Abstract
Vascular endothelial growth factor (VEGF) acting through VEGF receptor 2 (VEGFR2) on endothelial cells (ECs) is a key regulator of angiogenesis, a process essential for wound healing and tumor metastasis. Rap1a and Rap1b, 2 highly homologous small G proteins, are both required for angiogenesis in vivo and for normal EC responses to VEGF. Here we sought to determine the mechanism through which Rap1 promotes VEGF-mediated angiogenesis. Using lineage-restricted Rap1-knockout mice we show that Rap1-deficiency in endothelium leads to defective angiogenesis in vivo, in a dose-dependent manner. Using ECs obtained from Rap1-deficient mice we demonstrate that Rap1b promotes VEGF-VEGFR2 kinase activation and regulates integrin activation. Importantly, the Rap1b-dependent VEGF-VEGFR2 activation is in part mediated via integrin αvβ3. Furthermore, in an in vivo model of zebrafish angiogenesis, we demonstrate that Rap1b is essential for the sprouting of intersomitic vessels, a process known to be dependent on VEGF signaling. Using 2 distinct pharmacologic VEGFR2 inhibitors we show that Rap1b and VEGFR2 act additively to control angiogenesis in vivo. We conclude that Rap1b promotes VEGF-mediated angiogenesis by promoting VEGFR2 activation in ECs via integrin αvβ3. These results provide a novel insight into the role of Rap1 in VEGF signaling in ECs.
Introduction
Angiogenesis, sprouting of new blood capillaries from existing vasculature, is a central process in wound healing and tumor metastasis.1 One of the key regulators of these angiogenic responses in endothelial cells (ECs) is vascular endothelial growth factor-A (VEGF), which exerts its effects on ECs behavior via vascular endothelial growth factor receptor 2 (VEGFR2). VEGFR2 is essential for both: angiogenesis, sprouting from existing vasculature, and vasculogenesis, de novo vessel formation from EC precursors, angioblasts.2,3 VEGF-binding to VEGFR2 induces receptor dimerization, autophosphorylation resulting in increased VEGFR2 tyrosine kinase activity and phosphorylation of additional tyrosine residues. This, in turn, leads to the recruitment of several signaling molecules via adaptor proteins, triggering signaling cascades including p42/44 ERK1/2, p38 MAPK, and Akt promoting EC proliferation, migration, and survival.4 Thus, VEGFR2 activation is a key step regulating EC responses. Perturbation of VEGFR2 signaling by VEGF inhibitors has been shown to block angiogenic responses, and is a target of antitumor therapy.5
Signaling by VEGFR2 is regulated by several receptors including integrins, which are extracellular matrix receptors involved in bi-directional signaling.6 Of several endothelial integrins, integrins α5β1 and αvβ3 play a particularly important role in angiogenesis.7,8 These integrins, expressed at low levels in quiescent cells, are up-regulated during normal and tumor angiogenesis,9 which makes them attractive therapeutic targets. At the molecular level, signaling crosstalk between integrins and VEGFR2 is necessary for full realization of the angiogenic program.10 Upon ligand binding, VEGFR2 and integrin αvβ3 form a functional complex both in vitro and in vivo,11,12 which promotes the activation of each receptor, and is essential for full angiogenic response to VEGF.13,14 To date, intracellular signaling molecules responsible for VEGFR2 and integrin dual activation in ECs are not known.
Here, we propose that small GTPase Rap1, an evolutionarily conserved member of Ras family, is a likely candidate for this dual activation step in VEGF signaling in ECs. Rap1 regulates several basic cellular processes including adhesion, polarity, differentiation and growth.15,16 In vertebrates 2 rap1 genes encode highly homologous Rap1a and Rap1b proteins. Recently we and others have shown that total deletion of either Rap1a or Rap1b in mice leads to inhibition of angiogenesis in vivo.17-19 However, lack of Rap1a appeared to affect responses predominantly to FGF-2, while Rap1b−/− mice had a defect in response to both VEGF and FGF-2. In particular, Rap1b−/− mice have a defect in retinal neovascularization and VEGF-induced Matrigel invasion in vivo and defective aortic ring sprouting ex vivo.18 In vitro, several VEGF-induced responses including migration and proliferation are inhibited in Rap1-deficient ECs.20 However, mechanistically it is unclear how Rap1 promotes VEGF responses in ECs. Here, using lineage-restricted knockout mice we examined whether Rap1-deficiency in endothelium is responsible for the angiogenic defect. Further, we investigated whether Rap1 promotes VEGF responses by modulating VEGFR2 activity in ECs, and whether this signaling pathway is responsible for in vivo angiogenesis in zebrafish. Our results show that indeed, both Rap1a and Rap1b are required in endothelium for normal angiogenesis. We find that both isoforms promote VEGFR2 kinase activation, and this in part is mediated via integrin αvβ3 in ECs. Furthermore, Rap1b and VEGFR2 cooperate additively in mediating angiogenic responses in vivo.
Methods
The detailed information regarding chemicals, pharmacologic inhibitors and antibodies used as well as experimental procedures, including primer sequences, is provided in the expanded supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Animal studies
All mouse and zebrafish studies were performed according to Medical College of Wisconsin animal protocol guidelines under protocols 00001206 and 312-06-2, respectively. Generation of rap1b−/− and rap1af/frap1bf/f mice (with a mixed 129SvEv/C57BL/6, 50%/50% average, background) was described previously.21,22 Rap1-ECKO mice were generated by breeding rap1af/frap1bf/f, with Tie2-Cre+/0rap1a+/+rap1b+/+mice (strain C57BL/6),23 as described in supplemental Table 1. Integrin β3−/− mice24 were purchased from The Jackson Laboratory. flk1:EGFP zebrafish were grown and maintained in embryo water at 28.5°C.25 Embryos were generated by natural pair-wise mating and staged according to established protocols.26
Analysis of retinal neonatal vascularization
Retinal angiogenesis was analyzed as previously described.18 Retinas were isolated from 7-day-old Rap1-ECKO mutant mice and control littermates; 6-11 animals were analyzed from each group. Retinas were stained with TRITC-conjugated Griffonia simplicifolia isolectin B4 and imaged using Nikon inverted fluorescent microscope with a 4× objective and CoolSNAP ES CCD camera (Photometrics). Total and vascularized area were measured using Metamorph software Version 6.0 (Molecular Devices) and proportion of vascular to total retina area was calculated. For statistical analysis, the outcome variable (vascularized proportion of retina) was log-transformed, resulting in a multiplicative interpretation in the original scale of the additive models on the transformed scale. The effect of genotype was estimated using a mixed effects model with random litter effect and fixed genotype effects. The overall type I error rate was controlled at 5% over the 3 preplanned comparisons using a single-step method based on the joint normal distribution of the comparisons.
Matrigel plug in vivo angiogenesis assay
Matrigel plug assay was performed using a modification of a previously described protocol.27 Briefly, 500 μL of growth factor reduced Matrigel (BD Biosciences) mixed with 0.1% BSA/PBS or with 250 ng/mL VEGF-A165 (R&D Systems)/PBS and 20 U/mL heparin (Sigma-Aldrich) was injected subcutaneously in the mid-dorsal abdominal region of mutant and age-matched control mice. After 4 days Matrigel plugs were recovered and fixed in 10% formalin. Bright field images of Matrigel plugs were obtained using Zeiss SteREO Lumar.V12 stereoscope and 0.8× objective at 6.4× magnification and AxioCam MRc5 camera (Carl Zeiss MicroImaging). Microvessels were imaged in 5 μm paraffin sections stained with H&E using Nikon E400 microscope (Nikon Instruments Inc) with a 20×/0.50 objective and Spot Insight 11.2.X Color Mosaic camera (Diagnostic Instruments) and analyzed using Metamorph Version 6.0 software.
Determination of plasma VEGF levels
Whole blood was collected via retroorbital eyebleed from three 2-month-old Rap1b-ECKO and age-matched control mice that were anesthetized with isoflurane. VEGF levels in plasma were measured using Bio-Plex Mouse VEGF detection assay as previously described.18
EC culture
Rap1b−/−, integrin β3−/− and wild-type (WT) ECs were purified from lungs of neonatal mice, as described and characterized previously.18,28 After purification, cells were plated on tissue culture flasks coated with 2% bovine skin gelatin and cultured in VascuLife medium containing EnGS-Mv LifeFactors supplement (Lifeline Cell Technology) for a maximum of 2 additional passages.
siRNA knockdown
ECs were transfected with 50nM of siGENOMEsiRNA pool of Rap1b, ITGB3 (integrin β3) and ITGB1 (integrin β1) or control siRNA (all from Dharmacon) using RNAiMAX transfection reagent (Invitrogen). Functional assays were performed 24 hours after transfection. Knockdown efficiency was assessed by Western blotting and normalized to actin content.
Analysis of VEGFR2 phosphorylation
Quiescent ECs grown to 70%-80% confluence or ECs plated for 2 hours in serum-free media on collagen type I (150 μg/mL)– or poly-L-lysine (100 μg/mL, molecular weight 150 000-300 000)–coated and 0.1% BSA/PBS-blocked plates (as described in supplemental Methods) were stimulated with 40 ng/mL human recombinant VEGF-A165 for 5 minutes at 37°C. Cell lysates were prepared in RIPA lysis buffer and VEGFR2 was immunoprecipitated using a mouse monoclonal anti-VEGFR2 IgG (A-3; Santa Cruz Biotechnology). P-VEGFR2 level was measured by Western blotting with a phosphotyrosine-specific IgG cocktail (4G10 Platinum; Millipore). Phosphorylation of tyrosines 1054 and 1059 within tyrosine kinase domain of VEGFR2 was measured with phospho-VEGFR2 (Tyr1054/1059)–specific rabbit polyclonal antibody (Invitrogen). P-VEGFR2 band intensity was normalized to its respective total VEGFR2 or actin (in the pre-IP lysate), as described in supplemental Methods. Fold induction of normalized P-VEGFR in mutant EC samples was calculated relative to normalized P-VEGFR2 in WT/control EC samples. Average values of fold-induction obtained from at least 5 experiments were plotted.
Extracellular matrix adhesion assays
WT, Rap1b−/− and integrin β3−/− ECs were labeled with calcein-AM, suspended in serum-free DMEM in the absence or presence of β3 function blocking IgG (2C9. G3; Santa Cruz Biotechnology) and allowed to adhere to vitronectin (5 μg/mL, Sigma-Aldrich or R&D Systems)– or fibronectin (2.5 or 5 μg/mL for integrin β3−/− ECs, Invitrogen)–coated and 0.1% BSA/PBS-blocked wells of a 96-well plate. Fluorescence at 515 nm, measured using a Wallac VICTOR fluorescent plate reader (PerkinElmer), is proportional to the number of cells that adhered to the plate. Shown are average values from 3 experiments performed in triplicate.
Rap1b morpholino knockdown in zebrafish
Control (con-MO), rap1b ATG-targeted (rap1b-MO1) and Rap1b Exon2-Intron 3 targeted (rap1b-MO2) morpholino were designed as previously described29 and purchased from Gene Tools. Morpholino injections were performed as described previously.30 Rap1b knockdown efficiency was confirmed by Western blotting with a monoclonal IgG (M90) and semiquantitative PCR. Zebrafish embryo images were obtained using Leica MZ16 FA microscope (Leica Microsystems Inc) and 1× objective (FITC) with QImaging RETIGA Exi camera and ImageproMDA software (Media Cybernetics) and with Zeiss Axio Observer. Z1 fluorescent miscroscope and 10× objective (FITC) with AxioCam MRc5 camera and Axiovision 4.8 acquisition software (Carl Zeiss MicroImaging).
Inhibition of VEGFR2 activity in vivo; effect of Rap1b knockdown
EGFP embryos were incubated in embryo water at 28.5°C until they reached 20 somite stage, at which time they were dechorionated by enzymatic digestion with 1 mg/mL Proteinase K for 5 minutes at room temperature and incubated with pharmacologic inhibitors of VEGFR2: compounds SU5416 (EMD Chemicals) or ZM 323881 (Tocris Bioscience) at concentrations established in a dose-response experiment or in 0.1% DMSO as a control, for 60 minutes at 28°C. At prim-6 stage (∼ 28 hours postfertilization [hpf]), the ISV patterning was scored in GFP positive embryos. For the genetic interaction experiment, 2 ng of rap1b-MO1 was injected at 1 cell stage followed by 60 minutes incubation with VEGFR2 inhibitor at 20 hpf.
Statistical analysis
All data are represented as mean ± SEM. Statistical analysis of retinal neonatal vascularization was performed as described in “Analysis of retinal neonatal vascularization.” Statistical analysis of all other data were performed using 2-tailed Student t test, χ2 analysis with GraphPad Prism 5.0 (GraphPad Software) and Microsoft Office Excel 2003 software package, as appropriate. After comparison test, differences were considered significant at a P ≤ .05 level.
Results
Rap1b in endothelium is required for normal angiogenesis
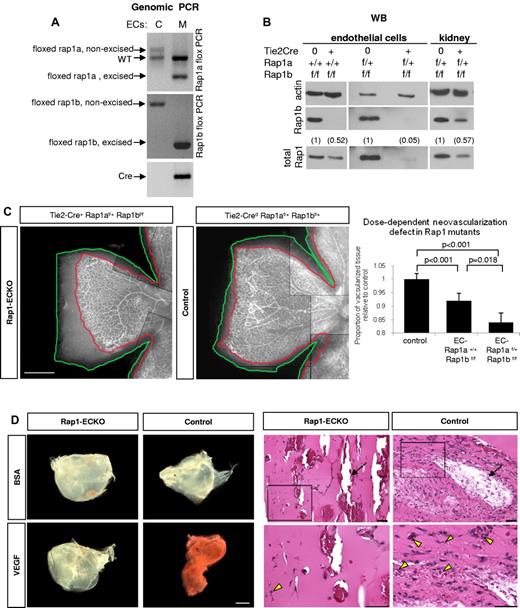
Total ablation of either rap1a or rap1b genes in mice leads to a defect in angiogenesis in vivo.17-19 To determine whether angiogenic defect was caused by defective endothelium, we created EC-lineage specific knockout mice (Rap1-ECKO) mice by crossing rap1af/frap1bf/f mice in which both rap1 genes are flanked with loxP cassettes,22 with Tie2 promoter-driven Cre recombinase expressing mice23 (supplemental Table 1). We confirmed Rap1 excision in ECs and vascularized tissues isolated from such generated Tie2-Cre+rap1a+/+rap1bf/f (henceforth Rap1b-ECKO) and Tie2-Cre+rap1af/+rap1bf/f (henceforth Rap1-ECKO) mice by Western blotting for Rap1b, total Rap1 and genomic PCR (Figure 1 and supplemental Figure 1). We found a complete deletion of Rap1b and ∼ 50% reduction in total Rap1 in endothelial cells from Rap1b-ECKO mice and a further reduction in total Rap1 in EC from Rap1-ECKO mice expressing only one allele of rap1a to ∼ 5% of that in littermate controls (Figure 1B). We also detected Rap1b excision in blood cells (supplemental Figure 1), consistently with previous reports of Tie2 promoter activity in the hematopoietic lineage.31
Angiogenic defect in Rap1-ECKO mice. (A-B) Rap1 expression in ECs and tissues from Rap1-ECKO mice and control littermates. (A) Genomic PCR shows excision of one rap1a and both rap1b floxed alleles in Tie2-Cre0rap1af/+rap1bf/f (Rap1-ECKO) mice. Only nonexcised floxed rap1a and rap1b alleles are present in Tie2-Cre0 controls. Inverted image of EtBr-stained gel is shown. (B) Western blots reveal lack of Rap1b and reduction of total Rap1 protein expression in ECs from Tie2-Cre+rap1a+/+rap1bf/f (Rap1b-ECKO) and a further reduction in Rap1-ECKO mice. (C) Dose-dependent reduction in retinal neoangiogenesis. P7 retinas stained with fluorescent isolectin from Rap1-ECKO and Tie2-Cre0 littermate controls were imaged using Nikon Eclipse TE200 inverted fluorescent microscope (Nikon Instruments Inc) and a 4×/0.13 objective and CoolSNAP ES CCD camera (Photometrics). Shown is a representative image of a quarter of each retina; scale bar indicates 500 μm. Lines have been inserted to indicate composite images. Graph represents mean ratio of vascularized (delineated in red) to total (delineated in green) retinal area in mutant mice relative to controls obtained from 6-11 mice. Vascularized area is significantly reduced in Rap1b-ECKO and deletion of additional Rap1a allele (Rap1-ECKO mice) significantly increases the defect. (D) Matrigel plug assay. Vascularization of VEGF-containing Matrigel plugs recovered from Rap1-ECKO mice after 4 days from Matrigel injection is decreased compared with Tie2-Cre0 controls, as visualized by decreased redness (bright field microscopy; left panel) and decreased microvessel density (H&E staining; right panel). There is no visible vascularization in BSA-containing Matrigel plugs. Bottom row of H&E stained sections are magnification of boxed areas; scale bars are 100 μm. Arrows indicate large vessels; arrowheads, RBCs-containing microvessels. Representative results (from n = 4) are shown.
Angiogenic defect in Rap1-ECKO mice. (A-B) Rap1 expression in ECs and tissues from Rap1-ECKO mice and control littermates. (A) Genomic PCR shows excision of one rap1a and both rap1b floxed alleles in Tie2-Cre0rap1af/+rap1bf/f (Rap1-ECKO) mice. Only nonexcised floxed rap1a and rap1b alleles are present in Tie2-Cre0 controls. Inverted image of EtBr-stained gel is shown. (B) Western blots reveal lack of Rap1b and reduction of total Rap1 protein expression in ECs from Tie2-Cre+rap1a+/+rap1bf/f (Rap1b-ECKO) and a further reduction in Rap1-ECKO mice. (C) Dose-dependent reduction in retinal neoangiogenesis. P7 retinas stained with fluorescent isolectin from Rap1-ECKO and Tie2-Cre0 littermate controls were imaged using Nikon Eclipse TE200 inverted fluorescent microscope (Nikon Instruments Inc) and a 4×/0.13 objective and CoolSNAP ES CCD camera (Photometrics). Shown is a representative image of a quarter of each retina; scale bar indicates 500 μm. Lines have been inserted to indicate composite images. Graph represents mean ratio of vascularized (delineated in red) to total (delineated in green) retinal area in mutant mice relative to controls obtained from 6-11 mice. Vascularized area is significantly reduced in Rap1b-ECKO and deletion of additional Rap1a allele (Rap1-ECKO mice) significantly increases the defect. (D) Matrigel plug assay. Vascularization of VEGF-containing Matrigel plugs recovered from Rap1-ECKO mice after 4 days from Matrigel injection is decreased compared with Tie2-Cre0 controls, as visualized by decreased redness (bright field microscopy; left panel) and decreased microvessel density (H&E staining; right panel). There is no visible vascularization in BSA-containing Matrigel plugs. Bottom row of H&E stained sections are magnification of boxed areas; scale bars are 100 μm. Arrows indicate large vessels; arrowheads, RBCs-containing microvessels. Representative results (from n = 4) are shown.
As a quantitative measure of angiogenesis, we analyzed retinal neonatal vascularization in isolectin-stained retinas from P7 animals.32 We found a significant reduction in the vascularized retina area in Rap1b-ECKO and a further reduction in Rap1-ECKO mice (Figure 1C), indicating that both Rap1 isoforms are required in EC for angiogenesis in vivo, with a dose-dependent effect. Similar analysis performed at P14 revealed full retinal vascularization of all mice (supplemental Figure 2), indicating that decreased angiogenesis at P7 results from a delay rather than complete inhibition of angiogenesis in Rap1-ECKO mice. To further analyze angiogenic defect we examined vessel branching and number of tip cells in P7 retinas and found that both were decreased in both mutants compared with littermate Tie2-Cre controls (supplemental Figure 3), consistently with decreased VEGF signaling.33 To determine whether decreased angiogenesis in Rap1-ECKO was because of decreased VEGF level, we determined plasma VEGF level in mutant and control animals. Similarly to total Rap1b−/− mice,18 we found an increase rather than a decrease in plasma VEGF level in Rap1b-ECKO mice, although the difference was not statistically significant (137 ± 32 vs 102 ± 9 pg/mL in control littermates; n = 3). Therefore, angiogenesis defect in Rap1b-ECKO animals is not caused by a decrease in VEGF level.
To further investigate the effect of Rap1-ECKO on VEGF-mediated angiogenesis in vivo, we performed Matrigel invasion assay.27 Matrigel plugs were recovered after 4 days from implantation to prevent vessel saturation and vascularization was analyzed. We found decreased number of large vessels, visualized by perfusion of mice with high-molecular-weight FITC-conjugated dextran before plug retrieval (supplemental Figure 4) and decreased density of microvessels, detected on H&E-stained thick sections of plugs (Figure 1D) in VEGF-containing Matrigel plugs recovered from Rap1-ECKO mice compared with that in control mice. BSA did not induce vascularization of plugs recovered from either group. These data further demonstrate defective VEGF responses in Rap1-ECKO.
Rap1 isoforms promote VEGF-induced VEGFR2 kinase activation in ECs
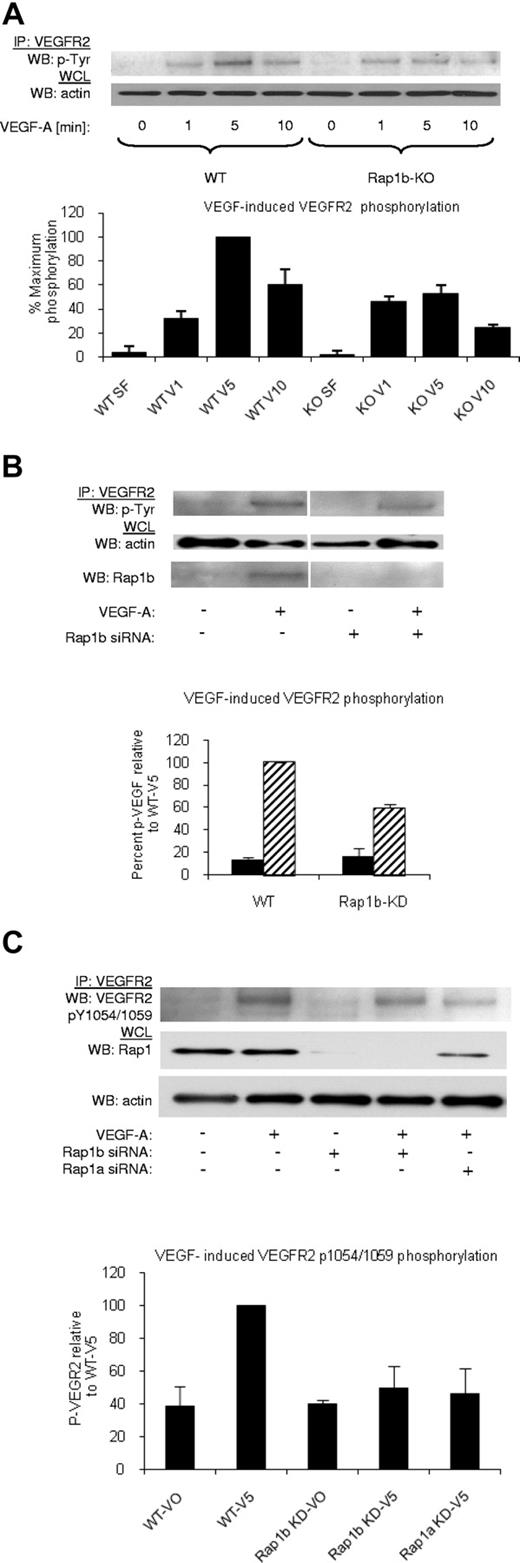
Rap1-deficiency attenuates VEGF responses in isolated ECs in vitro.20 To determine the mechanism through which this occurs, we systematically checked each step of VEGF signaling. First, we investigated the ability of VEGF to induce VEGFR2 phosphorylation in WT and Rap1b−/− ECs, as Rap1b is the predominant Rap1 isoform expressed in these cells.18 We found that maximal VEGFR2 phosphorylation was inhibited in Rap1b−/− ECs by 45% compared with WT ECs (Figure 2A), while VEGFR2 surface expression was unchanged (supplemental Figure 5A). This effect was a direct consequence of Rap1b deletion, as VEGF-induced VEGFR2 phosphorylation in WT ECs on silencing Rap1b expression with siRNA was reduced to the level observed in Rap1b−/− ECs (Figure 2B). In contrast, overexpression of constitutively active G12V Rap1b mutant in ECs led to enhanced VEGFR2 phosphorylation (supplemental Figure 6). Next, we investigated whether the decreased phosphorylation of VEGFR2 is a result of decreased VEGFR2 activation. To examine VEGFR2 activation, we investigated VEGF-induced phosphorylation of tyrosine residues 1054 and 1059 (Tyr 1054/1059) in VEGFR2 kinase domain. We found a significant decrease in Tyr 1054/1059 phosphorylation on knockdown of Rap1b in WT ECs (Figure 2C). Furthermore, Tyr 1054/1059 phosphorylation was also reduced when Rap1a expression was inhibited by Rap1a siRNA knockdown (Figure 2C), indicating that both Rap1 isoforms are required for full VEGFR2 activation in ECs. Because phosphorylation of these residues positively correlates with VEGFR2 kinase activity, we expect that attenuation of Tyr 1054/1059 phosphorylation will lead to attenuation of phosphorylation of other residues within VEGFR2 cytoplasmic domain, and consequently all VEGF-mediated VEGFR2 responses.
Decreased VEGF–induced VEGFR2 phosphorylation in Rap1-deficient ECs. (A) A time-course of VEGF-induced VEGFR2 phosphorylation in WT and Rap1b−/− ECs. Maximal VEGF-induced VEGFR2 phosphorylation occurring at 5 minutes in WT ECs is inhibited in Rap1b−/− ECs. (B-C) Analysis of VEGF- induced VEGFR2 total tyrosine phosphorylation (B) and VEGFR2 pY1054/1059 phosphorylation (C) in WT ECs and in WT ECs upon Rap1b or Rap1a siRNA knockdown, as indicated. Rap1 deficiency in WT ECs leads to reduced VEGFR2 pY1054/1059 phosphorylation (C) and total VEGFR2 tyrosine phosphorylation (B), similar to that observed in Rap1b−/− ECs in panel A. Efficiency of siRNA silencing was tested by Western blot for Rap1b (B) or total Rap1 (C). To normalize for protein content, a sample of lysates before IP was probed for actin (A-C bottom blots). Western blots shown are of typical experiments. Graphs represent mean fold induction in Rap1-deficient ECs normalized to WT EC values (“WT-V5”; n = 4 independent experiments; error bars are SEM).
Decreased VEGF–induced VEGFR2 phosphorylation in Rap1-deficient ECs. (A) A time-course of VEGF-induced VEGFR2 phosphorylation in WT and Rap1b−/− ECs. Maximal VEGF-induced VEGFR2 phosphorylation occurring at 5 minutes in WT ECs is inhibited in Rap1b−/− ECs. (B-C) Analysis of VEGF- induced VEGFR2 total tyrosine phosphorylation (B) and VEGFR2 pY1054/1059 phosphorylation (C) in WT ECs and in WT ECs upon Rap1b or Rap1a siRNA knockdown, as indicated. Rap1 deficiency in WT ECs leads to reduced VEGFR2 pY1054/1059 phosphorylation (C) and total VEGFR2 tyrosine phosphorylation (B), similar to that observed in Rap1b−/− ECs in panel A. Efficiency of siRNA silencing was tested by Western blot for Rap1b (B) or total Rap1 (C). To normalize for protein content, a sample of lysates before IP was probed for actin (A-C bottom blots). Western blots shown are of typical experiments. Graphs represent mean fold induction in Rap1-deficient ECs normalized to WT EC values (“WT-V5”; n = 4 independent experiments; error bars are SEM).
Taken together, these results suggest that Rap isoforms 1a and 1b are required for VEGF-induced VEGFR2 activation, and loss of Rap1 activity is expected to lead to attenuation of all VEGFR2-mediated EC responses, consistently with previously described attenuation of p38 MAPK, ERK, and Akt activation.18,19
Rap1b regulates integrin activity in ECs
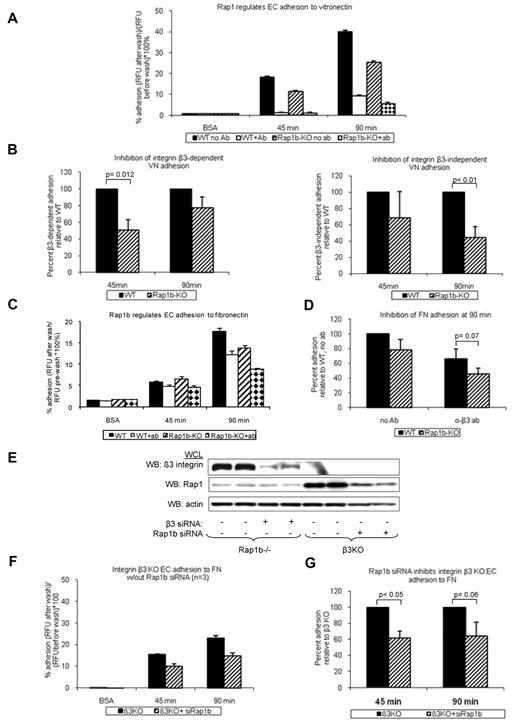
Activation and signaling downstream from VEGFR2 are regulated by several integrins.34 Integrins αvβ3 and α5β1 are both required for normal angiogenesis.7,8 Because Rap1b-deficiency leads to defective activation of platelet-specific β3 integrin, αIIbβ3,21 we investigated the effect of Rap1b-deficiency on integrin αvβ3 activation. Integrins αvβ3 and αvβ5 are major vitronectin receptors on ECs. As a measure of integrin αvβ3 activation, we examined the ability of WT and Rap1b−/− EC to adhere to vitronectin in the presence of integrin β3–function-blocking antibody. At 45 minutes, WT EC adhesion was completely inhibited in the presence of the antibody (Figure 3A open bars), indicating that αvβ3 is the major receptor mediating initial attachment. After 90 minutes, EC adhesion to vitronectin in the presence of the antibody was decreased by 76% (Figure 3A open bar), suggesting that other receptors, such as integrin αvβ5, may be involved during sustained adhesion. At both time points, respectively, integrin β3–dependent and integrin β3 –independent adhesion to vitronectin were decreased in Rap1b−/− ECs (Figure 3B), indicating that Rap1b regulates activity of integrins αvβ3 and αvβ5 in ECs. As surface and total expression analysis revealed increased integrin β3 expression in Rap1b−/− ECs (supplemental Figure 5B), we conclude that decreased adhesion results probably from decreased integrin β3 activation. Consistently with decreased integrin activation, the extent of EC spreading on vitronectin was inhibited in Rap1b−/− ECs (supplemental Figure 7A), indicating decreased integrin downstream signaling.
Inhibition of integrin activation in Rap1b-deficient ECs. Adhesion of calcein-AM-labeled WT and Rap1b−/− ECs to vitronectin- (A-B) or fibronectin- (C-D) coated 96-well plates in the absence or presence of β3-blocking antibody was measured using fluorescent plate reader. (E-G) Adhesion of integrin β3−/− ECs to fibronectin in the absence or presence of Rap1b siRNA. Efficiency of Rap1b knockdown and integrin β3 knockout was confirmed by Western blot with P3-specific and Rap1-specific antibodies (E). Panels B, D, and G are normalization of data in panels A, C, and F, respectively. Graphs represent mean value of mutant EC adhesion value relative to that of control ECs obtained in 4 independent experiments performed in triplicate. Error bars represent SEM. P values were obtained using paired t test.
Inhibition of integrin activation in Rap1b-deficient ECs. Adhesion of calcein-AM-labeled WT and Rap1b−/− ECs to vitronectin- (A-B) or fibronectin- (C-D) coated 96-well plates in the absence or presence of β3-blocking antibody was measured using fluorescent plate reader. (E-G) Adhesion of integrin β3−/− ECs to fibronectin in the absence or presence of Rap1b siRNA. Efficiency of Rap1b knockdown and integrin β3 knockout was confirmed by Western blot with P3-specific and Rap1-specific antibodies (E). Panels B, D, and G are normalization of data in panels A, C, and F, respectively. Graphs represent mean value of mutant EC adhesion value relative to that of control ECs obtained in 4 independent experiments performed in triplicate. Error bars represent SEM. P values were obtained using paired t test.
Because integrin α5β1 in ECs is also known to be involved in angiogenesis,35 we investigated whether Rap1b regulates activation of integrin α5β1 in ECs. We examined WT and Rap1b−/− EC adhesion to fibronectin in the presence of integrin β3 function blocking antibody. Under these conditions α5β1 is the major receptor, with αvβ1 also potentially involved.8,36 Adhesion of Rap1b−/− ECs to fibronectin (Figure 3C checkered bars) was inhibited compared with WT ECs (Figures 3C open bars and D). Similarly, Rap1b siRNA knockdown in integrin β3−/− ECs (Figure 3E), in which integrin α5β1 is the major fibronectin receptor, led to inhibition of adhesion to fibronectin (Figure 3F-G). Collectively, Rap1b-deficiency led to 25%-55% inhibition of integrin αvβ3, αvβ5 and integrins β1 (predominantly α5β1 but also potentially integrin αvβ1) in ECs, suggesting that Rap1 regulates the activity of several integrins in ECs. Consistently with decreased integrin β1 activation, Rap1b−/− EC spreading on collagen I was also inhibited (supplemental Figure 7B). Collectively, these data show that Rap1b is required for initial activation and signaling by several integrins in ECs.
Rap1b promotes VEGFR2 activation specifically via integrin αvβ3
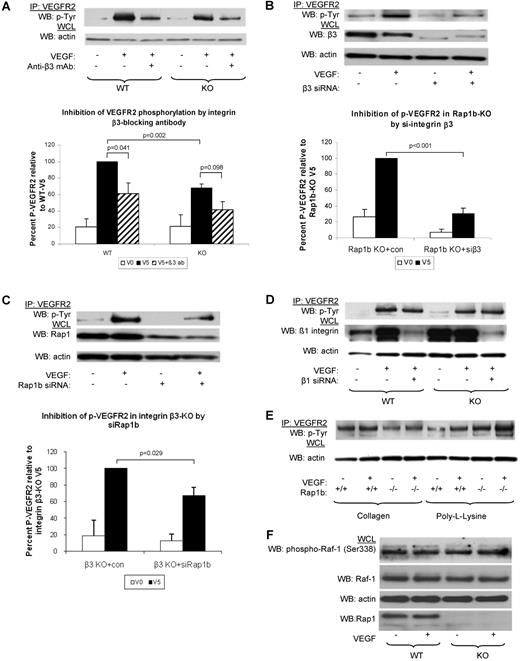
Next, we investigated whether integrins (β3 and β1) participate in Rap1b-VEGF signaling cascade. To this end, we examined the effect of integrin β3 function blocking antibody on VEGF-induced VEGFR2 phosphorylation. We found that VEGFR2 phosphorylation was reduced in WT ECs (Figure 4A). Thus, consistently with previous findings14 we conclude that functional integrin αvβ3 is required for full VEGFR2 activation in WT ECs. Inhibition of integrin β3 also led to a further decrease in VEGF-induced VEGFR2 phosphorylation in Rap1b−/− ECs, although the difference was not statistically significant (Figure 4A). However, silencing integrin β3 expression with siRNA led to an inhibition of VEGFR2 phosphorylation in WT ECs (data not shown) and a significant reduction of VEGFR2 phosphorylation in Rap1b−/− ECs (Figure 4B). Thus, lack of Rap1b in EC leads to a partial loss of integrin β3 activity (Figure 3B) and decreased signaling to VEGFR2.
Inhibition of signaling from integrin β3 to VEGFR2 in Rap1b-deficient ECs. (A-C). Analysis of VEGF-induced VEGFR2 phosphorylation in WT and Rap1b−/− ECs on inhibition of integrin β3 with function blocking antibody (A) or by siRNA silencing (B); and in integrin β3–knockout ECs on Rap1b siRNA knockdown (C). VEGFR2 immunoprecipitates were blotted with a phosphotyrosine-specific antibody (top blots). To normalize for protein content, a sample of lysates pre-IP was probed for actin (bottom blots). (D) Silencing integrin β1 in WT or Rap1b−/− ECs does not inhibit VEGF-induced VEGFR2 phosphorylation. Efficiency of siRNA silencing was tested by Western blot for integrin β3, Rap1b and β1 (B-D, respectively). (E) VEGF does not induce VEGFR2 phosphorylation in WT or Rap1b−/− ECs plated on collagen or poly-L-lysine. Blots shown are of typical experiments and graphs represent quantification of normalized percent VEGFR2 phosphorylation (shaded bars) relative to WT (A) or control siRNA-transfected (B-C) controls (filled bars). Values shown are mean of 4 independent experiments, error bars represent SEM. (F) C-Raf-1 phosphorylation is not changed in Rap1b−/− ECs. Serine 338 phosphorylation of C-Raf-1 was assessed in quiescent or VEGF-stimulated (5 minutes, 40 ng/mL) WT and Rap1b−/− ECs. Total C-Raf-1 and actin blots of the same gel were performed to ensure equal protein loading.
Inhibition of signaling from integrin β3 to VEGFR2 in Rap1b-deficient ECs. (A-C). Analysis of VEGF-induced VEGFR2 phosphorylation in WT and Rap1b−/− ECs on inhibition of integrin β3 with function blocking antibody (A) or by siRNA silencing (B); and in integrin β3–knockout ECs on Rap1b siRNA knockdown (C). VEGFR2 immunoprecipitates were blotted with a phosphotyrosine-specific antibody (top blots). To normalize for protein content, a sample of lysates pre-IP was probed for actin (bottom blots). (D) Silencing integrin β1 in WT or Rap1b−/− ECs does not inhibit VEGF-induced VEGFR2 phosphorylation. Efficiency of siRNA silencing was tested by Western blot for integrin β3, Rap1b and β1 (B-D, respectively). (E) VEGF does not induce VEGFR2 phosphorylation in WT or Rap1b−/− ECs plated on collagen or poly-L-lysine. Blots shown are of typical experiments and graphs represent quantification of normalized percent VEGFR2 phosphorylation (shaded bars) relative to WT (A) or control siRNA-transfected (B-C) controls (filled bars). Values shown are mean of 4 independent experiments, error bars represent SEM. (F) C-Raf-1 phosphorylation is not changed in Rap1b−/− ECs. Serine 338 phosphorylation of C-Raf-1 was assessed in quiescent or VEGF-stimulated (5 minutes, 40 ng/mL) WT and Rap1b−/− ECs. Total C-Raf-1 and actin blots of the same gel were performed to ensure equal protein loading.
To investigate whether Rap1b promotes VEGFR2 activation exclusively by regulating integrin β3 activity, we analyzed the effect of silencing Rap1b expression on VEGF-induced VEGFR2 phosphorylation in integrin β3−/− ECs. We found that VEGFR2 phosphorylation was decreased in these cells, compared with that in integrin β3−/− EC transfected with control siRNA (Figure 4C). This result would be expected either if Rap1b was acting independently of integrin β3 or in a pathway downstream from the integrin. However, as we did not detect Rap1 activation on integrin engagement (supplemental Figure 8), we conclude that Rap1b mediates VEGFR2 activation also via an integrin αvβ3–independent signaling pathway.
Integrin α5β1 is the major β1 integrin in ECs.8 To examine integrin α5β1 involvement in VEGFR2 activation, we analyzed VEGF-induced VEGFR2 phosphorylation in WT ECs upon down-regulation of integrin β1 expression. Integrin β1 siRNA knockdown did not lead to reduced VEGFR2 phosphorylation either in Rap1b−/− ECs or in WT ECs (Figure 4D and Mahabeleshwar et al37 ). Therefore, we conclude that integrin α5β1 is not involved in mediating Rap1b regulatory effect on VEGFR2.
To address the possibility that other integrins which are not fibronectin receptors are involved in the cross-talk to VEGFR2, we analyzed VEGF-induced VEGFR2 phosphorylation in ECs plated on collagen and found no induction of VEGFR2 phosphorylation (Figure 4E), consistent with previous reports.14 Like in WT ECs, we did not observe an induction of VEGFR2 phosphorylation in Rap1b−/− ECs plated on collagen, or when ECs were plated on poly-L-lysine, which does not engage integrins (Figure 4E). Taking all the biochemical data together, we conclude that Rap1b regulates activity of several integrins in ECs, but of these, only αvβ3 is specifically required for full VEGFR2 activation. Our data also suggest that Rap1 effectors other than integrins may be involved in promoting VEGFR2 activation. Serine/threonine kinase Raf-1 can act as Rap1 effector.38 However, Raf-1 activation downstream from VEGFR2 is unaffected in Rap1b−/− ECs (Figure 4F) and thus other Rap1 effectors must be involved in promoting VEGFR2 activation.
Rap1b is required for zebrafish angiogenesis in vivo
To investigate if the Rap1-VEGF signaling mechanism is responsible for EC signaling in vivo, we used zebrafish model of vessel formation, as signaling pathways governing vessel development are preserved between vertebrate species. Rap1b is highly conserved between human, mouse and zebrafish with 96% amino acid sequence identity, suggesting that Rap1b function is conserved across species. In the developing zebrafish embryo Rap1b is expressed in the vascular bed, including primary intersomitic vessels (ISVs),29 which form as sprouts from dorsal aorta between 20 and 28 hpf in a process dependent on VEGF and VEGFR2 (flk-1).39 We investigated Rap1b involvement in ISV formation by transiently silencing Rap1b expression in flk1: EGFP zebrafish embryos with Rap1b-specific morpholino29 injection at 1- to 2-cell stage (Figure 5A). Rap1b morphants did not show any obvious defects in the dorsal aorta (DA) or posterior cardinal vein (PCV) formation (data not shown) and did not have increased number of gastrulation defects compared with embryos injected with control MO (Figure 5C), suggesting that Rap1b is not required for early steps of vasculogenesis. However, injection of Rap1b-specific, but not control morpholinos led to a significant inhibition of ISV sprouting at 28 hpf (Figure 5A-B). While ISV patterning in Rap1b morphants remained defective until 28 hpf, no ISV growth or patterning defects were observed at 48 hpf, suggesting Rap1b plays a critical role in the initial events of ISV sprouting. This suggests that Rap1b is essential for angiogenesis in zebrafish.
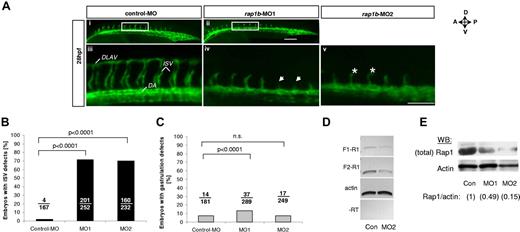
Knockdown of rap1b leads to angiogenesis defects in zebrafish in vivo. (A) Low- (i-ii) and high-power (iii-v) images of the trunk region of 28 hpf flk1:EGFP zebrafish embryos injected at 1- to 2-cell stage with 6 ng of MO, as indicated. Boxed areas (i-ii) indicate the region where intersomitic vessel (ISV) evaluation was performed. Missing ISVs (iv arrows) and “hammerhead” ISVs (v asterisks) are visible in embryos injected with rap1b-MOs but not with control-MOs. All images shown are lateral views with anterior side (A) oriented left and dorsal side (D) oriented up. DLAV indicates dorsolateral anastomotic vessel; DA, dorsal aorta. Scale bars 75 μm. (B-C) Quantitation of ISV (B) and gastrulation (C) defects in MO-injected embryos is expressed as number of affected embryos (numerator) in each group (denominator). No defects in ISV formation were observed in control MO injected embryos, whereas embryos injected with rap1b-MO1 (MO1) and rap1b-MO2 (MO2) displayed inhibition in ISV sprouting. (D-E) Analysis of Rap1b deletion efficacy (D) RT-PCR amplification of F1-R1 fragment (rap1b exon 1 to exon 4; 307bp) and F2-R1 fragment (exon 2 to exon 4; 212bp) of rap1b cDNA is substantially reduced in 6ng rap1b-MO2 injected zebrafish embryos compared with control. Ten to 20 embryos per group were injected at 1-2 cell stage. Total RNA was isolated at 28 hpf. (E) Rap1 expression in rap1b-MO (MO1, MO2) or control-MO (Con) injected embryos at 28 hpf was detected using M90 monoclonal IgG. Relative amount of Rap1 to actin is indicated in parentheses for each group; blot is representative of n = 3 independent experiments.
Knockdown of rap1b leads to angiogenesis defects in zebrafish in vivo. (A) Low- (i-ii) and high-power (iii-v) images of the trunk region of 28 hpf flk1:EGFP zebrafish embryos injected at 1- to 2-cell stage with 6 ng of MO, as indicated. Boxed areas (i-ii) indicate the region where intersomitic vessel (ISV) evaluation was performed. Missing ISVs (iv arrows) and “hammerhead” ISVs (v asterisks) are visible in embryos injected with rap1b-MOs but not with control-MOs. All images shown are lateral views with anterior side (A) oriented left and dorsal side (D) oriented up. DLAV indicates dorsolateral anastomotic vessel; DA, dorsal aorta. Scale bars 75 μm. (B-C) Quantitation of ISV (B) and gastrulation (C) defects in MO-injected embryos is expressed as number of affected embryos (numerator) in each group (denominator). No defects in ISV formation were observed in control MO injected embryos, whereas embryos injected with rap1b-MO1 (MO1) and rap1b-MO2 (MO2) displayed inhibition in ISV sprouting. (D-E) Analysis of Rap1b deletion efficacy (D) RT-PCR amplification of F1-R1 fragment (rap1b exon 1 to exon 4; 307bp) and F2-R1 fragment (exon 2 to exon 4; 212bp) of rap1b cDNA is substantially reduced in 6ng rap1b-MO2 injected zebrafish embryos compared with control. Ten to 20 embryos per group were injected at 1-2 cell stage. Total RNA was isolated at 28 hpf. (E) Rap1 expression in rap1b-MO (MO1, MO2) or control-MO (Con) injected embryos at 28 hpf was detected using M90 monoclonal IgG. Relative amount of Rap1 to actin is indicated in parentheses for each group; blot is representative of n = 3 independent experiments.
VEGFR2 and Rap1b function additively during angiogenic sprouting in vivo
Because our mechanistic results in ECs indicate that Rap1b acts upstream from VEGFR2, we investigated next whether the Rap1-VEGFR2 signaling axis was conserved in vivo. Chemical inhibition of VEGFR2 signaling using small molecules such as SU5416, a selective VEGFR2 inhibitor has been shown to effectively block angiogenesis in zebrafish.40 In fact, SU5416 was previously shown to inhibit ISV sprouting in a dose-dependent manner.41 Moreover, Rap1b knockdown also showed ISV sprouting defects in zebrafish (Figure 5A-B). To investigate whether Rap1b is required for VEGFR2 signaling in vivo, we combined the Rap1b LOF via MO with small molecule inhibition of VEGFR2 kinase activity via SU5416 and evaluated their effect on ISV formation. We hypothesized that if Rap1b promotes VEGFR2 activation in vivo, treatment of zebrafish embryos with combined inhibitors at sub-effective concentrations would have an additive or synergistic ISV sprouting defect.
In dose-response experiments we established that 0.25μM SU5416 and 2ng rap1b MO were the highest concentrations that did not lead to a significant ISV defects in flk1: EGFP zebrafish embryos (supplemental Figure 9). Strikingly, when the inhibitors were used in combination, ISV growth was almost completely ablated in the mid-trunk area (Figure 6Avi) and significantly inhibited in the anterior region (Figure 6Ax,D) in all injected embryos (Figure 6B), while ISV formation in control zebrafish was normal (Figure 6Av,ix,B,D). We obtained similar results when another class V VEGFR2 inhibitor, compound ZM 323881 was used (supplemental Figure 10). Thus, inhibition of Rap1b leads to increased ISV sensitivity to VEGFR2 inhibition, indicating that Rap1b and VEGFR2 act in the same signaling pathway.
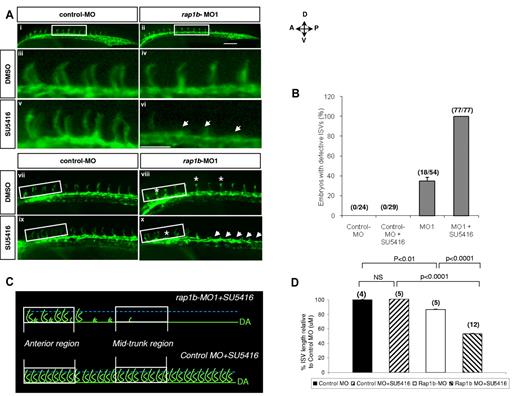
Suppression of rap1b expression increases ISV sensitivity to VEGFR2 inhibition. (A) Low- (i-ii,vii-x) and high- (iii-vi) power images of the lateral view of the mid-trunk (i-vi) and anterior (vii-x) region of 28 hpf flk1:EGFP zebrafish embryos injected at 1- to 2-cell stage with 2 ng of MO and treated at 20 hpf with 0.25μM SU5416 or DMSO control for 1 hour, as indicated. Treatment of embryos with a combination of sub-effective doses of SU5416 and rap1b-MO (vi,x) but not control MO (v,ix) led to a complete inhibition of ISV formation in the mid-trunk and decreased ISV length in anterior region of the trunk. Arrows indicate missing ISVs and asterisks indicate truncated ISVs; scale bars are 75 μm. (B) ISV defects in the mid-trunk region of rap1b or control morphants (boxed areas in Ai-ii) were quantified by counting the number of affected embryos (numerator) in each group (denominator). (C) Cartoon represents heterogeneous ISV phenotype of zebrafish embryos treated with a combination of sub-effective doses of SU5416 and rap1b-MO (top, image shown in panel Ax) compared with control embryos treated with SU5416 and control-MO (bottom, image shown in panel Aix). (D) ISV length in the anterior region of the trunk was quantified as diagrammed in panel C. Trajectories of 7 adjacent ISVs from the anterior region (boxed areas in panel Avii-x) were measured in 4-12 embryos per group, as indicated in parentheses above bars.
Suppression of rap1b expression increases ISV sensitivity to VEGFR2 inhibition. (A) Low- (i-ii,vii-x) and high- (iii-vi) power images of the lateral view of the mid-trunk (i-vi) and anterior (vii-x) region of 28 hpf flk1:EGFP zebrafish embryos injected at 1- to 2-cell stage with 2 ng of MO and treated at 20 hpf with 0.25μM SU5416 or DMSO control for 1 hour, as indicated. Treatment of embryos with a combination of sub-effective doses of SU5416 and rap1b-MO (vi,x) but not control MO (v,ix) led to a complete inhibition of ISV formation in the mid-trunk and decreased ISV length in anterior region of the trunk. Arrows indicate missing ISVs and asterisks indicate truncated ISVs; scale bars are 75 μm. (B) ISV defects in the mid-trunk region of rap1b or control morphants (boxed areas in Ai-ii) were quantified by counting the number of affected embryos (numerator) in each group (denominator). (C) Cartoon represents heterogeneous ISV phenotype of zebrafish embryos treated with a combination of sub-effective doses of SU5416 and rap1b-MO (top, image shown in panel Ax) compared with control embryos treated with SU5416 and control-MO (bottom, image shown in panel Aix). (D) ISV length in the anterior region of the trunk was quantified as diagrammed in panel C. Trajectories of 7 adjacent ISVs from the anterior region (boxed areas in panel Avii-x) were measured in 4-12 embryos per group, as indicated in parentheses above bars.
Discussion
In our previous study we have demonstrated that total Rap1b−/− mice have defective angiogenesis in vivo and that Rap1b−/− ECs show defective responses to VEGF.18 Here, through the use of lineage-specific Rap1b−/− mice we attributed the angiogenic defect to endothelium and we examined how Rap1b crosstalks with VEGF signaling to promote angiogenesis in vitro and in vivo. The salient findings of this study are: (1) Rap1a and Rap1b in endothelium are required for normal angiogenesis; (2) both Rap1a and Rap1b promote VEGF responses by promoting VEGFR2 activity in ECs; (3) Rap1-mediated VEGF-VEGFR2 activation is mediated in part via integrin αvβ3; (4) Rap1b is essential for zebrafish angiogenesis by promoting initial ISV sprouting; and (5) Rap1b and VEGFR2 signaling pathway act additively to control angiogenesis in vivo.
Previously, we and others have shown that several responses of Rap1-deficient ECs to VEGF were inhibited,20 and thus we have attributed the angiogenesis defect to ECs. Here we have explicitly shown that mice lacking Rap1 expression in ECs display an angiogenic defect similar to that in total Rap1b−/− mice.18 Further, we have shown that in the absence of Rap1b, Rap1a deficiency leads to a further inhibition of retinal neovascularization in mice, a process dependent on VEGF signaling.42 Therefore, both Rap1 isoforms are required in endothelium for VEGF-dependent angiogenesis in vivo. Expression of Tie2 in the hematopoietic lineage is a known limitation of this transgenic model31 ; consistently we see Rap1 excision in blood cells (supplemental Figure 1), and therefore we cannot exclude the contribution of the nonendothelial cells to the observed phenotype.
VEGF stimulation of ECs leads to a rapid and transient activation of Rap1b, implicating that Rap1b can act downstream from VEGFR2 activation.18 Here, we have shown that both Rap1a and Rap1b are required for full VEGFR2 activation and, therefore, act upstream from VEGFR2 as modulators of VEGFR2 kinase. Together, these results suggest that Rap1 is involved in a positive signaling feedback loop to VEGFR2 (Figure 7). However, in this model, the mechanism of how Rap1 activity is regulated via VEGF signaling loop in EC is unknown. Rap1 activation on disruption of adherens junctions has been described in epithelial cells.43,44 In these cells, E-cadherin internalization after junction disassembly leads to activation of Rap1, which in turn is required for integrin activation and the formation of focal adhesions.44 However, given that Rap1b activation in ECs is rapid, with a maximum within 1-5 minutes of VEGF stimulation,18 it most likely occurs more directly downstream from VEGFR2 and may not involve VE-cadherin outside-in signaling.
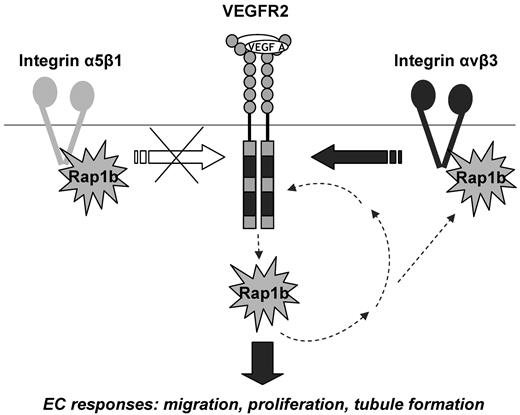
Positive signaling feedback between VEGFR2 and Rap1b modulates EC responses to VEGF stimulation: a model. Rap1b acts upstream from VEGFR2 by regulating the activity of integrin αvβ3 and thus feeding into integrin αvβ3 signaling to VEGFR2. There is a reciprocal up-regulation of Rap1b and integrin β3 in the absence of either protein (Figure 3E and supplemental Figure 5B), further suggesting the existence of a feedback mechanism between the two proteins. Because inhibition of Rap1b in integrin β3−/− ECs leads to a further inhibition of VEGFR2 activation, other mediators of Rap1b regulation of VEGFR2 exist beside integrin β3, however, they do not involve integrin α5β1. Rap1b also acts downstream from VEGFR2, as it becomes rapidly and transiently activated by VEGF.18 This creates a positive signaling feedback between Rap1b and VEGFR2 which promotes VEGF-induced blood vessel formation in vivo and EC responses in vitro; responses that are inhibited in mouse Rap1b-ECKO, zebrafish rap1b morphants and Rap1b−/− ECs, respectively.
Positive signaling feedback between VEGFR2 and Rap1b modulates EC responses to VEGF stimulation: a model. Rap1b acts upstream from VEGFR2 by regulating the activity of integrin αvβ3 and thus feeding into integrin αvβ3 signaling to VEGFR2. There is a reciprocal up-regulation of Rap1b and integrin β3 in the absence of either protein (Figure 3E and supplemental Figure 5B), further suggesting the existence of a feedback mechanism between the two proteins. Because inhibition of Rap1b in integrin β3−/− ECs leads to a further inhibition of VEGFR2 activation, other mediators of Rap1b regulation of VEGFR2 exist beside integrin β3, however, they do not involve integrin α5β1. Rap1b also acts downstream from VEGFR2, as it becomes rapidly and transiently activated by VEGF.18 This creates a positive signaling feedback between Rap1b and VEGFR2 which promotes VEGF-induced blood vessel formation in vivo and EC responses in vitro; responses that are inhibited in mouse Rap1b-ECKO, zebrafish rap1b morphants and Rap1b−/− ECs, respectively.
Our study shows that activation of several integrins, including αvβ3 and β1 is decreased in Rap1b−/− ECs. This is consistent with previous reports showing decreased β1 signaling to FAK and Akt and defective EC sprouting in Rap1-deficient ECs.19 However, we find that integrin αvβ3 but not β1 is required for VEGFR2 activation. This result is also in agreement with previous in vitro and in vivo studies showing that interaction between integrin αvβ3 and VEGFR2 is required for optimal migratory and angiogenic responses of ECs.10 This interaction involves formation of a complex between αvβ3 and VEGFR2, which, however does not appear to involve physical interaction with Rap1 and is not affected in Rap1b−/− ECs (supplemental Figure 11 and data not shown). The molecular connection between Rap1 and integrin αvβ3 in ECs is also poorly understood; in leukocyte and platelet models an adaptor protein RIAM has been implicated in this process. RIAM provides a scaffold connecting membrane-targeting sequences in Rap1 to talin and has been proposed to recruit talin to the plasma membrane leading to activation of integrins β1, β2 and αIIbβ3.45,46 In ECs grown on collagen, in which integrin β3 is not engaged, we find RIAM in a complex with β3 that is enhanced by VEGF stimulation (supplemental Figure 12A), a finding consistent with previously described models. In contrast, in cells grown under the conditions where integrin β3 is engaged, RIAM complex formation with integrin β3 is VEGF- and Rap1-independent (supplemental Figure 12A-B). Therefore, RIAM recruitment to integrin β3 is not the defect that leads to decreased VEGFR2 activation in Rap1−/− ECs. In addition to integrins, other Rap1b effectors of VEGFR2 activation exist since we observe decreased VEGFR2 activation in integrin β3−/− ECs on Rap1b knockdown. While serine/threonine kinase Raf-1 can act as Rap1 effector,38 Raf-1 activation downstream from VEGFR2 is unaffected in Rap1b−/− ECs (Figure 4F), suggesting that Rap1 might promote VEGFR2 activation by regulating NADPH oxidases.47,48
The biochemical results clearly highlight the molecular link between Rap1, integrin αvβ3 and VEGFR2 activation in ECs, and provide a mechanistic explanation for the diminished responses of Rap1-deficient ECs to VEGF stimulation in vitro. However, more importantly, these results provide a basis for the observed defects in VEGF-dependent vessel formation in zebrafish in vivo. In zebrafish, VEGF is required for ISV formation, an angiogenic process.49 Rap1b LOF also leads to severe ISV defect, with a differential effect on the anterior and mid-trunk ISV formation (Figure 6). This differential anatomic effect may result from a delayed initiation of ISV sprouting in Rap1b morphants along the embryonic axis, which normally develops in a gradient manner, beginning from the anterior of the embryo and moves dorsally as development proceeds. Moreover, partial inhibition of Rap1b expression combined with a sub-effective dose of VEGFR2 kinase inhibitor, results in a similar ISV defect, supporting a model in which Rap1 and VEGFR2 act in the same pathway to control VEGF angiogenic phenotypic responses in vivo. This model implies that Rap1 is required for the majority of signals downstream from VEGFR2 in ECs.
Our finding that Rap1 is a positive modulator of VEGFR2 activity may implicate a broader role for Rap1 in maintaining blood vessel homeostasis. Previously, Rap1 activation in ECs has been linked with promoting barrier function. Specifically, cAMP-induced activation of Rap1 via Epac promotes adherens junction and EC barrier formation50 inhibiting VEGF-induced EC chemotaxis.51 Furthermore, activation of Epac leads to the inhibition of angiogenesis in vivo, an effect partly mimicked by transfection of cells with a CA mutant of Rap1.52 These studies strongly suggest that dynamic regulation of Rap1 activity is required for EC homeostasis and underscore the importance of identifying regulators and effectors of Rap1 involved in the specific EC response.
In conclusion, we have identified a novel mechanism for Rap1 in regulating VEGF responses in ECs. Rap1 positively modulates VEGFR2 activation via regulation of integrin αvβ3 and works additively with VEGF signaling in vivo. This finding has potential therapeutic implications for antineovascularization therapy and therefore warrants further investigation of Rap1 in regulation of VEGF-induced EC responses.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs A. Morozov and M. Martin for generously providing rap1af/frap1bf/f mice and Dr A. Szabo for statistical analysis of mouse in vivo data.
This study was supported by American Heart Association grant 0950118G and start-up funds from Blood Research Institute to M.C.-W. and with start-up funds from Children's Research Institute and National Institutes of Health grant HL090712 (to R.R.). C.C. was supported in part by Advancing Healthier Wisconsin funds to R.R.
National Institutes of Health
Authorship
Contribution: S.L. designed and performed research, analyzed data, performed statistical analysis and wrote the manuscript; M.S. designed and performed research, analyzed data, performed statistical analysis, and wrote the manuscript; C.C. designed and performed research, analyzed and interpreted data, and approved the manuscript; A.H. performed research, collected and analyzed data, and approved the manuscript; J.D. performed research, collected data, and approved the manuscript; R.R. designed research, analyzed data, and critically revised the manuscript; and M.C.-W. designed research, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Magdalena Chrzanowska-Wodnicka, Blood Research Institute, BloodCenter of Wisconsin, PO Box 2178, Milwaukee, WI 53201; e-mail: magdalena.wodnicka@bcw.edu.