Abstract
IL-7 is an important cytokine for lymphocyte differentiation. Similar to what occurs in vivo, human CD19+ cells developing in human/murine xenogeneic cultures show differential expression of the IL-7 receptor α (IL-7Rα) chain (CD127). We now describe the relationship between CD127 expression/signaling and Ig gene rearrangement. In the present study, < 10% of CD19+CD127+ and CD19+CD127− populations had complete VDJH rearrangements. IGH locus conformation measurements by 3D FISH revealed that CD127+ and CD127− cells were less contracted than pediatric BM pro-B cells that actively rearrange the IGH locus. Complete IGH rearrangements in CD127+ and CD127− cells had smaller CDR3 lengths and fewer N-nucleotide insertions than pediatric BM B-lineage cells. Despite the paucity of VDJH rearrangements, microarray analysis indicated that CD127+ cells resembled large pre-B cells, which is consistent with their low level of Ig light-chain rearrangements. Unexpectedly, CD127− cells showed extensive Ig light-chain rearrangements in the absence of IGH rearrangements and resembled small pre-B cells. Neutralization of IL-7 in xenogeneic cultures led to an increase in Ig light-chain rearrangements in CD127+ cells, but no change in complete IGH rearrangements. We conclude that IL-7–mediated suppression of premature Ig light-chain rearrangement is the most definitive function yet described for IL-7 in human B-cell development.
Introduction
Mammalian B-lineage cells develop from hematopoietic stem cells in fetal liver and adult BM, and differentiate through well-characterized stages before migrating to secondary lymphoid tissues as naive B lymphocytes.1,2 Successful development of a broad Ig repertoire is accomplished by selection of cells with functional rearrangements at the Ig heavy- and light-chain loci. Ig gene rearrangement generally proceeds through an ordered process that is initiated at the IGH locus, with DH to JH rearrangements occurring in pro-B cells3,4 and mediated by RAG1 and RAG2 proteins.5,6 Distinct VH segments then rearrange to the DJH element, and in-frame VDJH rearrangements are expressed as Igμ heavy chains, which can then pair with the surrogate light-chain components VpreB and λ57 to form the pre-BCR. Expression of the pre-BCR initiates several cycles of proliferation at the large pre-B-cell stage, at which time the Ig light-chain loci are epigenetically silenced. After this proliferation phase, Ig light-chain rearrangement is initiated in small pre-B cells. If a functional Ig light chain (Igκ or Igλ) is able to assemble with Igμ, then the cell expresses surface Ig as a BCR+ immature B cell.
The role of transcription factors in regulating Ig gene rearrangement and the expression/function of RAG1 and RAG2 is well chronicled.8 Cytokine signaling also plays a regulatory role. Mice lacking a functional IL-7 receptor (IL-7R) pathway by virtue of targeted deletion of genes encoding the IL-7Rα (IL-7Rα) chain9 or signal transducer and activator of transcription 5 (STAT5)10 exhibit reductions in IGH locus germline transcription and rearrangement of distal VH genes. STAT5-mediated IL-7 signaling can also influence Ig rearrangement via induction of the early B-cell transcription factor (EBF)11 and by germline transcription of distal VH genes.12 Recent studies using a Stat5 conditional deletion mutant mouse showed that IL-7 signaling served to repress premature Igk rearrangement in pro-B cells, thereby maintaining the Ig heavy chain to light-chain rearrangement hierarchy in developing B-lineage cells.13
Efforts to elucidate the role of IL-7 in human B-cell development date back to the mid-1990s.14 Interestingly, SCID patients with mutations in genes encoding the IL-7Rα (CD127) or the common γ subunit (CD132) have normal numbers of circulating B cells at 3-6 months of age.15,16 These experiments of nature have been the basis for arguments that human B-cell development is IL-7 independent. Although CD127 is expressed on human B-lineage cells at several stages of differentiation, its function has remained elusive. We17,18 and others19-22 have used the human cord blood/murine MS-5 stromal cell xenogeneic culture as a model to study human B-cell development and as a resource for generating sufficient numbers of B-lineage cells for biochemical and molecular studies. We previously identified 2 populations of CD19+ B-lineage cells that could be distinguished by expression of CD127. CD127+ cells were more blastic than CD127− cells,17 and the former responded to IL-7 by induction of pSTAT5 and pERK1/2.18 However, whether CD127+ and CD127− cells differ by other characteristics, suggesting different stages or pathways in B-cell development, is unknown. To characterize the role of IL-7 signaling in human B-cell lymphopoiesis in greater detail, we examined the Ig gene rearrangement patterns and gene-expression profiles of these IL-7R signaling competent and incompetent B-lineage populations in the MS-5 xenogeneic model. Our results provide new insight into the consequences of IL-7R expression and IL-7 signaling on Ig gene rearrangement and expression in human B-lineage cells.
Methods
Isolation and culture of cells
Cord blood CD34+ hematopoietic progenitor cells (HPCs) were isolated and plated on the murine MS-5 stromal cell line as described previously.17,18 Pediatric BM was obtained from allogeneic transplantation donors.23 Thymic specimens were obtained from children requiring surgery for congenital heart disease. All tissues were obtained following protocols approved by the institutional review board at the University of Minnesota and the medical ethics committee at the Erasmus MC Rotterdam.
Neutralization experiments
Xenogeneic cultures were maintained as described previously.17 After 3 weeks of culture, goat anti–murine IL-7–neutralizing antibody (AB-407-NA; R&D Systems) was added to a final concentration of 10 μg/mL, and cultures were maintained for 1 additional week without medium changes. After 4 or 7 days, cells were harvested by anti-CD19 bead–positive selection19 and sorted into CD127+Igμ− and CD127−Igμ− populations to > 95% purity. For short-term neutralizing cultures, 0.5-1 × 105 CD19+ cells were sorted to be CD98hi/sIgμ− and plated on confluent monolayers of MS-5 in 96-well plates, as described previously.17 Neutralizing anti-murine IL-7 was added to a final concentration of 10 μg/mL. Cells were harvested at day 3 or 6 for surface phenotyping and quantitation as described previously.17
Other
Details for flow cytometry, cell sorting, probe preparation, 3D FISH, image acquisition, distance calculations, statistics, quantitation of Ig gene rearrangements, junctional region analysis, quantitative RT-PCR, and gene-expression profiling are provided in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All microarray data are available in ArrayExpress under accession number E-MEXP-384.
Results
Biologic characteristics of CD19+CD127+ and CD19+CD127− cells
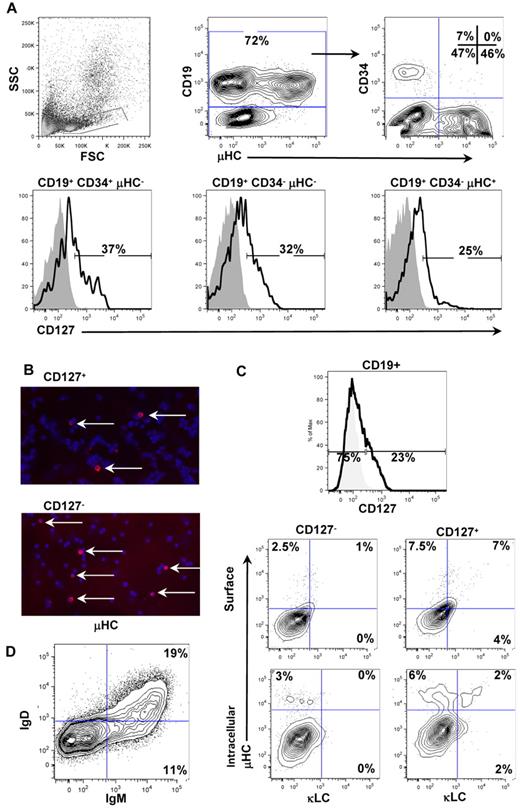
Previous studies of freshly isolated human B-lineage cells from fetal24 and adult25,26 BM have revealed that the IL-7Rα chain (CD127) is expressed on multiple stages of B-cell development. We extended this analysis to pediatric BM and, as shown in Figure 1A, CD127 was expressed on ∼ 30% of pro-, pre-, and immature/naive B-lineage cells. This pattern of expression is consistent with our previous study of fetal BM24 and a recent study using adult BM.26 Therefore, in normal BM, CD127 expression may confer distinct functions within the pro-B and pre-B compartments. Alternatively, CD127 expression may discriminate populations independently of the historical definition of CD19+CD34+ pro-B and CD19+CD34− pre-B cells. We tested the latter possibility using a human/murine xenogeneic culture system to generate CD19+CD127+IgM− and CD19+CD127−IgM− cells (hereafter referred to as CD127+ and CD127−). We reported previously that CD19+CD127−IgM− cells from xenogeneic cultures were small cells with low forward light-scattering characteristics, whereas CD19+CD127+IgM− cells were more blastic.17 To determine whether these properties affected survival, we enriched the 2 populations from xenogeneic cultures and replated them in the absence of exogenous cytokines and MS-5 stromal cells. Both populations underwent gradual cell death over 7 days, with CD127+ cells showing slightly greater survival capability (data not shown). We next determined whether CD127+ and CD127− populations harbored differences in Ig protein expression. As shown in Figure 1B, FACS-purified CD127+ and CD127− populations both contained < 5% Igμ+ cells. The frequency of Igμ+, Igμ+Igκ+, and Igμ+Igλ+ cells in the CD127+ and CD127− populations were similar when the cells were examined by cell-surface staining and after fixation and permeabilization (Figure 1C and data not shown). Although xenogeneic cultures generally undergo a loss of stromal cell integrity and lymphoid cell survival between 4 and 6 weeks, a minority can survive longer. An example of B-cell differentiation occurring in a 7-week culture is shown in Figure 1D, where well-defined populations of Igμ+Igδ− immature B cells and Igμ+Igδ+ naive B cells can be seen. Therefore, xenogeneic cultures can support a full range of B-cell development that occurs in normal human BM.
Expression of CD127 and Ig proteins on normal pediatric BM B-lineage cells and CD19+ cells from xenogeneic cultures. (A) CD127 expression was assayed on normal pediatric BM by flow cytometry. Total CD19+ cells in the lymphoid light-scatter gate were analyzed for surface μHC and CD34 expression (top right contour plot). CD127 expression was then analyzed and plotted on the 3 major populations of B-lineage cells in human BM: pro-B cells (CD19+/CD34+/μHC−), pre-B cells (CD19+/CD34−/μHC−), and immature/naive B cells (CD19+/CD34−/μHC+). CD127 expression was detected on all 3 subpopulations of B-lineage cells in human BM. (B) Cytocentrifuge slides of FACS-purified CD127+ and CD127− cells from xenogeneic cultures were stained for expression of Igμ heavy chains using goat anti–human IgM TRITC (red) and counterstained with DAPI (blue). Mounting medium (2.3% wt/vol DABCO; Sigma D-2522/10% 1× PBS/87.7% glycerol) was used to cover the stained slides and slides were stored at 4°C and visualized at room temperature. Images were acquired using the Plan-Apochromat 10×/0.45 numerical aperture (NA) objective on an Axiovert 2 fluorescent microscope (Carl Zeiss) equipped with a Spot CCD camera (Diagnostic Instruments) and Spot Advanced 4.6 acquisition software. Adobe Photoshop 7.0 was used to create image overlays. Approximately 5% of cells in each population expressed Igμ. (C) CD19+ cells were isolated from 4-week xenogeneic cultures and stained for expression of CD127, Igμ, Igκ, and Igλ. (D) CD19+ cells were isolated from a 7-week xenogeneic cultures and stained for expression of Igμ and Igδ heavy chains.
Expression of CD127 and Ig proteins on normal pediatric BM B-lineage cells and CD19+ cells from xenogeneic cultures. (A) CD127 expression was assayed on normal pediatric BM by flow cytometry. Total CD19+ cells in the lymphoid light-scatter gate were analyzed for surface μHC and CD34 expression (top right contour plot). CD127 expression was then analyzed and plotted on the 3 major populations of B-lineage cells in human BM: pro-B cells (CD19+/CD34+/μHC−), pre-B cells (CD19+/CD34−/μHC−), and immature/naive B cells (CD19+/CD34−/μHC+). CD127 expression was detected on all 3 subpopulations of B-lineage cells in human BM. (B) Cytocentrifuge slides of FACS-purified CD127+ and CD127− cells from xenogeneic cultures were stained for expression of Igμ heavy chains using goat anti–human IgM TRITC (red) and counterstained with DAPI (blue). Mounting medium (2.3% wt/vol DABCO; Sigma D-2522/10% 1× PBS/87.7% glycerol) was used to cover the stained slides and slides were stored at 4°C and visualized at room temperature. Images were acquired using the Plan-Apochromat 10×/0.45 numerical aperture (NA) objective on an Axiovert 2 fluorescent microscope (Carl Zeiss) equipped with a Spot CCD camera (Diagnostic Instruments) and Spot Advanced 4.6 acquisition software. Adobe Photoshop 7.0 was used to create image overlays. Approximately 5% of cells in each population expressed Igμ. (C) CD19+ cells were isolated from 4-week xenogeneic cultures and stained for expression of CD127, Igμ, Igκ, and Igλ. (D) CD19+ cells were isolated from a 7-week xenogeneic cultures and stained for expression of Igμ and Igδ heavy chains.
Ig gene rearrangement patterns in CD127+ and CD127− cells
We next determined whether competency to respond to IL-7 was correlated with differences in Ig gene rearrangement patterns. Sorted CD19+CD127+Igμ− and CD19+CD127−Igμ− populations were assessed for IGH, IGK, and IGL rearrangements by quantitative RT-PCR. Rearrangements were quantified relative to monoallelically rearranged control cell lines, which were defined as “100% rearranged.” In both populations, approximately 150% of IGH alleles (ie, three-fourths of the total alleles) had undergone DJH rearrangement, and similar DH gene family usage profiles were present (Figure 2A). However, complete VH to DJH gene rearrangements were low, occurring in only ∼ 10% of cells from both populations (Figure 2B), similar to the frequency of cells in each population expressing the Igμ protein (Figure 1B). These rearrangements largely used the VH1-3 gene families (4.6%-7.6% compared with VH4-7 at 0.01%-0.02%). This was somewhat unexpected, because VH4 is one of the most commonly used families in fetal BM27 and pediatric BM CD19+CD34+ pro-B cells.23 However, although not visible in the bar graph shown in Figure 2B, VH4-DJ rearrangements were detectable. GeneScan size analysis of VH-DJH rearrangements showed large size variations (Figure 2G). Therefore, both cell populations have their IGH loci in immature configuration with high levels of DH-JH rearrangements and low levels of VH-DJH rearrangements that do not demonstrate in-frame selection.
Unique Ig rearrangement patterns in purified CD19+CD127+ and CD19+CD127− populations. (A-F) The levels of DH-JH, VH-DJH, Vκ-Jκ, Vκ-Kde, IntronRSS-Kde, and Vλ-Jλ rearrangements were quantified in FACS-purified CD127+ and CD127− populations and compared with freshly isolated pediatric BM pro-B cells (dotted line) and small pre-B cells (gray shaded area). The quantitative standards used to determine the percentage of rearranged alleles were a mixture of leukemic cell lines with monoallelic rearrangements set at 100%. All data were obtained from triplicate experiments using cells isolated from 3 separate xenogeneic cultures. (G-I) The size distributions of complete VH-DJH, Vκ-Jκ, and Vλ-Jλ were assessed using GeneScan analysis. These patterns are not consistent with in-frame selection, which would be indicated by a characteristic trinucleotide spacing (triplet peaks) shown on the x-axis of each plot. Size standard peaks are shown in light gray at positions 139, 160, 246, and 300.
Unique Ig rearrangement patterns in purified CD19+CD127+ and CD19+CD127− populations. (A-F) The levels of DH-JH, VH-DJH, Vκ-Jκ, Vκ-Kde, IntronRSS-Kde, and Vλ-Jλ rearrangements were quantified in FACS-purified CD127+ and CD127− populations and compared with freshly isolated pediatric BM pro-B cells (dotted line) and small pre-B cells (gray shaded area). The quantitative standards used to determine the percentage of rearranged alleles were a mixture of leukemic cell lines with monoallelic rearrangements set at 100%. All data were obtained from triplicate experiments using cells isolated from 3 separate xenogeneic cultures. (G-I) The size distributions of complete VH-DJH, Vκ-Jκ, and Vλ-Jλ were assessed using GeneScan analysis. These patterns are not consistent with in-frame selection, which would be indicated by a characteristic trinucleotide spacing (triplet peaks) shown on the x-axis of each plot. Size standard peaks are shown in light gray at positions 139, 160, 246, and 300.
Multiple types of Ig light-chain rearrangements were assayed, including the Vκ-Jκ and Vλ-Jλ rearrangements, which can yield functional Ig light chains, as well as Ig κ–deleting (Kde) rearrangements that render an IGK allele nonfunctional (Vκ-Kde and IntronRSS-Kde). Vκ-Kde rearrangements delete Jκ gene segments, thereby removing the previously formed Vκ-Jκ rearrangements from the genome. In contrast, IntronRSS-Kde rearrangements do not delete Jκ gene segments, allowing for the detection of both IntronRSS-Kde and Vκ-Jκ joints on the same allele.23,28 The majority of Ig light-chain gene rearrangements are induced in small pre-B cells,23 with Vκ-Jκ rearrangements preceding Kde and Vλ-Jλ rearrangements.29 Very few Ig light-chain rearrangements were detected in CD127+ cells (Figure 2C-F). Interestingly, CD127− cells had extensive rearrangement activity at the Ig light-chain loci despite the paucity of VDJH rearrangements. Abundant Vκ-Jκ rearrangements were present (Figure 2C), exceeding the 200% level that corresponds to 2 rearranged alleles per cell. The levels of Kde (Figure 2D-E) and Vλ-Jλ rearrangements (Figure 2F) were comparable to those found in freshly isolated small pre-B cells, but were dramatically higher than in CD127+ cells. GeneScan analysis of Vκ-Jκ and Vλ-Jλ rearrangements showed size distributions similar to those detected in small pre-B cells.23 Despite the high levels of gene rearrangements in CD127− cells, this analysis did not reveal uniform triplet peaks, indicating that in-frame selection had not occurred. These results show that CD127− cells have high levels of Ig light-chain gene rearrangements in the absence of complete VDJH rearrangements.
Limited TdT activity in cultured human B-lineage cells
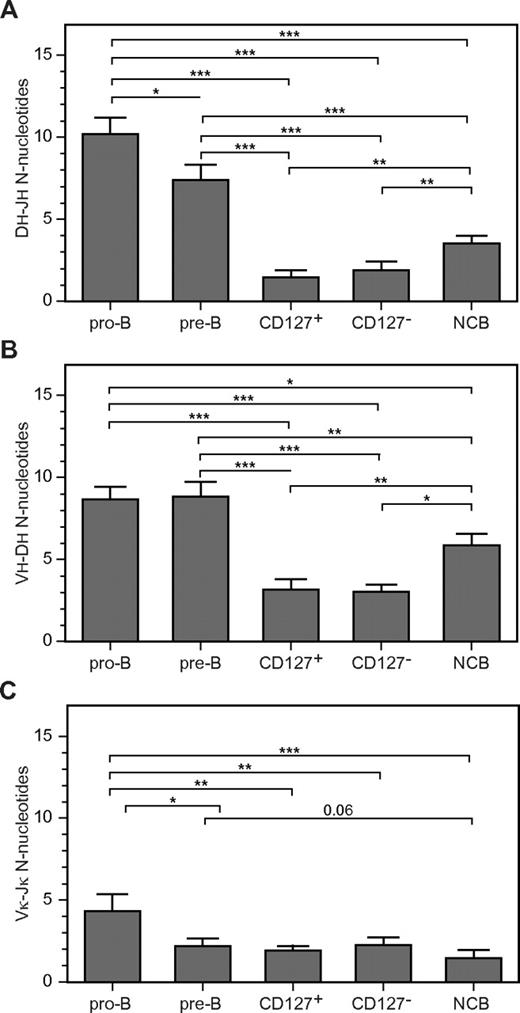
We next analyzed coding joint formation in CD127+ and CD127− cells. The results were compared with IGH and IGK rearrangements occurring in pro- and pre-B cells from pediatric BM and B cells from cord blood. CD127+ and CD127− cells had significantly lower N-nucleotide insertions at VH-DH and DH-JH junctions compared with pediatric BM pro-B and pre-B cells (Figure 3A-B), resulting in shorter CDR3 lengths (Table 1). In addition, their junctions had significantly more nucleotides deleted from the 5′ end of the JH segment than did pro-B and pre-B cells (Table 1). These results suggest that IGH gene rearrangements were formed in the presence of less TdT compared with B-lineage cells from normal pediatric BM. Cord blood B cells (that represent a mixture of immature, transitional, and mature populations30 ) had CDR3 lengths comparable to CD127+ and CD127− cells (Table 1). The number of N-nucleotide insertions in cord blood B-cell DJH and VDJH junctions was significantly larger than CD127+ and CD127− cells (Figure 3A-B), but smaller than pediatric BM.
Limited N-nucleotide insertions in complete VH-DJH and Vκ-Jκ gene rearrangements of cultured CD19+CD127+ and CD19+CD127− cells. Bar graphs show the number of N-nucleotides (y-axis) in DH-JH (A), VH-DH (B), and Vκ-Jκ (C) junctions. Values represent the means ± SD of multiple sequences from FACS-purified CD127+ and CD127− populations from 3 donors, normal cord blood B cells (NCB) from 2 donors, and FACS-purified pediatric pro-B and pre-B-cell populations from 2-3 donors. The nonparametric Mann-Whitney test was used to calculate significance levels between paired populations (horizontal bars). *P < .05; **P < .01; ***P < .001.
Limited N-nucleotide insertions in complete VH-DJH and Vκ-Jκ gene rearrangements of cultured CD19+CD127+ and CD19+CD127− cells. Bar graphs show the number of N-nucleotides (y-axis) in DH-JH (A), VH-DH (B), and Vκ-Jκ (C) junctions. Values represent the means ± SD of multiple sequences from FACS-purified CD127+ and CD127− populations from 3 donors, normal cord blood B cells (NCB) from 2 donors, and FACS-purified pediatric pro-B and pre-B-cell populations from 2-3 donors. The nonparametric Mann-Whitney test was used to calculate significance levels between paired populations (horizontal bars). *P < .05; **P < .01; ***P < .001.
IGH junction characteristics
| . | Sequence no. . | CDR3 length . | del, VH . | P, VH . | N, VH-DH . | P, 5′DH . | del, 5′DH . | del, 3′DH . | P, 3′DH . | N, DH-JH . | P, JH . | del, J . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aa . | nt . | ||||||||||||
| pro-B | 92 | 20.41 | 60.55 | 2.53 | 0.34 | 8.68 | 0.18 | 4.47 | 4.89 | 0.11 | 10.21 | 0.14 | 5.34 |
| pre-B | 53 | 19.91 | 59.81 | 2.15 | 0.11 | 8.83 | 0.11 | 4.62 | 5.21 | 0.04 | 7.42 | 0.08 | 6.08 |
| CD127+ | 43 | 15.43 | 45.65 | 1.22 | 0.24 | 3.16 | 0.05 | 5.76 | 5.16 | 0.08 | 1.46 | 0.05 | 3.08 |
| CD127− | 37 | 14.91 | 44.12 | 1.53 | 0.16 | 3.02 | 0.00 | 6.35 | 4.44 | 0.19 | 1.91 | 0.11 | 3.30 |
| NCB | 65 | 16.02 | 47.97 | 1.41 | 0.25 | 5.88 | 0.03 | 5.57 | 5.26 | 0.08 | 3.52 | 0.14 | 3.52 |
| . | Sequence no. . | CDR3 length . | del, VH . | P, VH . | N, VH-DH . | P, 5′DH . | del, 5′DH . | del, 3′DH . | P, 3′DH . | N, DH-JH . | P, JH . | del, J . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aa . | nt . | ||||||||||||
| pro-B | 92 | 20.41 | 60.55 | 2.53 | 0.34 | 8.68 | 0.18 | 4.47 | 4.89 | 0.11 | 10.21 | 0.14 | 5.34 |
| pre-B | 53 | 19.91 | 59.81 | 2.15 | 0.11 | 8.83 | 0.11 | 4.62 | 5.21 | 0.04 | 7.42 | 0.08 | 6.08 |
| CD127+ | 43 | 15.43 | 45.65 | 1.22 | 0.24 | 3.16 | 0.05 | 5.76 | 5.16 | 0.08 | 1.46 | 0.05 | 3.08 |
| CD127− | 37 | 14.91 | 44.12 | 1.53 | 0.16 | 3.02 | 0.00 | 6.35 | 4.44 | 0.19 | 1.91 | 0.11 | 3.30 |
| NCB | 65 | 16.02 | 47.97 | 1.41 | 0.25 | 5.88 | 0.03 | 5.57 | 5.26 | 0.08 | 3.52 | 0.14 | 3.52 |
The data shown are the means ± SD from 2-3 donors. Bolded values indicate significantly different values compared to all freshly isolated BM and NCB cells.
Analysis of Vκ-Jκjunctions showed that pediatric BM pro-B cells had longer CDR3 lengths than pre-B cells (Table 2), mainly due to the inclusion of N-nucleotides (Figure 3C). This finding is consistent with the lower TdT expression in normal pre-B cells, as shown in quantitative RT-PCR and microarray analysis (Table 3). In addition, pre-B cells showed more deletions and fewer P-nucleotides than pro-B cells at the Vκ site of the Vκ-Jκ junction (Table 2). The Vκ-Jκjunctions in CD127+ and CD127− cells were indistinguishable from those in pre-B cells (Figure 3C), but were significantly different from pro-B cells with respect to CDR3 lengths, addition of N-nucleotides (Figure 3C), formation of P-nucleotides, and deletions at the Vκ site of the junction (Table 2).
IGK junction characteristics
| . | Sequence no. . | CDR3 length . | del, Vκ . | P, Vκ . | N, Vκ − Jκ . | P, Jκ . | del, Jκ . | |
|---|---|---|---|---|---|---|---|---|
| aa . | nt . | |||||||
| pro-B | 17 | 10.46 | 30.59 | 1.59 | 0.65 | 4.29 | 0.24 | 3.29 |
| pre-B | 28 | 9.30 | 27.11 | 3.15 | 0.04 | 2.07 | 0.25 | 2.64 |
| CD127+ | 39 | 9.5 | 27.68 | 2.98 | 0.15 | 1.8 | 0.15 | 2.08 |
| CD127− | 40 | 9.31 | 27.18 | 3.38 | 0.08 | 2.13 | 0.13 | 2.23 |
| NCB | 32 | 8.4 | 24.53 | 3.03 | 0 | 1.38 | 0.18 | 2.16 |
| . | Sequence no. . | CDR3 length . | del, Vκ . | P, Vκ . | N, Vκ − Jκ . | P, Jκ . | del, Jκ . | |
|---|---|---|---|---|---|---|---|---|
| aa . | nt . | |||||||
| pro-B | 17 | 10.46 | 30.59 | 1.59 | 0.65 | 4.29 | 0.24 | 3.29 |
| pre-B | 28 | 9.30 | 27.11 | 3.15 | 0.04 | 2.07 | 0.25 | 2.64 |
| CD127+ | 39 | 9.5 | 27.68 | 2.98 | 0.15 | 1.8 | 0.15 | 2.08 |
| CD127− | 40 | 9.31 | 27.18 | 3.38 | 0.08 | 2.13 | 0.13 | 2.23 |
| NCB | 32 | 8.4 | 24.53 | 3.03 | 0 | 1.38 | 0.18 | 2.16 |
The data shown are the means ± SD of 2-3 donors. Bolded values indicate significantly different values compared to all freshly isolated BM and NCB cells.
3D architecture of the IGH locus in human B-lineage cells
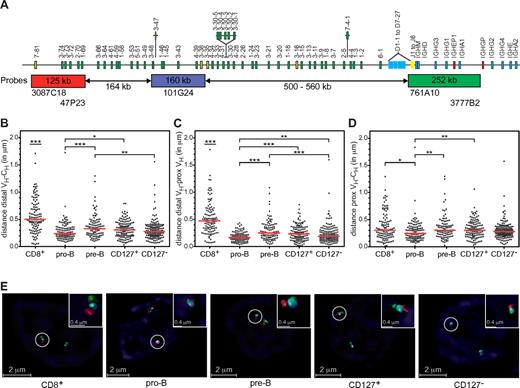
The IGH locus undergoes contraction before VH to DJH recombination in murine pro-B cells.31-34 Subsequent differentiation to pre-B cells leads to IGH locus decontraction, which is thought to prevent further rearrangements and contribute to allelic exclusion.34 To determine whether the relative paucity of VH-DJH rearrangements in CD19+ cells from 4-week xenogeneic cultures resulted from impaired contraction, we measured the spatial distances between 3 probe sets recognizing distal VH, proximal VH, and CH regions (Figure 4A). Similar analyses were conducted on pediatric BM B-lineage cells and CD8+ thymocytes (the latter serving as a control for maximum decontraction of the IGH locus). Spatial distances between distal VH and CH regions, distal VH and proximal VH regions, and proximal VH and CH regions were the shortest in pediatric BM pro-B cells (Figure 4B-D). The distances between distal VH and proximal VH probes (Figure 4C), as well as distal VH and CH probes (Figure 4B), were significantly shorter in pre-B cells compared with CD8+ thymocytes. However, distances between proximal VH and CH probes were similar between pre-B and CD8+ thymocytes (Figure 4D), indicating that this region is close to or fully decontracted in pre-B cells. Contraction distances in CD127+ cells were identical to pre-B cells, but significantly longer than pro-B cells (Figure 4B-D). Contraction of the IGH locus in CD127− cells was intermediate between pro-B and pre-B cells (Figure 4B-D).
Impaired IGH locus compaction in cultured CD19+CD127+ and CD19+CD127− cells. (A) Schematic representation of the human IGH locus and the combinations of bacterial artificial chromosome clones used as 3D FISH probes are shown. The distance separating each of the 3 probes and their positions within the IGH locus were determined from the IMGT database35 Distal VH probes were labeled with Alexa Fluor 568 (red), proximal VH probes with Cy5 (blue), and CH probes with Alexa Fluor 488 (green). Numbers in the rectangles represent the size of the regions recognized by the probes and the numbers below the arrows represent genomic distances between probe sets. (B-D) Scatter plots showing the distances in micrometers (y-axis) separating distal VH, proximal VH, and CH regions in FACS-purified pediatric BM pro-B and small pre-B cells, CD8+ thymocytes, and FACS-purified CD19+CD127+ and CD19+CD127− populations from xenogeneic cultures. Except for pro-B cells (1 donor) at least 2 different donors and at least 100 alleles were analyzed per population. Red horizontal lines represent median distances between indicated probes in each population. The nonparametric Mann-Whitney test was used to calculate significance levels between paired populations (horizontal bars). *P < .05; **P < .01; ***P < .001. (E) Representative images of IGH loci in the primary and cultured cells populations captured with an SP5 confocal microscope (Leica Microsystems). Using a 63×/1.4 NA lens, images of ∼ 70 serial optical sections spaced by 0.148 μm were acquired at room temperature using LAS AF 1.8.2 software (Leica Microsystems). The datasets were deconvolved and analyzed with Huygens Professional software (Scientific Volume Imaging).
Impaired IGH locus compaction in cultured CD19+CD127+ and CD19+CD127− cells. (A) Schematic representation of the human IGH locus and the combinations of bacterial artificial chromosome clones used as 3D FISH probes are shown. The distance separating each of the 3 probes and their positions within the IGH locus were determined from the IMGT database35 Distal VH probes were labeled with Alexa Fluor 568 (red), proximal VH probes with Cy5 (blue), and CH probes with Alexa Fluor 488 (green). Numbers in the rectangles represent the size of the regions recognized by the probes and the numbers below the arrows represent genomic distances between probe sets. (B-D) Scatter plots showing the distances in micrometers (y-axis) separating distal VH, proximal VH, and CH regions in FACS-purified pediatric BM pro-B and small pre-B cells, CD8+ thymocytes, and FACS-purified CD19+CD127+ and CD19+CD127− populations from xenogeneic cultures. Except for pro-B cells (1 donor) at least 2 different donors and at least 100 alleles were analyzed per population. Red horizontal lines represent median distances between indicated probes in each population. The nonparametric Mann-Whitney test was used to calculate significance levels between paired populations (horizontal bars). *P < .05; **P < .01; ***P < .001. (E) Representative images of IGH loci in the primary and cultured cells populations captured with an SP5 confocal microscope (Leica Microsystems). Using a 63×/1.4 NA lens, images of ∼ 70 serial optical sections spaced by 0.148 μm were acquired at room temperature using LAS AF 1.8.2 software (Leica Microsystems). The datasets were deconvolved and analyzed with Huygens Professional software (Scientific Volume Imaging).
Gene-expression profiling of CD19+CD127+ and CD19+CD127− cells
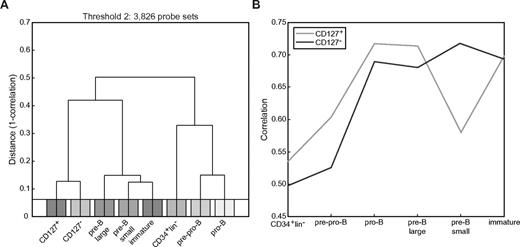
The data presented above revealed some prominent differences in Ig gene rearrangement and expression in CD127+ and CD127− cells. To identify additional differences, we performed gene-expression profiling on the 2 populations and compared them with each other and with pediatric BM B-lineage cell subsets.23 Unbiased clustering analysis was performed based on probe sets that best discriminated between cultured cells and the 4 most mature B-lineage cell stages from BM (Figure 5). This analysis showed that the cultured cells were most comparable to each other and to the later stages of precursor-B-cell development. Specifically, CD127+ cells showed the highest correlation with pro-B and large pre-B cells, whereas CD127− cells showed the highest correlation with small pre-B cells (Figure 5).
Unbiased clustering analysis of genome-wide expression profiles indicate that CD19+CD127+ and CD19+CD127− cells are most similar to pro-B and large pre-B cells and small pre-B cells, respectively. Clustering analysis was performed without bias for known genes or subsets. All probe sets were ranked based on the maximum difference in expression that was observed between any 2 arrays (excluding the CD34+Lin− and pre-pro-B cells). (A) Hierarchical clustering (complete linkage) using 1 − r as a distance measure based on the 3826 (threshold at log2 value 2) probe sets that showed the most variation between any 2 samples. (B) Correlation of the CD127+ and CD127− cells with each of the freshly isolated cord blood CD34+Lin− cells and pediatric BM B-lineage cell subsets.
Unbiased clustering analysis of genome-wide expression profiles indicate that CD19+CD127+ and CD19+CD127− cells are most similar to pro-B and large pre-B cells and small pre-B cells, respectively. Clustering analysis was performed without bias for known genes or subsets. All probe sets were ranked based on the maximum difference in expression that was observed between any 2 arrays (excluding the CD34+Lin− and pre-pro-B cells). (A) Hierarchical clustering (complete linkage) using 1 − r as a distance measure based on the 3826 (threshold at log2 value 2) probe sets that showed the most variation between any 2 samples. (B) Correlation of the CD127+ and CD127− cells with each of the freshly isolated cord blood CD34+Lin− cells and pediatric BM B-lineage cell subsets.
In addition to unbiased clustering analysis, expression patterns for specific sets of well-studied genes in B-cell differentiation were determined. Table 3 shows the gene-array results for selected genes and the confirmation by quantitative RT-PCR (the last column in Table 3). As expected based on membrane protein expression, CD127 transcripts were dramatically higher in CD127+ cells than in CD127− cells. These levels were still higher than those in pediatric BM B-lineage subsets, of which large pre-B cells showed the highest IL7R expression. STAT5A and STAT5B, which encode transcription factors in the CD127 pathway, did not differ in expression between CD127+ cells and CD127− cells. The increased expression of CCND2 and MKI67 in CD127+ cells was correlated with the more blastic characteristics reported previously for these cells.17 Furthermore, CD127+ cells showed higher expression of BCL2, but not MCL1, which is consistent with the Western blotting analysis of sorted CDl27+ and CD127− cells (data not shown). Finally, CD127− cells showed higher BCL6 expression than CD127+ cells, which is consistent with the findings that IL-7R signaling negatively regulates BCL6.36
Gene expression in freshly isolated B-lineage cell subsets from pediatric BM* and sorted CD19+CD127+ and CD19+CD127− cells from 4-week xenogeneic cultures
| Gene . | Probe set . | CD34+Lin− . | Pre-pro-B . | Pro-B . | Pre-B large . | Pre-B small . | Immature B . | CD127+ . | CD127− . | CD127− vs CD127+, -fold difference† . | CD127− vs CD127+, -fold difference† . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Survival/proliferation | |||||||||||
| CD127 (IL7R) | 226218_at | 114.5 | 681.6 | 377.1 | 919.8 | 706.5 | 630.8 | 5242.1 | 2074.0 | −2.5‡ | −5.6# |
| STAT5A | 203010_at | 1085.3 | 1201.7 | 693.3 | 456.5 | 523.2 | 375.9 | 468.4 | 448.5 | 1.0 | ND |
| STAT5B | 205026_at | 76.2 | 116.1 | 158.1 | 128.5 | 99.5 | 109.6 | 97.2 | 82.4 | −1.2 | ND |
| CCND2 | 200951_s_at | 332.5 | 377.9 | 167.5 | 130.9 | 99.0 | 117.1 | 334.5 | 135.9 | −2.5‡ | −5.0¶ |
| SLC2A1 | 201250_s_at | 263.0 | 255.5 | 295.3 | 409.3 | 370.1 | 458.3 | 376.9 | 244.8 | −1.5 | −1.4§ |
| BCL2 | 203685_at | 117.8 | 283.9 | 144.5 | 143.7 | 76.4 | 110.3 | 796.7 | 283.7 | −2.8‡ | ND |
| BCL6 | 203140_at | 383.8 | 1006.1 | 367.7 | 759.3 | 798.0 | 1292.0 | 470.6 | 645.0 | 1.4‡ | ND |
| MCL1 | 200797_s_at | 4372.5 | 5049.2 | 3264.3 | 4421.2 | 5347.8 | 4184.0 | 1786.4 | 1538.9 | −1.2 | ND |
| MKI67 | 212021_s_at | 134.1 | 533.0 | 1082.9 | 1562.2 | 578.9 | 1025.0 | 900.0 | 460.9 | −2.0‡ | ND |
| Ig rearrangement | |||||||||||
| IGH | 209374_s_at | 2814.2 | 5512.7 | 7720.9 | 13 182.0 | 14 308.4 | 14 294.9 | 4709.9 | 8866.8 | 1.9 | ND |
| IGKC | 224795_x_at | 437.2 | 320.3 | 4931.0 | 12 559.6 | 12 982.2 | 11 829.2 | 1698.8 | 5090.4 | 3.0‡ | 1.8§ |
| IGLC | 234764_x_at | 106.3 | 63.6 | 93.0 | 150.9 | 383.6 | 269.6 | 86.1 | 208.2 | 2.4‡ | ND |
| RAG1 | 206591_at | 38.1 | 99.7 | 780.6 | 146.0 | 534.5 | 51.8 | 898.9 | 3160.0 | 3.5‡ | 6.7# |
| RAG2 | 215117_at | 24.6 | 70.9 | 389.9 | 219.2 | 289.8 | 58.7 | 657.1 | 1186.5 | 1.8‡ | 1.9§ |
| LIG4 | 227766_at | 52.4 | 37.6 | 253.7 | 93.3 | 215.0 | 120.5 | 269.2 | 780.6 | 2.9‡ | ND |
| XRCC4 | 210813_s_at | 40.6 | 45.9 | 66.5 | 75.3 | 53.3 | 61.6 | 199.2 | 133.6 | −1.5‡ | ND |
| EZH2 | 203358_s_at | 216.6 | 1094.0 | 1627.9 | 1503.6 | 1046.8 | 1175.2 | 2763.2 | 2056.0 | −1.3‡ | −2.5 |
| DNTT | 210487_at | 117.9 | 9204.1 | 14 100.0 | 1860.8 | 2970.3 | 943.7 | 2728.3 | 4067.0 | 1.5 | 2.2§ |
| IGLL | 206660_at | 413.9 | 2378.5 | 6067.5 | 5344.0 | 3150.2 | 2447.8 | 2330.3 | 2730.3 | 1.2 | 1 |
| VPREB1 | 221349_at | 60.6 | 1365.2 | 4780.5 | 3257.6 | 3175.3 | 1882.2 | 1793.3 | 3753.6 | 2.1 | 1.8§ |
| Transcription factors | |||||||||||
| TCF3 | 209153_s_at | 648.9 | 1599.9 | 3078.6 | 3917.8 | 3805.8 | 1805.9 | 2259.9 | 4272.3 | 1.9‡ | 1.5§ |
| EBF1 | 229487_at | 134.2 | 980.4 | 1124.6 | 1072.3 | 946.3 | 744.0 | 1389.4 | 2245.7 | 1.6‡ | 1.3 |
| FOXO1 | 202723_s_at | 2784.6 | 1108.4 | 4723.5 | 3275.3 | 4808.2 | 3672.9 | 985.0 | 2758.4 | 2.8‡ | 2.7¶ |
| FOXO3 | 204131_s_at | 4404.1 | 4002.6 | 2646.4 | 691.8 | 1026.1 | 686.4 | 541.2 | 643.4 | 1.2 | 1.1 |
| ID2 | 201565_s_at | 1738.6 | 384.3 | 727.1 | 1071.5 | 761.5 | 864.9 | 1461.3 | 787.4 | −1.9‡ | −2.9§ |
| IKZF1 | 227346_at | 676.3 | 760.0 | 988.7 | 1139.8 | 885.1 | 1152.6 | 2956.7 | 3374.2 | 1.1 | −1.1 |
| IRF4 | 204562_at | 120.8 | 280.4 | 1068.1 | 4161.9 | 5581.8 | 3580.5 | 1179.8 | 2652.8 | 2.2‡ | 4.3§ |
| IRF8 | 204057_at | 530.5 | 911.0 | 560.4 | 729.6 | 543.7 | 2693.6 | 795.4 | 559.2 | −1.4 | −1.2 |
| PAX5 | 221969_at | 83.6 | 2709.9 | 4850.0 | 6006.2 | 7351.0 | 6083.7 | 4152.5 | 5177.0 | 1.2 | 1.2 |
| Other | |||||||||||
| CD19 | 206398_s_at | 100.1 | 310.3 | 2104.5 | 5010.5 | 6853.9 | 4997.7 | 3469.2 | 3621.7 | 1.0 | −1.5 |
| CD22 | 38521_at | 255.3 | 2038.1 | 1316.3 | 2086.9 | 1833.0 | 1841.4 | 732.3 | 958.5 | 1.3 | ND |
| CD79A | 205049_s_at | 190.4 | 3114.3 | 2194.5 | 5099.8 | 2450.2 | 3802.6 | 1748.8 | 1445.9 | −1.2 | 1 |
| CD79B | 205297_s_at | 154.3 | 2338.0 | 6444.1 | 8468.3 | 9375.5 | 4908.8 | 3257.4 | 7916.3 | 2.4‡ | ND |
| CD98 (SLC3A2) | 200924_s_at | 413.0 | 636.1 | 555.7 | 696.8 | 414.0 | 774.2 | 494.5 | 275.9 | −1.8 | ND |
| CD98 (SLC7A5) | 201195_s_at | 1215.8 | 1733.8 | 876.9 | 1697.9 | 1375.3 | 2014.7 | 968.1 | 151.0 | −6.4‡ | ND |
| Gene . | Probe set . | CD34+Lin− . | Pre-pro-B . | Pro-B . | Pre-B large . | Pre-B small . | Immature B . | CD127+ . | CD127− . | CD127− vs CD127+, -fold difference† . | CD127− vs CD127+, -fold difference† . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Survival/proliferation | |||||||||||
| CD127 (IL7R) | 226218_at | 114.5 | 681.6 | 377.1 | 919.8 | 706.5 | 630.8 | 5242.1 | 2074.0 | −2.5‡ | −5.6# |
| STAT5A | 203010_at | 1085.3 | 1201.7 | 693.3 | 456.5 | 523.2 | 375.9 | 468.4 | 448.5 | 1.0 | ND |
| STAT5B | 205026_at | 76.2 | 116.1 | 158.1 | 128.5 | 99.5 | 109.6 | 97.2 | 82.4 | −1.2 | ND |
| CCND2 | 200951_s_at | 332.5 | 377.9 | 167.5 | 130.9 | 99.0 | 117.1 | 334.5 | 135.9 | −2.5‡ | −5.0¶ |
| SLC2A1 | 201250_s_at | 263.0 | 255.5 | 295.3 | 409.3 | 370.1 | 458.3 | 376.9 | 244.8 | −1.5 | −1.4§ |
| BCL2 | 203685_at | 117.8 | 283.9 | 144.5 | 143.7 | 76.4 | 110.3 | 796.7 | 283.7 | −2.8‡ | ND |
| BCL6 | 203140_at | 383.8 | 1006.1 | 367.7 | 759.3 | 798.0 | 1292.0 | 470.6 | 645.0 | 1.4‡ | ND |
| MCL1 | 200797_s_at | 4372.5 | 5049.2 | 3264.3 | 4421.2 | 5347.8 | 4184.0 | 1786.4 | 1538.9 | −1.2 | ND |
| MKI67 | 212021_s_at | 134.1 | 533.0 | 1082.9 | 1562.2 | 578.9 | 1025.0 | 900.0 | 460.9 | −2.0‡ | ND |
| Ig rearrangement | |||||||||||
| IGH | 209374_s_at | 2814.2 | 5512.7 | 7720.9 | 13 182.0 | 14 308.4 | 14 294.9 | 4709.9 | 8866.8 | 1.9 | ND |
| IGKC | 224795_x_at | 437.2 | 320.3 | 4931.0 | 12 559.6 | 12 982.2 | 11 829.2 | 1698.8 | 5090.4 | 3.0‡ | 1.8§ |
| IGLC | 234764_x_at | 106.3 | 63.6 | 93.0 | 150.9 | 383.6 | 269.6 | 86.1 | 208.2 | 2.4‡ | ND |
| RAG1 | 206591_at | 38.1 | 99.7 | 780.6 | 146.0 | 534.5 | 51.8 | 898.9 | 3160.0 | 3.5‡ | 6.7# |
| RAG2 | 215117_at | 24.6 | 70.9 | 389.9 | 219.2 | 289.8 | 58.7 | 657.1 | 1186.5 | 1.8‡ | 1.9§ |
| LIG4 | 227766_at | 52.4 | 37.6 | 253.7 | 93.3 | 215.0 | 120.5 | 269.2 | 780.6 | 2.9‡ | ND |
| XRCC4 | 210813_s_at | 40.6 | 45.9 | 66.5 | 75.3 | 53.3 | 61.6 | 199.2 | 133.6 | −1.5‡ | ND |
| EZH2 | 203358_s_at | 216.6 | 1094.0 | 1627.9 | 1503.6 | 1046.8 | 1175.2 | 2763.2 | 2056.0 | −1.3‡ | −2.5 |
| DNTT | 210487_at | 117.9 | 9204.1 | 14 100.0 | 1860.8 | 2970.3 | 943.7 | 2728.3 | 4067.0 | 1.5 | 2.2§ |
| IGLL | 206660_at | 413.9 | 2378.5 | 6067.5 | 5344.0 | 3150.2 | 2447.8 | 2330.3 | 2730.3 | 1.2 | 1 |
| VPREB1 | 221349_at | 60.6 | 1365.2 | 4780.5 | 3257.6 | 3175.3 | 1882.2 | 1793.3 | 3753.6 | 2.1 | 1.8§ |
| Transcription factors | |||||||||||
| TCF3 | 209153_s_at | 648.9 | 1599.9 | 3078.6 | 3917.8 | 3805.8 | 1805.9 | 2259.9 | 4272.3 | 1.9‡ | 1.5§ |
| EBF1 | 229487_at | 134.2 | 980.4 | 1124.6 | 1072.3 | 946.3 | 744.0 | 1389.4 | 2245.7 | 1.6‡ | 1.3 |
| FOXO1 | 202723_s_at | 2784.6 | 1108.4 | 4723.5 | 3275.3 | 4808.2 | 3672.9 | 985.0 | 2758.4 | 2.8‡ | 2.7¶ |
| FOXO3 | 204131_s_at | 4404.1 | 4002.6 | 2646.4 | 691.8 | 1026.1 | 686.4 | 541.2 | 643.4 | 1.2 | 1.1 |
| ID2 | 201565_s_at | 1738.6 | 384.3 | 727.1 | 1071.5 | 761.5 | 864.9 | 1461.3 | 787.4 | −1.9‡ | −2.9§ |
| IKZF1 | 227346_at | 676.3 | 760.0 | 988.7 | 1139.8 | 885.1 | 1152.6 | 2956.7 | 3374.2 | 1.1 | −1.1 |
| IRF4 | 204562_at | 120.8 | 280.4 | 1068.1 | 4161.9 | 5581.8 | 3580.5 | 1179.8 | 2652.8 | 2.2‡ | 4.3§ |
| IRF8 | 204057_at | 530.5 | 911.0 | 560.4 | 729.6 | 543.7 | 2693.6 | 795.4 | 559.2 | −1.4 | −1.2 |
| PAX5 | 221969_at | 83.6 | 2709.9 | 4850.0 | 6006.2 | 7351.0 | 6083.7 | 4152.5 | 5177.0 | 1.2 | 1.2 |
| Other | |||||||||||
| CD19 | 206398_s_at | 100.1 | 310.3 | 2104.5 | 5010.5 | 6853.9 | 4997.7 | 3469.2 | 3621.7 | 1.0 | −1.5 |
| CD22 | 38521_at | 255.3 | 2038.1 | 1316.3 | 2086.9 | 1833.0 | 1841.4 | 732.3 | 958.5 | 1.3 | ND |
| CD79A | 205049_s_at | 190.4 | 3114.3 | 2194.5 | 5099.8 | 2450.2 | 3802.6 | 1748.8 | 1445.9 | −1.2 | 1 |
| CD79B | 205297_s_at | 154.3 | 2338.0 | 6444.1 | 8468.3 | 9375.5 | 4908.8 | 3257.4 | 7916.3 | 2.4‡ | ND |
| CD98 (SLC3A2) | 200924_s_at | 413.0 | 636.1 | 555.7 | 696.8 | 414.0 | 774.2 | 494.5 | 275.9 | −1.8 | ND |
| CD98 (SLC7A5) | 201195_s_at | 1215.8 | 1733.8 | 876.9 | 1697.9 | 1375.3 | 2014.7 | 968.1 | 151.0 | −6.4‡ | ND |
ND indicates not determined.
The results for CD34+Lin−, pre-pro-B, pro-B, pre-B large, pre-B small, and immature B were obtained from a new analysis on previously published data.25
The -fold difference was calculated by determining the absolute value of the ratio of transcript expression between each population. Values were assigned a negative value if gene expression was higher in CD127+ compared with CD127− and values were assigned a positive value if gene expression was higher in CD127− compared with CD127+. Left column is microarray data and right column is quantitative RT-PCR data.
Statistically significant difference between CD127+ and CD127− cells based on DNA microarray data.
P < .05 as determined by Student t test on quantitative RT-PCR data.
P < .01 as determined by Student t test on quantitative RT-PCR data.
P < .001 as determined by Student t test on quantitative RT-PCR data.
Analysis of genes involved in V(D)J recombination revealed that CD127− cells had increased expression of RAG1, RAG2, and LIG4, which is consistent with increased rearrangement at the Ig L chain loci in these cells (Figure 2). Furthermore, these cells contained increased levels of IGH, IGK, and IGL transcripts. The probes for these transcripts are specific for constant regions and therefore likely represent transcripts from both rearranged alleles and germline (sterile) transcripts. Finally, several genes encoding transcription factors involved in the regulation of Ig gene rearrangement were up-regulated in CD127− cells, including TCF3, EBF1, FOXO1, and IRF4. PAX5, FOXO3, IKZF1, and IRF8 were not differentially expressed, and EZH2 and ID2 (inhibitor of E2A) were down-regulated in CD127− cells compared with CD127+ cells.
IL-7R signaling inhibits Ig light-chain gene rearrangements in human precursor B cells
IL-7 signaling has been shown to repress premature Igk rearrangements in murine pro-B cells.13 We have demonstrated previously that MS-5–derived IL-7 mediates effects on CD19+ cells in our xenogeneic model.17 Given the paucity of IGK rearrangements in CD127+ cells (Figure 2), in the present study, we investigated whether suppression of IL-7 signaling would initiate the onset of IGK rearrangements in CD127+ cells.
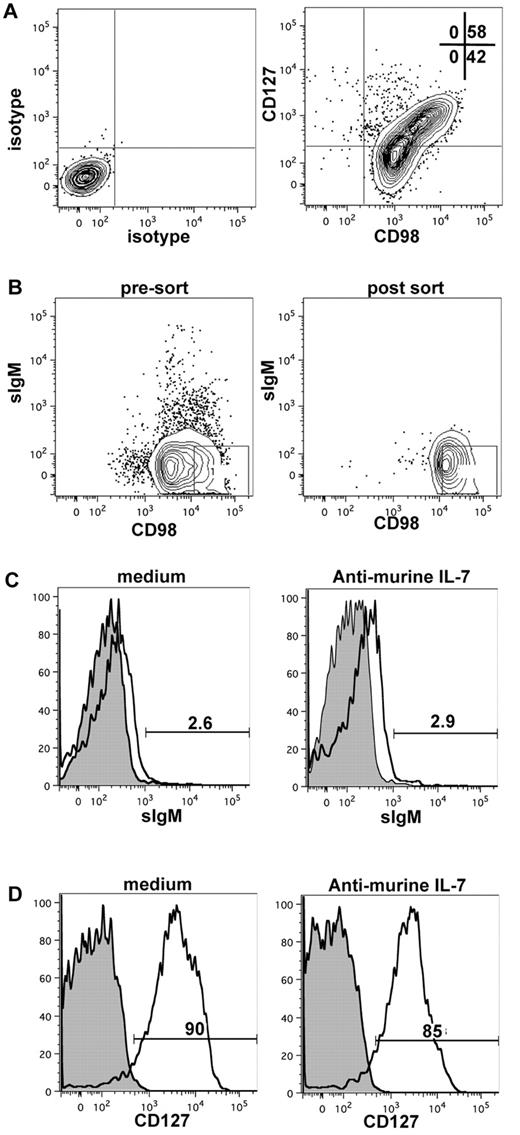
To initially exclude the possibility that suppressing an IL-7 signal would lead to selective cell death of a subpopulation of CD127+ cells or induction of B-cell differentiation, we determined the effect of neutralizing mIL-7 on the viability and maturity of sorted CD127+ cells. To eliminate any contribution that the anti-CD127 used in a sorting protocol could have on recultured CD127+ cells (via either a positive agonistic signal or blocking access to IL-7 binding), we used an antibody to CD98 for purification. The rationale was based on the fact that our gene microarray data revealed that CD98 was more highly expressed on CD127+ cells compared with CD127− cells (Table 3). Although CD98 was expressed on all CD19+ B-lineage cells from xenogeneic cultures, CD127+ cells segregated into the CD98hi fraction (Figure 6A). Therefore, by sorting CD19+CD98hi cells, we could obtain a population highly enriched for CD127+ cells (Figure 6B). Using this strategy, we showed that the IL-7 signal did not induce acquisition of cell-surface Igμ (Figure 6C) or the development of CD127− cells from CD127+ cells (Figure 6D). Neutralization of mIL-7 had no effect on the survival of CD127+ cells when assayed at 3-6 days after plating of sorted cells (data not shown).
Effect of neutralizing murine IL-7 on CD127 and surface IgM expression on sorted CD98hi (CD127+) cells. (A) CD19+ cells from MS-5 xenogeneic cultures were stained for expression of CD98 and CD127; the expression of CD127 is correlated with high CD98 expression. (B) Pre-sort sIgM and CD98 phenotype (left), post-sort analysis of sorted sIgM-CD98hi cells (middle), and CD127 expression on sorted sIgM-CD98hi (right). (C-D) Surface IgM (C) and CD127 (D) expression on CD19+CD98hisIgM− cells after 6 days of culture on MS-5 in the absence or presence of neutralizing anti–mIL-7. Filled histograms indicate unstained control cells (B-D).
Effect of neutralizing murine IL-7 on CD127 and surface IgM expression on sorted CD98hi (CD127+) cells. (A) CD19+ cells from MS-5 xenogeneic cultures were stained for expression of CD98 and CD127; the expression of CD127 is correlated with high CD98 expression. (B) Pre-sort sIgM and CD98 phenotype (left), post-sort analysis of sorted sIgM-CD98hi cells (middle), and CD127 expression on sorted sIgM-CD98hi (right). (C-D) Surface IgM (C) and CD127 (D) expression on CD19+CD98hisIgM− cells after 6 days of culture on MS-5 in the absence or presence of neutralizing anti–mIL-7. Filled histograms indicate unstained control cells (B-D).
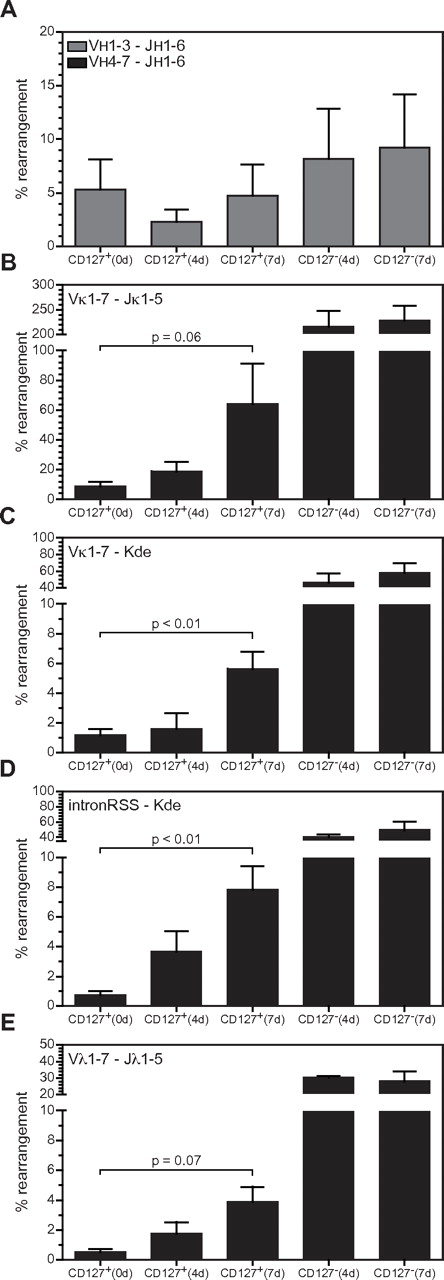
To address directly whether IL-7 signaling suppresses Ig light-chain rearrangements, anti–mIL-7 was added to xenogeneic cultures at 3 weeks and CD127+ and CD127− cells were sorted 4-7 days later. As shown in Figure 7, the frequency of all types of rearrangements in the IGK locus increased in CD127+ cells as the duration of mIL-7 neutralization increased. The frequency of Vκ-Kde and IntronRSS-Kde rearrangements was significantly greater after 7 days of IL-7 neutralization, whereas the increase in Vκ-Jκ rearrangements was close to significant (P = .064). IGL locus rearrangements showed a trending increase in CD127+ cells in the presence of neutralizing anti–mIL-7. There was no change in VH to DJH rearrangements. Rearrangements in CD127− cells are shown for comparison, and demonstrate high levels of rearrangements at the L-chain loci, as shown in Figure 2. Therefore, inhibition of IL-7R signaling initiates Ig light-chain rearrangements in human precursor B cells.
IL-7R signaling and complete Ig gene rearrangements. (A-E) The levels of VH-DJH, Vκ-Jκ, Vκ-Kde, IntronRSS-Kde, and Vλ-Jλ rearrangements were quantified as in Figure 2 in FACS-purified CD127+ and CD127− populations from cultures in which endogenous IL-7 was neutralized for either 4 or 7 days. Each bar represents the mean ± SD of triplicate experiments except CD127+ (0 days), which represents quadruplicate values (the triplicate values from Figure 2 plus 1 additional experiment donor matched to one of the experiments in which IL-7 was neutralized).
IL-7R signaling and complete Ig gene rearrangements. (A-E) The levels of VH-DJH, Vκ-Jκ, Vκ-Kde, IntronRSS-Kde, and Vλ-Jλ rearrangements were quantified as in Figure 2 in FACS-purified CD127+ and CD127− populations from cultures in which endogenous IL-7 was neutralized for either 4 or 7 days. Each bar represents the mean ± SD of triplicate experiments except CD127+ (0 days), which represents quadruplicate values (the triplicate values from Figure 2 plus 1 additional experiment donor matched to one of the experiments in which IL-7 was neutralized).
Discussion
We embarked on this study with the expectation that evaluating human B-lineage cells that are competent versus incompetent to respond to IL-7 would facilitate our understanding of how development of the human immune system is regulated. The MS-5 murine stromal cell line17-22 and human BM mesenchymal stromal cells24,26 have both been useful for analyzing the development of human B-lineage cells. However, an advantage of the MS-5 xenogeneic model is the opportunity to analyze the contribution of stromal cell–derived IL-7 to the developmental biology of human B-lineage cells. Such an analysis requires that IL-7 signaling can be controlled, and the efficiency of neutralizing anti–IL-7 in the xenogeneic model fulfills this important criterion. The goals of the current study were: (1) to undertake a comprehensive analysis of Ig gene rearrangements in CD127+ and CD127− cells present in MS-5 xenogeneic cultures, and (2) to determine whether CD127 expression and/or IL-7 signaling conferred functional consequences on Ig gene rearrangement and global gene expression in B-cell precursors.
The MS-5 xenogeneic model can support up to 30% of CD19+ B-lineage cells expressing cell-surface Igμ (Figure 1D). This is similar to the frequency of CD19+/cell-surface Igμ+ B-lineage cells in normal human BM and comparable to results using human BM mesenchymal stromal cells.26,37 However, we focused on earlier time points in the xenogeneic model: 4 weeks, at which time the cultures are enriched for cells that do not express cell-surface Igμ. CD127+ and CD127− populations derived from 4-week cultures both have a small fraction of cells expressing Igμ, in agreement with the low frequency of complete VDJH rearrangements determined by quantitative RT-PCR (Figure 2B). However, it is unclear why VH to DJH rearrangements exist at low frequency (Figure 2B), when the frequency of DJH rearrangements is much higher (Figure 2A). One approach to answering this question was to use 3D FISH to assess the nuclear topography of the IGH locus. Murine studies have shown that the entire repertoire of VH genes juxtaposes to DH elements at the pro-B-cell stage,32 which means that the locus undergoes contraction. A comparable analysis of IGH locus topography has not been reported for human B-lineage cells. Therefore, we initially addressed whether freshly isolated pediatric pro-B and pre-B cells show differences in IGH locus contraction. As shown in Figure 4B-D, pro-B cells exhibit significantly greater IGH locus contraction than pre-B cells, as measured by the 3 probe sets. Analysis of IGH locus contraction showed that CD127+ cells are less contracted than normal pediatric BM pro-B cells (Figure 4B-D), providing at least one potential explanation for the low frequency of VDJH rearrangements in CD127+ cells. In the case of CD127− cells, only one probe set (distal VH-proximal VH; Figure 4C) showed a significant difference compared with normal pro-B cells. Pax5 gene–targeted mice harbor early B-lineage cells that are severely impaired in their capacity to undergo VH to DJH rearrangement,38 and Pax5 also promotes IGH locus contraction39 and transactivates the RAG complex.40 PAX5 expression in CD127+ and CD127− cells from xenogeneic cultures was comparable to expression in normal pro-B cells (Table 3), suggesting that simple differences in PAX5 expression do not explain why complete VDJH rearrangements occur at low frequency in cells from 4-week xenogeneic cultures. It remains possible that the subcellular distribution of Pax5, expression of different Pax5 isoforms, or Pax5 transcriptional activity differ in CD127+ and CD127− cells in 4-week xenogeneic cultures compared with normal BM pro-B cells.
Both CD127+ and CD127− populations have a small number of cells expressing cell-surface Igμ/κ or Igμ/λ (Figure 1C). Surprisingly, analysis of Ig light-chain locus recombination revealed extensive rearrangements in CD127− cells that greatly exceeded CD127+ cells (Figure 2C). There are several possible explanations for the existence of CD19+CD127− cells with extensive Ig light-chain rearrangements but few VDJH rearrangements, including heightened recombinatorial flexibility41,42 and IgμH chain deficiency.43 However, Ig light-chain rearrangement before Ig heavy chain rearrangement still appears to be a rare event. CD19+CD127− cells may be normally destined to die in the BM because of the absence of a functional VH to DJH rearrangement. In this scenario, they would likely be eliminated (after cell death) by macrophages and would be difficult to detect in vivo. Their presence in xenogeneic cultures may reflect the absence of a normal clearing mechanism.
The high level of Vκ-Jκ rearrangements present in CD127− cells (Figure 2C) may have been due to amplification of Vκ-Jκ rearrangements present on excision circles formed by ongoing rearrangements between upstream Vκ and downstream Jκ gene segments. It is also possible that the elevated number of Vκ-Jκ rearrangements included in-frame IGK rearrangements, which would predict that cytoplasmic Igκ protein should be present (and in a larger fraction of cells than those expressing the BCR; Figure 1B). However, efforts to detect cytoplasmic Igκ protein by flow cytometry or Western blotting were inconclusive, suggesting that Igκ protein levels may be beneath the sensitivity of the assays we used. This could be a consequence of a low rate of transcription and/or translation of Igκ in these small, resting cells, or Igκ proteins may have a short half-life in the absence of pairing with Igμ.
Figure 1D shows that xenogeneic cultures can support the development of immature (Igμ+Igδ−) and naive mature (Igμ+Igδ+)populations. CD127 expression and IL-7 signaling are generally assumed to be restricted to cells that have not reached the immature/naive mature B-cell stage.44,45 However, we found that ∼ 25% of peripheral blood B cells expressd CD127 (Figure 1A), as did cord blood transitional (CD10+IgM+IgD+) and naive mature (CD10−IgM+IgD+) B cells (S.E.N. and T.W.L, unpublished data, March 2010). The detection of CD127 on immature/naive B cells was unexpected, and the functional significance is under investigation. Therefore, the CD127+BCR+ cells that develop in xenogeneic cultures (Figure 1C) could be developmentally similar to the CD127+BCR+ cells we detect in peripheral and cord blood.
IGH rearrangements in pediatric BM pro-B cells occur in the presence of high levels of TdT.23 These rearrangements therefore have a high content of N-nucleotides, which are retained in pre-B cells (Figure 3A-B). The rare Vκ-Jκ rearrangements present in pro-B cells contain several N-nucleotides, whereas the high levels of Vκ-Jκ rearrangements in pre-B cells contain significantly fewer N-nucleotides (Figure 3C). In contrast to pediatric BM, cord blood B cells contain fewer N-nucleotides in IGH gene rearrangements (Figure 3A-B). CD127+ and CD127− cells showed few N-nucleotides, resulting in junctions that were significantly smaller than the IGH junctions formed in pediatric BM pro-B cells and in cord blood B cells. Therefore, the B cells that develop from cord blood CD34+ HPCs plated on MS-5 may have a more restricted VH repertoire, likely reflecting the lower expression of TdT.
Gene-array analysis using unbiased hierarchical clustering indicated was further used to characterize CD127+ and CD127− populations. The blastic features of CD127+ cells—the absence of VDJH rearrangements and decreased expression of genes associated with VDJH rearrangement—suggests a resemblance to pro-B cells/large pre-B cells. By contrast, CD127− cells are morphologically small cells17 and have a high frequency of Ig light-chain gene rearrangements and increased expression of RAG1 and RAG2. Excluding the absence of complete VDJH rearrangements in the majority of CD127− cells, this population most closely resembles small pre-B cells. Pro-B and pre-B populations in normal pediatric BM contain both CD127+ and CD127− subpopulations (Figure 1A). Future studies comparing CD19+CD127+ and CD19+CD127− cells from xenogeneic cultures with subpopulations isolated from normal pediatric BM will be informative, and these complementary approaches will yield deeper insights into the developmental biology of the human immune system.
E2A proteins have been shown to promote access to the Jκ1 RSS by RAG1 and RAG2,46 and can induce recombination at the Igκ locus when coexpressed with RAG proteins in nonlymphoid cells. In the present study, increased levels of E2A transcripts were detected in CD127− cells compared with CD127+ cells (Table 3), but whether this is an instructive or permissive event for Ig light-chain rearrangement before complete IGH rearrangement is unknown. The increased expression of Foxo1 in CD127− cells could reflect the heightened expression of RAG1 and RAG2, because Foxo1 is known to regulate expression of these genes in the mouse.47,48
Several studies have reported that IL-7 signaling leads to changes in chromatin accessibility at the Igh locus via induction of histone acetylation at distal VH genes.9,10,12,49 Malin et al readdressed the role of STAT5 and IL-7 signaling using murine pro-B cells with a conditional deletion in the Stat5 gene,15 and found that IL-7 signaling did not regulate VH expression, but rather functioned to suppress rearrangement at the Igk locus in pro-B cells that had not completed a functional VH to DJH rearrangement. This represents a change in our mechanistic understanding of how IL-7 functions in regulating Ig rearrangement in the mouse. Given that human B-cell development in the murine MS-5 xenogeneic culture depends on IL-7 signaling,17,18 we exploited this model to determine whether IL-7 might function to suppress human IGK rearrangements. The results shown in Figure 7 reveal that CD127+ cells isolated from IL-7–neutralized cultures had increased IGK rearrangements but no change in IGH rearrangements. Furthermore, results shown in Figure 6 reveal that the increase in IGK rearrangements is not (1) likely to be explained by a simple loss of CD127+ cells and concomitant enrichment of CD127− cells, and (2) accompanied by an increase in cell-surface Igμ or differentiation of CD127+ cells into CD127− cells. This may be the most definitive function yet ascribed to a role for IL-7 signaling in human B-cell development and appears to be conserved between mice and humans.
Despite the widely cited T−/B+/NK+ phenotype in SCID patients with mutations in IL2RG, IL7R, and JAK315,16 as evidence that human B-cell development does not require IL-7 signaling, evidence supporting a role for IL-7 signaling in BM B-lineage cell proliferation in vivo has been described. In a phase 1 trial of recombinant human IL-7 therapy for T-cell reconstitution in 16 cancer patients, a statistically significant increase in the frequency of BM CD19+CD45dim and CD19+CD10+ cells was observed after treatment in approximately 80% of patients.50 Based on the results in the present study, it is possible that these SCID patients have promiscuous IGK rearrangements in cells with incompletely rearranged IGH loci because of the absence of IL-7 signaling. Future studies investigating the rearrangement status of the Ig loci in B-lineage cells from these SCID patients will be important to validate the consequences of IL-7 signaling described herein.
In conclusion, we report unappreciated functional relationships between the expression of the IL-7R, IL-7 signaling, and Ig gene rearrangement in human B-lineage cells. These relationships were delineated using a model wherein CD127+ and CD127− B-lineage cells develop from CD34+ HPCs and B-cell development is IL-7 dependent. The emergence of CD19+CD127+ cells provided the means to examine the role of IL-7 signaling and led to the revelation that suppression of IGK rearrangement before the onset of VDJH rearrangement is the most specific function yet attributable to IL-7 in human B-cell development. Similarly, the absence of IL-7 signaling in CD127− B-lineage cells was associated with extensive IGK and some IGL gene rearrangements, and this was independent of IGH gene rearrangements. Stochastic differences in this regulatory network at the single-cell level could lead to asynchronous (premature) IGK and IGL rearrangements and thereby to dysfunctional rearrangements. The xenogeneic model was invaluable for the generation of the new results reported herein, but further studies will be necessary to validate and extend its physiologic relevance. Future studies will be needed to explore the network position of IL-7 signaling in the suppression of IGK rearrangement in mice and in humans to determine differences in the effect of IL-7 on proliferation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Margaret MacMillan and other physician-scientist members of the Blood and Marrow Transplant Program at the University of Minnesota for assisting with acquisition of pediatric BM specimens, with a special thanks to Tim Krepski for facilitating this process; the Erasmus MC Optical Imaging Center for support with confocal microscopy; and S. de Bruin-Versteeg for assistance with preparing the manuscript.
This work was supported by the National Institutes of Health (T32-HL07062, S.E.N.), the Leukemia Research Fund at the Masonic Cancer Center (T.W.L.), the Apogee Enterprises Chair in Cancer Research (T.W.L.), and by a VENI grant from the Dutch Organization for Scientific Research (NWO/ZonMW 91611090, M.C.v.Z.).
National Institutes of Health
Authorship
Contribution: S.E.N. designed the research, performed the experiments, analyzed the results, created the figures, and wrote the manuscript; M.A.B. performed the experiments, analyzed the results, created the figures, and commented on the manuscript; A.A.B. and N.S. performed the experiments; D.d.R. analyzed the results and created the figures; J.J.M.v.D. analyzed the results and commented on the manuscript; T.W.L. designed the experiments, analyzed the results, and wrote the manuscript; and M.C.v.Z. performed the experiments, analyzed the results, created figures, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tucker W. LeBien, Masonic Cancer Center, University of Minnesota, MMC 806, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: lebie001@umn.edu.
References
Author notes
T.W.L. and M.C.v.Z. are joint senior authors of this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal