 6 ± 1 | 8 ± 1* | 4 ± 1 | 5 ± 1 | 5 ± 1† |
| MPAP, mmHg | |||||
| Control | 18 ± 2 | 18 ± 2 | 11 ± 3 | 10 ± 2 | 9 ± 2 |
| PCC35 | 17 ± 3 | 20 ± 3 | 12 ± 4 | 13 ± 3 | 13 ± 5† |
| PCC50DIC− | 17 ± 2 | 18 ± 2* | 15 ± 4 | 15 ± 1† | 16 ± 2† |
| PCC50DIC+ | 18 ± 5 | 17 ± 2 | 13 ± 4 | 11 ± 2§ | 11 ± 1§ |
| CO, L/min | |||||
| Control | 4.2 ± 0.5 | 4.3 ± 0.4 | 3.0 ± 1.2 | 2.4 ± 1.3 | 1.3 ± 0.5 |
| PCC35 | 4.3 ± 0.4 | 4.4 ± 0.5 | 3.5 ± 0.4 | 3.3 ± 0.4† | 3.3 ± 0.5† |
| PCC50DIC− | 3.9 ± 0.4 | 4.3 ± 0.6 | 3.7 ± 0.7 | 3.7 ± 0.6† | 3.2 ± 0.6† |
| PCC50DIC+ | 4.1 ± 0.5 | 3.9 ± 0.2 | 2.6 ± 0.9 | 2.4 ± 0.9 | 1.7 ± 0.2‡§ |
Data are mean ± SD.
CO indicates cardiac output; CVP, central venous pressure; HR, heart rate; MAP, mean arterial pressure; and MPAP, mean pulmonary pressure
P < .05 versus each group over time.
P < .05 versus control.
P < .05 versus PCC35.
P < .05 versus PCC50DIC−.
Prothrombin time. Prothrombin time was measured on a coagulometer to determine the effect of PCC therapy. The data are mean values. *P < .05 vs control. †P < .05 vs PCC35; ‡P < .05 vs PCC50DIC−. ◊, not detectable.
Prothrombin time. Prothrombin time was measured on a coagulometer to determine the effect of PCC therapy. The data are mean values. *P < .05 vs control. †P < .05 vs PCC35; ‡P < .05 vs PCC50DIC−. ◊, not detectable.
Thromboelastometry (ROTEM) parameters. The ROTEM coagulation analyzer was used to assess blood clotting. The EXTEM assay was used according to the manufacturer's instructions. Clotting was activated by the EXTEM reagent, which contains tissue factor as the starting reagent. (A) CT, representing the initiation of clot formation; this corresponds to the reaction time. (B) CFT, the time between clot initiation and attainment of an amplitude of 20 mm. (C) MCF, reflecting the strength of the resulting clot. *P < .05 vs control; †P < .05 vs PCC35; ‡P < .05 vs PCC50DIC−. ◊, not detectable.
Thromboelastometry (ROTEM) parameters. The ROTEM coagulation analyzer was used to assess blood clotting. The EXTEM assay was used according to the manufacturer's instructions. Clotting was activated by the EXTEM reagent, which contains tissue factor as the starting reagent. (A) CT, representing the initiation of clot formation; this corresponds to the reaction time. (B) CFT, the time between clot initiation and attainment of an amplitude of 20 mm. (C) MCF, reflecting the strength of the resulting clot. *P < .05 vs control; †P < .05 vs PCC35; ‡P < .05 vs PCC50DIC−. ◊, not detectable.
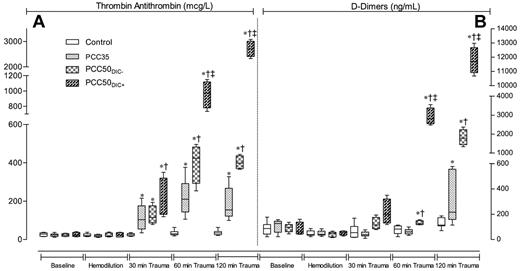
TAT complexes and D-dimers. Markers of coagulation activation at baseline (after splenectomy), after hemodilution, and at 30, 60, and 120 minutes after trauma. (A) TAT complexes. (B) D-dimers. Data are shown as box plots (minimum, first quartile, median, third quartile, and maximum). *P < .05 vs control; †P < .05 vs PCC35; ‡P < .05 vs PCC50DIC−.
TAT complexes and D-dimers. Markers of coagulation activation at baseline (after splenectomy), after hemodilution, and at 30, 60, and 120 minutes after trauma. (A) TAT complexes. (B) D-dimers. Data are shown as box plots (minimum, first quartile, median, third quartile, and maximum). *P < .05 vs control; †P < .05 vs PCC35; ‡P < .05 vs PCC50DIC−.
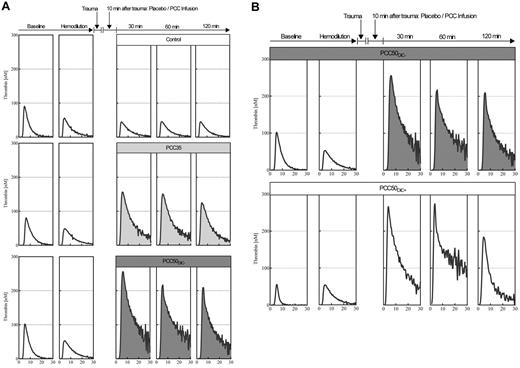
Representative curves from the thrombin generation assays. (A) Plasma thrombin generation, for assessment of hypercoagulability after PCC administration. Thrombin generation in platelet-poor plasma was assessed by means of the CAT according to standard in-house procedures, using 1pM tissue factor and 4μM phospholipids to trigger the assay. Representative curves are shown for baseline, hemodilution, and 30, 60, and 120 minutes after trauma, following the administration of saline (top row: open curves); 35 IU/kg PCC (middle row: light gray curves); and 50 IU/kg PCC (bottom row: dark gray curves). Independent of the dose of PCC, plasma thrombin generation was characterized by a prolonged time to tail, indicating attenuated inhibition of coagulation. (B) 50 IU/kg PCC can induce a hypercoagulable state associated with DIC. Top row: thrombin generation curves in plasma from an animal receiving 50 IU/kg PCC and not developing DIC. Bottom row: thrombin generation curves in plasma from an animal receiving 50 IU/kg PCC and developing DIC (PCC50DIC+). Remarkably, no difference was observed in thrombin generation before and after hemodilution.
Representative curves from the thrombin generation assays. (A) Plasma thrombin generation, for assessment of hypercoagulability after PCC administration. Thrombin generation in platelet-poor plasma was assessed by means of the CAT according to standard in-house procedures, using 1pM tissue factor and 4μM phospholipids to trigger the assay. Representative curves are shown for baseline, hemodilution, and 30, 60, and 120 minutes after trauma, following the administration of saline (top row: open curves); 35 IU/kg PCC (middle row: light gray curves); and 50 IU/kg PCC (bottom row: dark gray curves). Independent of the dose of PCC, plasma thrombin generation was characterized by a prolonged time to tail, indicating attenuated inhibition of coagulation. (B) 50 IU/kg PCC can induce a hypercoagulable state associated with DIC. Top row: thrombin generation curves in plasma from an animal receiving 50 IU/kg PCC and not developing DIC. Bottom row: thrombin generation curves in plasma from an animal receiving 50 IU/kg PCC and developing DIC (PCC50DIC+). Remarkably, no difference was observed in thrombin generation before and after hemodilution.
Measurements after standardized liver trauma
Control group.
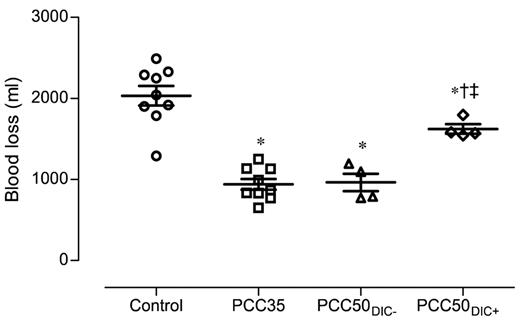
The control animals developed severe coagulopathy with persistent prolongation of PT, CT, and CFT (Figures 1–2). These parameters continued to deteriorate over time, resulting in significant prolongation compared with that in all other study groups. Platelet count, MCF, and the concentrations of fibrinogen and hemoglobin were also significantly lower in this group than in the PCC35 and PCC50DIC− groups (Table 1; Figure 2). Compared with the PCC35 and PCC50DIC− groups, controls had a lower mean arterial pressure and a higher heart rate (Table 2). Correspondingly, blood loss was highest among controls (2033 ± 363 mL), lowest in the PCC35 (941 ± 199 mL) and PCC50DIC− (964 ± 215 mL) groups, and intermediate in the PCC50DIC+ group (1624 ± 115 mL; Figure 5). Four animals in the control group (44%) died before the end of the observation period, a significantly higher percentage than that in the PCC35 group (P = .010).
Total blood loss, measured 120 minutes after liver injury. Horizontal lines depict the means. *P < .05 vs control; †P < .05 vs PCC35; ‡P < .05 vs PCC50DIC−.
Total blood loss, measured 120 minutes after liver injury. Horizontal lines depict the means. *P < .05 vs control; †P < .05 vs PCC35; ‡P < .05 vs PCC50DIC−.
Substitution with 35 IU/kg PCC.
PCC35 animals recovered from trauma after PCC administration, and this was reflected by significant decreases in PT, CT, and CFT (Table 1; Figure 2). Directly after PCC application (30 minutes after trauma), thrombin generation increased: ETP, 2726 ± 531nM · min; peak height; 168 ± 10nM (P < .001 vs control for both parameters; Figure 4). Although both parameters gradually declined during the observation period, thrombin generation remained higher than baseline at 120 minutes after trauma (ETP, 1525 ± 38nM · min; peak height, 128 ± 4nM). Levels of TAT complexes were significantly higher than those in controls at all time points after PCC administration (P < .001; Figure 5), whereas significantly higher levels of D-dimers were only observed 120 minutes after trauma (P < .001 vs control). All animals in the PCC35 group survived until the end of the experiment. No significant difference in survival (P = .145) was observed between the PCC35 and PCC50groups.
Substitution with 50 IU/kg PCC.
In animals not developing DIC (5 of 9 [56%] in the original PCC50 group), the coagulation parameters, blood loss and hemodynamic stability were comparable with those of animals in the PCC35 group (Tables 1–2; Figures 1–2 and 5). Animals developing DIC (n = 4, 44% of the original PCC50 group) exhibited significantly prolonged PT, CT, and CFT (Figures 1–2). This was accompanied by significant but temporary decreases in the concentrations of fibrinogen and clot strength (MCF; Table 1; Figure 2). Levels of platelets, hemoglobin, and antithrombin decreased over time in the PCC50DIC+ group, such that they were lower in this group than in all other groups at 120 minutes after trauma (Table 1).
Immediately after PCC infusion, TAT complexes and D-dimers had increased in the PCC50DIC+ group, and these levels continued to increase throughout the observation period. Conversely, in the PCC50DIC− group, there was no increase 60 minutes after trauma in levels of TAT complexes, although D-dimers started to increase 60 minutes after trauma (Figure 3). Overall trends in levels of TAT complexes and D-dimers were similar in the PCC50DIC− and PCC35 groups.
Thrombin generation increased immediately after PCC administration in all animals receiving the 50 IU/kg dose. At 30 minutes after trauma, ETP was 3455 ± 494nM · min, and peak height was 253 ± 4nM (pooled data from PCC50DIC+ and PCC50DIC− animals; P < .001 vs PCC35 for both parameters; Figure 4). Both of these parameters remained higher than baseline at 120 minutes after trauma (ETP, 2143 ± 982nM · min; peak height, 196 ± 15nM). Thrombin generation curves from animals in the PCC50DIC+ group showed a lack of reduction in thrombin generation after hemodilution. One animal in both in the PCC50DIC− and PCC50DIC+ groups died before the end of the observation period. No significant difference in survival was observed between the PCC50DIC+ and PCC50DIC− group compared with that of the control group (P = .937).
Histopathologic analysis
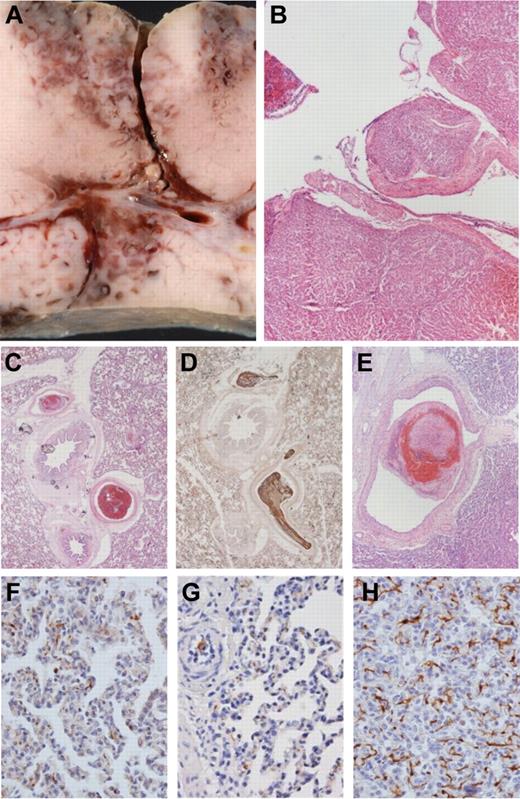
The histopathologic examination of injured liver sections revealed homogeneous tissue damage and comparable lacerations of venous vessels up to 4 mm in diameter (Figure 6A-B). No thromboemboli or other pathologic changes were present in kidneys or nontraumatized liver tissue. In the myocardium, there were no thromboemboli, but signs of premortal hypoxia (“waving”) were observed.
Macroscopic liver injury and microscopic histology of lung tissue: representative samples. Postmortem internal organs were removed to evaluate the degree of liver injury and to assess the occurrence of any thromboembolic events. (A-B) Macroscopic and histologic images of liver trauma, with laceration of venous vessels up to 4 mm in diameter. (B) H&E stain. (C-E) Multiple thromboemboli in the PCC50 group (up to 4 mm in panel E). (C,E) H&E stain. (D) Immunohistochemical staining against fibrinogen. (F-G) Samples from the control group and PCC50 without DIC with rare, irregular, small deposits of fibrinogen in lung capillaries. (H) Net-like staining pattern for fibrinogen in lung capillaries of animals of the PCC50 group wth DIC. (F-H) Immunohistochemical stain against fibrinogen. Original magnification: panels B and E, 20×; panels C-D, 40×; panels F-H, 400×.
Macroscopic liver injury and microscopic histology of lung tissue: representative samples. Postmortem internal organs were removed to evaluate the degree of liver injury and to assess the occurrence of any thromboembolic events. (A-B) Macroscopic and histologic images of liver trauma, with laceration of venous vessels up to 4 mm in diameter. (B) H&E stain. (C-E) Multiple thromboemboli in the PCC50 group (up to 4 mm in panel E). (C,E) H&E stain. (D) Immunohistochemical staining against fibrinogen. (F-G) Samples from the control group and PCC50 without DIC with rare, irregular, small deposits of fibrinogen in lung capillaries. (H) Net-like staining pattern for fibrinogen in lung capillaries of animals of the PCC50 group wth DIC. (F-H) Immunohistochemical stain against fibrinogen. Original magnification: panels B and E, 20×; panels C-D, 40×; panels F-H, 400×.
Two control animals had singular thromboemboli, 1-2 mm in diameter, inside the lung arterioles. In 3 animals from the PCC35 group, several thromboemboli 1-2 mm in diameter were found. All animals from the PCC50 group had disseminated thromboemboli in lung arterioles and arteries up to 4 mm in diameter (Figure 6C-E). Three of 4 animals from the PCC50DIC+ group also showed signs of a reticular (net-like) pattern of fibrinogen deposits within lung capillaries (Figure 6H). In contrast, fibrinogen deposits in lung capillaries of all other animals were small in both size and number (Figure 6F-G).
Discussion
This experimental animal study is the first to examine the effects of 2 doses of PCC on blood loss and coagulation parameters after trauma. PCC at a dose of 35 IU/kg was effective in reversing coagulopathy related to hemodilution and trauma, reducing blood loss compared with that in controls. At 50 IU/kg, PCC treatment was followed by DIC in approximately half of the animals. Histopathologic assessment showed thromboemboli in all animals receiving the higher dose, including those not developing DIC. This was accompanied by a net-like pattern of pulmonary fibrinogen deposits in 3 of 4 animals developing DIC.
Our finding that PCC can, at an appropriate dose, be effective in treating coagulopathy is in agreement with several animal studies of PCC in dilutional coagulopathy.5-7 However, we also found that 50 IU/kg of PCC had no greater impact on blood loss than 35 IU/kg. This could be related to the fact that all thrombin-generating drugs are dependent on an adequate level of the substrate fibrinogen to be effective. Accordingly, it has been shown that treatment with both recombinant factor VIIa and fibrinogen is more effective in correcting coagulopathy than treatment with either agent alone.10,36 Likewise, results from 2 different studies performed in a porcine model of trauma indicated considerably greater reduction in blood loss after coadministration of fibrinogen plus PCC,8 compared with administration of fibrinogen alone.37 Thus, combined treatment with fibrinogen and PCC has been advocated in trauma, with fibrinogen supplementation as the first priority on the basis that critically low levels of fibrinogen are encountered early in massive bleeding.8,23,38 It would certainly be of interest to repeat our study with this treatment regimen. Studies have shown that coagulopathy-related bleeding may be independently associated with low concentrations of either thrombin or fibrinogen.39 For targeted therapy with coagulation factor concentrates, it is necessary to identify the cause of coagulation deficiency. This approach allows therapy to be tailored to each patient's specific needs.23 In contrast, formula-driven general treatment with allogeneic blood products (eg, administration of red blood cells/FFP/platelets in a 1:1:1 ratio) may lead to unnecessary use of blood products.
In our experimental animal model, the EXTEM assay did not point to a hypercoagulable state after PCC infusion: CT was not shortened (relative to baseline) in any of the treatment groups. In addition, only small differences were observed between the 35 and 50 IU/kg doses of PCC in all of the EXTEM parameters (considering animals not developing DIC). Likewise, PT showed little difference between the PCC35 and PCC50DIC− groups, with little change after hemodilution in these animals. In contrast, the thrombin generation assay showed a clear, dose-dependent increase in thrombin generation after PCC administration. Several different factors may have contributed to these findings. First, the end point of the CT and PT parameters is formation of fibrin, meaning that they depend on an adequate level of fibrinogen. Second, the relationship between coagulation factor levels and CT/PT values is not linear: in hemodilution, the concentration of coagulation factors needs to drop to < 40% before prolongation of CT or PT is observed.40,41 On the other hand, there is a linear relationship between thrombin generation and levels of coagulation factors, making this assay more sensitive to changes in these proteins. Third, neither EXTEM CT nor PT is influenced by antithrombin, whereas thrombin generation is strongly influenced by antithrombin. Fourth, much higher levels of tissue factor are used in the PT and EXTEM assays than in the thrombin generation assay. Prolongation of EXTEM CT has already been used as a trigger for the administration of PCC in a goal-directed algorithm of trauma bleeding management.23,38 The question of whether EXTEM CT may be used to guide the dose of PCC has not yet been investigated, but the present data suggest that it might not be appropriate for this purpose.
The present study suggests that a clinical trial program (phases II and III) is needed to establish the safety and efficacy of PCC in patients with trauma-induced or dilutional coagulopathy and different underlying risk factors for thromboembolic events. In accordance with our expectations for clinical practice, such a program would ideally investigate PCC therapy in combination with fibrinogen supplementation (with PCC being administered as the second of these 2 interventions). Establishing the optimal PCC dose would, of course, be a key reason for pursuing the clinical trial program. The choice of 50 IU/kg as the highest dose in our study was in accordance with previous studies. A clinical study of individualized PCC dosing for the reversal of vitamin K antagonists included 50 IU/kg as the highest dose,33 whereas in another publication 50 IU/kg PCC was recommended for vitamin K antago-nist patients with an international normalized ratio ≥ 6.0.42 Furthermore, a pharmacokinetic study of PCC conducted in healthy volunteers used a dose of 50 IU/kg.32 The lower dose (35 IU/kg) was chosen on the basis that this dose was shown in previous porcine studies to be effective in the treatment of dilutional coagulopathy.5,8 The median PCC dose administered in a trial of combined administration of fibrinogen concentrate and PCC in trauma patients was approximately 22 IU/kg.23 Clinical doses of PCC for trauma-induced coagulopathy have not yet been established; therefore, the optimal doses to include in investigation of the safety or efficacy of PCC in this setting are poorly defined.
In the present study, protracted elevation of TAT and D-dimers accompanied by fibrin precipitation in lung capillaries in a subset of animals indicated an excessive activation of coagulation. The increase in TAT immediately after the application of PCC was expected. However, the continuous increase throughout the observation period is surprising and indicates ongoing activation of the coagulation system. In a study of 50 IU/kg PCC for reversal of rivaroxaban, thrombin generation (ETP) was measured for 24 hours after PCC administration.43 ETP increased within 15 minutes of PCC administration and continued to increase over the observation period to reach a level 50% higher than the pre-rivaroxaban baseline level. It remains unclear whether ETP would have returned to the pre-rivaroxaban level. The protracted activation of coagulation after PCC administration may be attributable to an imbalance of prohemostatic factors and inhibitors, specifically an insufficient level of antithrombin compared with the potential for thrombin generation. This hypothesis corresponds with in vitro and in vivo data that identified prothrombin overload (and insufficient inhibition of newly generated thrombin by antithrombin) as the most likely cause of thrombosis.18,44 It is also supported by our observations of prolonged tails as well as large amplitudes in the thrombin generation curves, indicating a lack of thrombin inhibition. In a biochemical comparison of 7 commercially available PCCs, none of the products contained antithrombin at a sufficient level to balance the thrombin-generating potential of prothrombin.11 Thus, the application of antithrombin might be considered before PCC infusion for trauma-induced coagulopathy to protect against excessive systemic thrombin levels.
The apparent potential for increased risk of thromboembolism and DIC when PCC is used to treat trauma-induced coagulopathy is of major clinical significance. The European Medicines Agency core summary of product characteristics for PCCs gives “treatment of bleeding and perioperative prophylaxis of bleeding in acquired deficiency of the prothrombin complex coagulation factors” as an indication.45 This provides authorization for the use of PCC in trauma-induced coagulopathy. Physicians may be assured by statements in the literature that PCCs are balanced products because of the presence of anticoagulant proteins.11,46,47 However, we believe that PCCs cannot be considered as balanced unless they include antithrombin at an quantity equivalent to that of prothrombin.
Several limitations of our study need to be acknowledged. Hemodilution had to be induced before the infliction of injury to standardize the degree of coagulopathy. Clinically, hemodilution results from blood loss (caused by, eg, major trauma). This is followed by the infusion of large volumes, and hemostatic agents are administered as coagulopathy occurs. In addition, in the present study, liver injury was induced in anesthetized healthy pigs. Physiologic responses to factors such as pain and inflammation may have additional effects on hemostasis, and these are not reflected in our model. It is possible that fibrinogen levels were overestimated in the present study, because hydroxyethyl starch had been administered.48-50 The method used to determine the incidence of DIC in this animal study was based on clinical judgment, with consideration of the International Society on Thrombosis and Hemostasis criteria, which were designed for humans.35 There are no established diagnostic criteria for porcine DIC, but the clinical signs of DIC were clear in this study. Coagulation profiles differ between species; therefore, the extent to which the present results can be extrapolated to humans needs to be confirmed.
In conclusion, coagulopathy associated with hemodilution and trauma was reversed in this study by early administration of exogenous PCC at a dose of 35 IU/kg. Animals receiving this dose showed lower blood loss and a higher survival rate. However, data from this study show that PCCs can also induce a prothrombotic state. Given the lack of clinical safety data for PCCs in this setting, the present data provide the best available insight. Clinical studies, particularly in patients with comorbidities, are needed to optimize dosing and ascertain the safety of PCCs for coagulopathy associated with hemodilution and trauma.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Thaddäus Stopinski (Institute of Laboratory Animal Science, RWTH Aachen University Hospital) for animal experiments and Professor Dietrich Henzler (Department of Anesthesia and Division of Critical Care, Dalhousie University Halifax) for his critical reading.
This research was supported by the START program of the Faculty of Medicine, RWTH Aachen, Biotest (Dreieich, Germany), and Sanquin (Amsterdam, The Netherlands).
Authorship
Contribution: O.G. conceived and conducted the experimental laboratory work and drafted the manuscript; T.B. performed the pathologic analyzes and drafted the manuscript; H.M.H.S., R.v.O., and H.t.C. performed the CAT assays and helped to write the manuscript; S.E. and A.D.R. helped to perform the study; C.F. performed the statistical analyses; R.T. helped to prepare the manuscript; and R.R. participated in the study design and helped to write the manuscript. All authors read and approved the final manuscript.
Conflict-of-interest disclosure: O.G. has received research funding from Novo Nordisk (Denmark) and R.R. has received honoraria for lectures and consultancy from CSL Behring (Germany), Novo Nordisk (Germany), Bayer Healthcare (Germany) and Air Liquide (France). The remaining authors declare no competing financial interests. The sponsors had no influence on the design of the study or the interpretation of results.
Correspondence: Oliver Grottke, Department of Anesthesiology, RWTH Aachen University Hospital, Pauwelsstrasse 30, 52074 Aachen, Germany; e-mail: ogrottke@ukaachen.de.