Abstract
Filamin A (FlnA) is a large cytoplasmic protein that crosslinks actin filaments and anchors membrane receptors and signaling intermediates. FlnAloxP PF4-Cre mice that lack FlnA in the megakaryocyte (MK) lineage have a severe macrothrombocytopenia because of accelerated platelet clearance. Macrophage ablation by injection of clodronate-encapsulated liposomes increases blood platelet counts in FlnAloxP PF4-Cre mice and reveals the desintegration of FlnA-null platelets into microvesicles, a process that occurs spontaneously during storage. FlnAloxP PF4-Cre bone marrows and spleens have a 2.5- to 5-fold increase in MK numbers, indicating increased thrombopoiesis in vivo. Analysis of platelet production in vitro reveals that FlnA-null MKs prematurely convert their cytoplasm into large CD61+ platelet-sized particles, reminiscent of the large platelets observed in vivo. FlnA stabilizes the platelet von Willebrand factor receptor, as surface expression of von Willebrand factor receptor components is normal on FlnA-null MKs but decreased on FlnA-null platelets. Further, FlnA-null platelets contain multiple GPIbα degradation products and have increased expression of the ADAM17 and MMP9 metalloproteinases. Together, the findings indicate that FlnA-null MKs prematurely release large and fragile platelets that are removed rapidly from the circulation by macrophages.
Introduction
Filamins link membrane glycoproteins to the actin cytoskeleton and collect partner proteins to serve as signaling hubs. Filamins translate receptor and intracellular signals into cell movements, modulate cytoskeleton dynamics, and regulate cell transcription.1 The filamin family is composed of 3 isoforms: filamin A (FlnA) and FlnB, which are ubiquitously expressed, and FlnC, which is restricted to skeletal and cardiac muscles.
Filamins are crucial for human development because mutations in the FLNA and FLNB genes lead to brain, bone, cardiovascular, and other abnormalities.2 Mutations in the X-linked FLNA gene that cause early truncation of FlnA lead to periventricular heterotopia, characterized by central nervous system, gut, and cardiovascular malformations, vascular defects and hemorrhage.3 Missense mutations of FLNA cause otopalatodigital spectrum disorders, characterized by bone malformations.4
FlnA promotes high angle branching of actin filaments, organizing them into a 3-dimensional network that gives mechanical stability to the cell. M2 melanoma cells that lack FlnA have unstable surfaces and are distinguished by extensive blebbing of the plasma membrane.5-7 FlnA and actin filaments are enriched at the sites of local force treatment in fibroblasts, and M2 cells have greatly increased susceptibility to force-induced membrane leakage.8 Thus, FlnA stabilizes plasma membranes when damage is induced by tension.
FlnA has > 70 binding partners. In platelets, FlnA attaches the von Willebrand Factor receptor (VWFR) GPIb-IX-V to F-actin.9,10 Studies in CHO cells expressing mutated GPIbα that cannot bind FlnA showed increased cell detachment from VWF surfaces at high shear.11,12 Further, disruption of FlnA-GPIbα interaction with peptides causes inhibition of shear-dependent VWF-induced platelet aggregation and protein tyrosine phosphorylation in human platelets.13,14 Recently, we have shown that FlnAloxP GATA1-Cre mice that lack FlnA in platelets have a macrothrombocytopenia, decreased expression, and altered surface distribution of GPIbα, as well as platelet signaling and functional defects.10 Platelet FlnA was found to interact with Syk and this interaction was particularly indispensable for platelet activation through the collagen receptor GPVI and the C-type lectin-like receptor 2.
Here, we sought to investigate the mechanisms that lead to low platelet counts in the absence of FlnA. Mice that lack FlnA in the megakaryocyte (MK) lineage were generated by pairing FlnAloxP mice with PF4-Cre mice. FlnAloxP PF4-Cre mice had a severe macrothrombocytopenia because of the rapid clearance of FlnA-null platelets from the circulation. Ablation of macrophages partially rescued the thrombocytopenia but resulted in the intravascular appearance of microvesicles. Further, FlnA was important for the final steps of platelet formation because FlnAloxP PF4-Cre bone marrows and spleens had increased megakaryopoiesis and FlnA-null proplatelets released platelets more readily than controls in vitro. Together, the data show that FlnA-null MKs prematurely produce large and fragile platelets that are rapidly removed from the circulation by macrophages.
Methods
Mice
FlnAloxP mice10 were paired with PF4-Cre mice (The Jackson Laboratory) to inactivate the FLNA gene in the MK lineage.15 The FlnAloxP PF4-Cre males obtained were viable, fertile, and further paired with FlnAloxP females. Mice were treated according to the National Institutes of Health and Children's Hospital Animal Care and Use Committee guidelines.
Materials
Anti–β-tubulin and anti-actin antibodies were purchased from Sigma-Aldrich. Antibodies directed against FlnA (Invitrogen), FlnB, GAPDH (Chemicon), ADAM17 (Abcam), and MMP9 (Cell Signaling) were used. Secondary AlexaFluor-568–labeled goat anti–mouse antibody, DMEM, FCS, penicillin/streptomycin, and chloromethylfluorescein diacetate (CMFDA) were from Invitrogen. GM6001 was from Calbiochem and calpeptin from Tocris. Clodronate- and PBS-encapsulated liposomes were obtained from Dr Nico van Rooijen (Vrije Universiteit, Amsterdam, The Netherlands).
Platelet counts and preparation
Blood was collected in EDTA from the retro-orbital plexus of mice, and platelet counts were determined by HEMAVET 950 automated hematologic analyzer (Drew Scientific) or by flow cytometry using 5.5-μm diameter SPHERO rainbow beads as reference (Spherotech).
Blood was collected in acid-citrate-dextrose anticoagulant and platelet-rich plasma was obtained by centrifugation of the blood at 100g for 8 minutes, followed by the centrifugation of the supernatant and the buffy coat at 100g for 6 minutes. After washing them twice in washing buffer (140mM NaCl, 5mM KCl, 12mM trisodium citrate, 10mM glucose, and 12.5mM sucrose, pH 6.0), platelets were resuspended at 2 to 4 × 108 platelets/mL in resuspension buffer (140mM NaCl, 3mM KCl, 0.5mM MgCl2, 5mM NaHCO3, 10mM glucose, 10mM HEPES, pH 7.4).
Platelet clearance
Platelets were fluorescently labeled with 1.8μM CMFDA in resuspension buffer for 20 minutes at 37°C. Unincorporated dye was removed by centrifugation. Platelet transfusions were performed by retro-orbital injection of 200 μL of 4 × 108 platelets/mL. Blood was collected by retro-orbital eye bleeding at time points of 1 minute and 2, 24, 48, and 72 hours, and the percentage of fluorescent platelets in platelet-rich plasma determined by flow cytometry. Data were normalized by adjusting the amount of fluorescent control platelets at 1 minute as 100%.
Macrophage depletion
Bone marrow and spleen histology
Femurs and spleens of FlnAloxP PF4-Cre and FlnAloxP mice were fixed overnight in 4% paraformaldehyde/PBS. Bones were decalcified in 0.5M EDTA, pH 7.8, for 7 days, exchanging EDTA twice a day. Tissues were paraffin-embedded, and sections were stained with H&E at the Dana-Farber Cancer Institute pathology core.
Isolation of mouse fetal liver cells and MK differentiation
Pregnant mice were anesthetized and killed by CO2 asphyxiation 14.5 days after coitus. Mouse fetal livers were isolated from mouse embryos, and cells were prepared as described previously.18 Briefly, 5 × 106/mL cells were cultured in DMEM/10% FCS/penicillin-streptomycin, with 10 ng/mL of recombinant thrombopoietin (R&D Systems). Enriched MKs were prepared by a 2-step BSA density gradient on day 2 or 3. MK cultures were used for experiments or were incubated for additional 18 or 72 hours to induce proplatelet formation and release of nascent platelets.
Platelets produced from FlnA-null and control MK cultures were counted. Briefly, at day 3, MKs were seated in 24-well plates at 1 × 105 cells/mL per well. The supernatants containing released platelets were collected after 18 and 72 hours and centrifuged at 330g. The pellet was resuspended in 100 μL PBS/0.5% BSA, and stained for CD61 or an isotype antibody as control. CD61+ platelet-sized particles were counted by flow cytometry in a gate adjusted for blood platelets together with beads.
Flow cytometry
For ploidy measurements, MK suspensions were centrifuged for 5 minutes at 200g and resuspended in 40mM sodium citrate, 0.25M sucrose, pH 7.4. MKs were incubated for 15 minutes with 20 μg/mL propidium iodide in 0.5% Nonidet P-40, 0.5mM EDTA, 0.5 mg/mL RNase and analyzed by flow cytometry on a FACSCalibur using CELLQuest software (BD Biosciences).
MKs and platelets were stained with fluorescein isothiocyanate-labeled anti-GPIbα, GPIbβ, GPIX, GPV, and CD61 (Emfret Analytics), and incubated for 20 minutes at room temperature in the dark. For phosphatidylserine (PS) exposure, 107 platelets were stained with phycoerythrin-labeled annexin V (BD Biosciences) in 140mM NaCl, 10mM HEPES and 2.5mM CaCl2.
Immunofluorescence and videomicroscopy
Cells were fixed in 4% paraformaldehyde and centrifuged onto poly-L-lysine–coated coverslips. After washing in PBS, cells were permeabilized with 0.1% Triton X-100/PBS and blocked in 5% goat serum/PBS. Cells were incubated with a mouse anti-β-tubulin antibody and probed with a fluorescently conjugated secondary goat antibody. Coverslips were mounted onto microscope slides with Aqua-Mount (Polysciences). Images were taken by Nikon Eclipse TE-2000E fluorescent microscope with 60× NA 1.4 using differential interference contrast objectives with a 1.5 optivar (Nikon) and captured with an Orca-II ER cooled CCD camera (Hamamatsu); shutter and image acquisition were controlled by MetaMorph software (MDS Analytical Technologies). Marginal band stained by β-tubulin was used to determine the diameter of in vitro and in vivo platelets and was analyzed by MetaMorph Version 7.7 software.
Movies of proplatelet-forming MKs were obtained by time-lapse video microscopy on IncuCyte automated, phase-contrast microscope (Essen BioScience).
Electron microscopy of microtubules
Platelets were fixed with 0.25% paraformaldehyde, 0.03% picric acid, and 2.5% glutaraldehyde in 0.1M cacodylate buffer, pH 7.4 for 1 hour, postfixed with 1% osmium tetroxide, dehydrated through series of alcohols, infiltrated with propylene oxide, and embedded in epoxy resin. Ultrathin sections were stained and examined with an electron microscope (JEOL JEM-1200 EX) at an accelerating voltage of 100 kV.
Negative staining
Platelets were fixed with 0.25% formaldehyde, lysed in 60mM Pipes, 25mM HEPES, 10mM EDTA, 2mM MgCl2 (PHEM buffer) containing 0.5% Triton X-100, and immediately centrifuged on the surface of carbon-formar-coated copper grids at 280g. Grids were stained with 1% uranyl acetate and viewed in a JEOL 1200 electron microscope at an accelerating voltage of 60 kV.
Western blotting and cytoskeleton isolation
Cells were lysed in PHEM buffer containing 1% Triton X-100, 10μM phallacidin, and Complete protease inhibitor cocktail (Roche). The actin cytoskeletons were isolated by centrifugation at 100 000g for 30 minutes at 4°C in a Beckman Optima TL ultracentrifuge using polycarbonate tubes and a TLA 100 rotor. SDS-PAGE buffer was added to cell lysates, soluble and insoluble fractions with or without 5% β-mercaptoethanol. Proteins were resolved by SDS-PAGE and transferred onto nitrocellulose membrane (Sigma-Aldrich). After blocking overnight with 1% BSA in 0.2% Tween-20, 100mM NaCl, 20mM Tris, pH 7.4, membranes were probed with antibodies directed against proteins of interest. Detection was performed using an enhanced chemiluminescence system (Thermo Fisher Scientific).
Data analysis
All experiments were performed at least in triplicate, and data are mean ± SD. Data were analyzed using Student t test or analysis of variance followed by Bonferroni test. Differences were considered significant when P < .05.
Results
Severe thrombocytopenia because of increased platelet clearance in FlnAloxP PF4-Cre mice
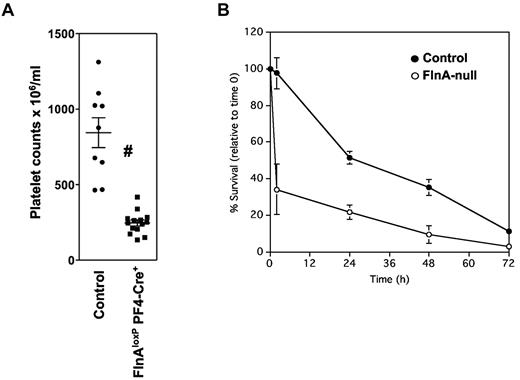
FlnAloxP mice were bred with PF4-Cre mice to excise the FLNA gene in the MK lineage.15 By pairing FlnAloxP PF4-Cre males with FlnAloxP females, 30 (45%) of 67 embryos were FlnAloxP PF4-Cre at embryonic day 14.5, not statistically different from the expected 50%; only few embryos exhibited bleeding with no obvious developmental abnormalities. However, 95 (41%) of 233 newborn mice were FlnAloxP PF4-Cre, statistically lower than the expected 50% (P < .05), indicating increased lethality in late embryogenesis. FlnAloxP PF4-Cre mice had a severe thrombocytopenia (Figure 1A), with 247 ± 74 platelets/μL, compared with 844 ± 296 platelets/μL in control FlnAloxP mice, consistent with our previous observations.10
Platelet counts and clearance. (A) Peripheral platelet counts in control and FlnAloxP PF4-Cre mice. Results represent mean ± SD (n = 9). #P < .05. (B) Average survival of transfused platelets in mice. CMFDA-labeled control and FlnA-null platelets were injected into control mice. Results are the percentage of labeled platelets in the circulation relative to time 1 minute, represent the mean ± SD of 3 mice, and are representative of 3 independent experiments.
Platelet counts and clearance. (A) Peripheral platelet counts in control and FlnAloxP PF4-Cre mice. Results represent mean ± SD (n = 9). #P < .05. (B) Average survival of transfused platelets in mice. CMFDA-labeled control and FlnA-null platelets were injected into control mice. Results are the percentage of labeled platelets in the circulation relative to time 1 minute, represent the mean ± SD of 3 mice, and are representative of 3 independent experiments.
To test whether the thrombocytopenia was the result of increased platelet removal, platelets isolated from FlnAloxP PF4-Cre mice (FlnA-null platelets) were fluorescently labeled with CMFDA and injected into control recipients (Figure 1B). Control FlnAloxP platelets disappeared at a constant rate over time for approximately 72 hours with a half-life time of 36 hours, as described previously.19 In contrast, approximately 60% of labeled FlnA-null platelets were cleared from the circulation 2 hours after injection. After 24 hours, 80% of labeled FlnA-null and 50% of control platelets were removed from the circulation. The results indicate that the thrombocytopenia observed in FlnAloxP PF4-Cre mice is caused by the fast elimination of FlnA-null platelets from the circulation.
Microvesicles in circulation of FlnAloxP PF4-Cre mice after macrophage removal
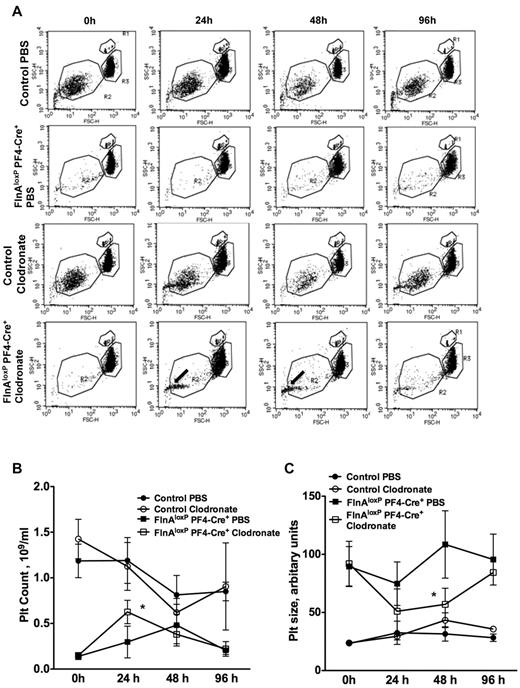
To determine whether macrophages were responsible for the fast removal of FlnA-null platelets, clodronate-encapsulated or control PBS liposomes were intravenously injected into FlnAloxP PF4-Cre and control FlnAloxP mice to deplete macrophages in vivo.16,17 Platelet counts were then followed for 96 hours (Figure 2A). Macrophage removal enhanced the survival of FlnA-null platelets, as platelet counts increased from 290 ± 170 × 103/μL in PBS-treated FlnAloxP PF4-Cre mice to 620 ± 120 × 103/μL in clodronate-treated FlnAloxP PF4-Cre mice (mean ± SD; P < .05; n = 4; Figure 2B).
Macrophage depletion and microvesicle formation. (A) Control and FlnAloxP PF4-Cre mice were injected with PBS- or clodronate-encapsulated liposomes to ablate macrophages. Blood was collected at indicated time points, and platelet count and size were analyzed by flow cytometry. Results are forward/side scatter dot plots of each condition and are representative of 4 independent experiments. Gates represent beads (R1), platelets (R2), and erythrocytes (R3). Note the appearance of a population of small microvesicles in clodronate-treated FlnAloxP PF4-Cre mice (arrows). (B) Platelet counts increase and (C) platelet size decrease in FlnAloxP PF4-Cre mice after clodronate-liposome injection. Platelet size is measured by forward scatter and expressed as arbitrary units. Data represent the mean ± SD (n = 4). *P < .05.
Macrophage depletion and microvesicle formation. (A) Control and FlnAloxP PF4-Cre mice were injected with PBS- or clodronate-encapsulated liposomes to ablate macrophages. Blood was collected at indicated time points, and platelet count and size were analyzed by flow cytometry. Results are forward/side scatter dot plots of each condition and are representative of 4 independent experiments. Gates represent beads (R1), platelets (R2), and erythrocytes (R3). Note the appearance of a population of small microvesicles in clodronate-treated FlnAloxP PF4-Cre mice (arrows). (B) Platelet counts increase and (C) platelet size decrease in FlnAloxP PF4-Cre mice after clodronate-liposome injection. Platelet size is measured by forward scatter and expressed as arbitrary units. Data represent the mean ± SD (n = 4). *P < .05.
Macrophage removal also revealed that FlnA-null platelets microvesiculate in blood. A population of particles smaller than 1 μm appeared in the circulation of FlnAloxP PF4-Cre mice 24 hours after treatment with clodronate-encapsulated liposomes. This population remained in blood 48 hours after injection and diminished at 96 hours. Further, the average platelet size was 57.0 ± 13.8 arbitrary units 48 hours after clodronate treatment, compared with 108.5 ± 28.0 arbitrary units in PBS-treated animals (mean ± SD; P < .05; n = 3), as evidenced by flow cytometry forward scatter. Clodronate treatment of control FlnAloxP mice caused the appearance of a small population of microvesicles after 24 hours. However, this increase was not significant and disappeared after 48 hours. Platelet counts and size remained similar in PBS- and clodronate-treated control FlnAloxP mice throughout the experiment (Figure 2B-C).
Increased megakaryocyte numbers in FlnAloxP PF4-Cre mice
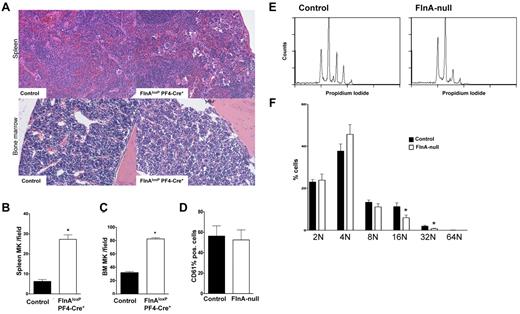
Bone marrow and spleen megakaryopoiesis were further investigated in FlnAloxP PF4-Cre mice. Spleens of FlnAloxP PF4-Cre mice were normal in size compared with control FlnAloxP mice (data not shown). However, paraffin-embedded spleen sections of FlnAloxP PF4-Cre mice had 27 ± 5 MKs per visual field, compared with 6 ± 2 in control FlnAloxP control animals (mean ± SD; P < .001; n = 5), as revealed by H&E staining (Figure 3A-B). Similarly, paraffin-embedded bone marrow sections of FlnAloxP PF4-Cre mice had 83 ± 4 MKs per visual field, compared with 32 ± 3 in FlnAloxP control animals (mean ± SD; P < .001; n = 5; Figure 3A,C). Immunohistochemistry analysis revealed similar platelet numbers in FlnAloxP PF4-Cre and FlnAloxP bone marrows (data not shown).
In vivo and in vitro megakaryopoiesis. (A) Sections of mouse spleen and femur bone marrow of control and FlnAloxP PF4-Cre mice were stained with H&E to reveal megakaryocytes. Sections shown are representative of 5 mice for each genotype. (B-C) Spleen and bone marrow MKs were quantified per field of view. Data represent the mean ± SD of 5 or 6 fields (5 mice for each genotype). *P < .05. (D) Percentage of CD61+ MKs differentiated from fetal livers in control and FlnA-null cultures. Results are expressed as mean ± SD (n = 8). (E) Ploidy analysis of fetal liver-derived control and FlnA-null MKs. Results are representative of 6 independent experiments. (F) Percentage of MK ploidy. Data are mean ± SD (n = 6). *P < .05.
In vivo and in vitro megakaryopoiesis. (A) Sections of mouse spleen and femur bone marrow of control and FlnAloxP PF4-Cre mice were stained with H&E to reveal megakaryocytes. Sections shown are representative of 5 mice for each genotype. (B-C) Spleen and bone marrow MKs were quantified per field of view. Data represent the mean ± SD of 5 or 6 fields (5 mice for each genotype). *P < .05. (D) Percentage of CD61+ MKs differentiated from fetal livers in control and FlnA-null cultures. Results are expressed as mean ± SD (n = 8). (E) Ploidy analysis of fetal liver-derived control and FlnA-null MKs. Results are representative of 6 independent experiments. (F) Percentage of MK ploidy. Data are mean ± SD (n = 6). *P < .05.
Normal MK differentiation in the absence of FlnA
The role of FlnA on the differentiation of MKs from fetal liver cells (FLCs) was further investigated. Cell counts and percentages of CD61+ cells were similar in FlnAloxP PF4-Cre and FlnAloxP cultures (Figure 3D; and data not shown), indicating normal proliferation and commitment from stem cells to MKs in the absence of FlnA. The data are in agreement with increased megakaryopoiesis in the spleen and bone marrow of FlnAloxP PF4-Cre mice. In vitro differentiated FlnA-null MKs had slightly reduced ploidy (Figure 3E-F). The percentages of 16N and 32N containing FlnA-null MKs were decreased by 48% and 61%, respectively, compared with control MKs (n = 6; P < .05).
Altered platelet production in FlnA-null MKs
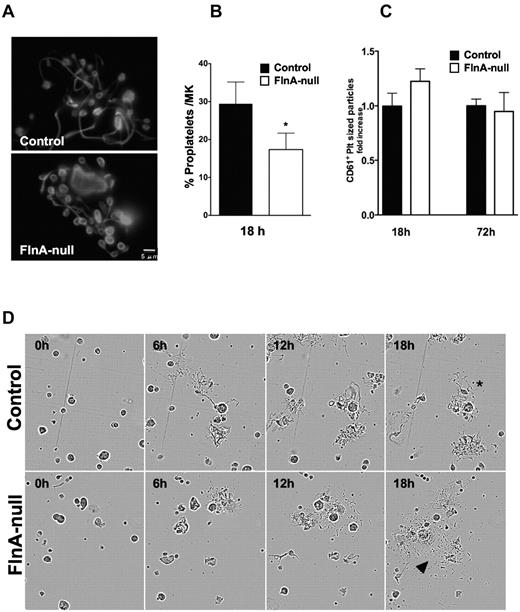
To further investigate the role of FlnA in thrombopoiesis, day 3 FLC-derived FlnA-null and control MKs were cultured for additional 18 and 72 hours and analyzed for proplatelet formation and platelet release. FlnA-null MKs produced proplatelets (Figure 4A). Swellings along the shafts and numbers of branches appeared similar between FlnA-null and control proplatelets. However, a number of FlnA-null proplatelets had enlarged tips compared with controls. The release of CD61+ platelet-sized particles was evaluated at 18 and 72 hours by flow cytometry. At 18 hours, the numbers of proplatelet-forming MKs was reduced to 17% ± 4% (Figure 4B), compared with 29% ± 6% in controls (mean ± SD; P < .005; n = 7), and FlnA-null MKs released 23% ± 2% more platelets than controls (Figure 4C). By 72 hours, FlnA-null and control MKs released similar numbers of platelets.
Altered thrombopoiesis in FlnA-null MKs. (A) Control and FlnA-null MK cultures with proplatelets on day 4 were visualized by immunofluorescence for β-tubulin. Images are representative of 4 different experiments. (B) The number of MKs extending proplatelets was counted 18 hours after BSA gradient (day 4 of culture) and expressed as the percentage of total MKs. Results are mean ± SD (n = 7). *P < .05. FlnA-null MKs have fewer proplatelets than control MKs. (C) Platelets in MK cultures were counted at 18 and 72 hours by flow cytometry. Data are expressed as ratio relative to control platelet counts and are mean ± SD (n = 4). (D) Control and FlnA-null MKs were subjected to time-lapse video microscopy and monitored for proplatelet formation. Frames presented were captured at 0, 6, 12, and 18 hours. Released platelets were observed in FlnA-null MK cultures (arrowheads). *Control cultures mostly contained proplatelet bearing MKs.
Altered thrombopoiesis in FlnA-null MKs. (A) Control and FlnA-null MK cultures with proplatelets on day 4 were visualized by immunofluorescence for β-tubulin. Images are representative of 4 different experiments. (B) The number of MKs extending proplatelets was counted 18 hours after BSA gradient (day 4 of culture) and expressed as the percentage of total MKs. Results are mean ± SD (n = 7). *P < .05. FlnA-null MKs have fewer proplatelets than control MKs. (C) Platelets in MK cultures were counted at 18 and 72 hours by flow cytometry. Data are expressed as ratio relative to control platelet counts and are mean ± SD (n = 4). (D) Control and FlnA-null MKs were subjected to time-lapse video microscopy and monitored for proplatelet formation. Frames presented were captured at 0, 6, 12, and 18 hours. Released platelets were observed in FlnA-null MK cultures (arrowheads). *Control cultures mostly contained proplatelet bearing MKs.
The data suggest that FlnA-null proplatelet-bearing MKs release platelets more rapidly than controls. To further examine this possibility, time-lapse video microscopy was performed to monitor proplatelet formation in the MK cultures (Figure 4D). FlnA-null proplatelets matured more readily into platelets than control proplatelets.
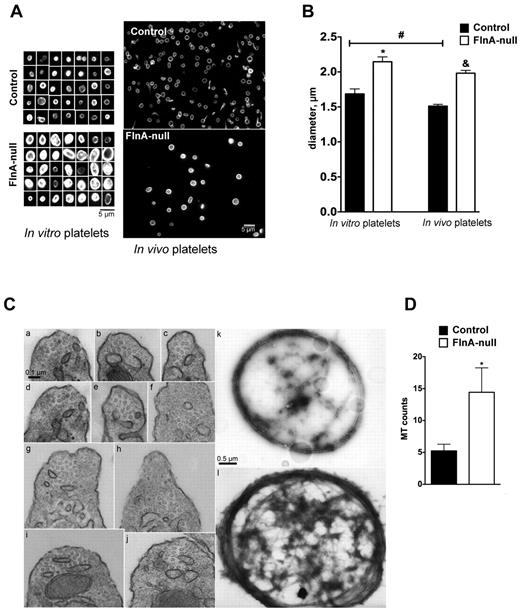
Increased size and microtubule coils in FlnA-null platelets
The size of peripheral blood platelets and in vitro released platelets was measured by the diameters of their microtubule coils after β-tubulin staining (Figure 5A-B). Peripheral blood FlnA-null platelets had a diameter of 1.98 ± 0.33 μm (mean ± SD; P < .05; n = 100 from 3 different experiments), compared with 1.51 ± 0.22 μm for control platelets, consistent with the approximately 2.5-fold increased volume described previously.10 Similarly, FlnA-null platelets released in culture were enlarged, with a diameter of 2.14 ± 0.70 μm, compared with 1.68 ± 0.64 μm for control platelets (mean ± SD; P < .05; n = 100 from 3 different experiments), indicating that FlnA-null MKs aberrantly released enlarged platelets. Although FlnA-null blood platelets appeared smaller than in vitro released platelets, the difference in diameter did not reach statistical significance. On the other hand, control blood platelets were smaller than in vitro released platelets (P < .05).
Microtubule coils in FlnA-null platelets. (A) Size of microtubule coils in control and FlnA-null platelets matured in vitro or in vivo. Microtubule coils were visualized by immunofluorescence for β-tubulin. Images are representative of 4 separate MK cultures and 3 mice. FlnA-null platelets had larger and brighter microtubule coils. (B) Quantification of platelet size. The diameters of microtubule coils were analyzed using MetaMorph Version 7.7 software. (C) Electron micrographs of mouse peripheral blood platelets in transverse sections show the increased number of microtubules in the marginal coils of FlnA-null platelets compared with controls: (i-v) control platelets; (vi-x) FlnA-null platelets. Electron micrographs of negative stained platelets show the increased thickness of the microtubule coils in FlnA-null platelets (xii) compared with control platelets (xi). Representative images of 3 independent experiments are shown. (D) Quantification of the number of microtubule coils in the marginal band in transverse images of thin sections of platelets. Results are mean ± SD (n = 10). *P < .05.
Microtubule coils in FlnA-null platelets. (A) Size of microtubule coils in control and FlnA-null platelets matured in vitro or in vivo. Microtubule coils were visualized by immunofluorescence for β-tubulin. Images are representative of 4 separate MK cultures and 3 mice. FlnA-null platelets had larger and brighter microtubule coils. (B) Quantification of platelet size. The diameters of microtubule coils were analyzed using MetaMorph Version 7.7 software. (C) Electron micrographs of mouse peripheral blood platelets in transverse sections show the increased number of microtubules in the marginal coils of FlnA-null platelets compared with controls: (i-v) control platelets; (vi-x) FlnA-null platelets. Electron micrographs of negative stained platelets show the increased thickness of the microtubule coils in FlnA-null platelets (xii) compared with control platelets (xi). Representative images of 3 independent experiments are shown. (D) Quantification of the number of microtubule coils in the marginal band in transverse images of thin sections of platelets. Results are mean ± SD (n = 10). *P < .05.
Staining of β-tubulin also revealed a brighter marginal band in cultured and peripheral blood FlnA-null platelets (Figure 5A). In agreement with this finding, the microtubule ring of FlnA-null platelets was dramatically thickened in negative stained samples, compared with that of control platelets (Figure 5C). Electron microscopy of transverse thin sections revealed that FlnA-null platelets had 14.4 ± 3.8 microtubule coils, compared with 5.3 ± 1.0 in control platelets (mean ± SD; n = 10; P < .05; Figure 5C-D).
Normal GPIb expression in FlnA-null MKs
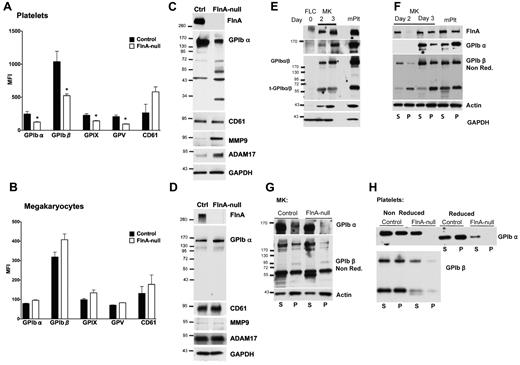
Platelets isolated from FlnAloxP PF4-Cre mice showed reduced surface expression of all VWFR subunits, as well as an increase in CD61, as evidenced by flow cytometric analysis (Figure 6A). We further examined the surface expression of major glycoproteins in maturing control and FlnA-null MKs (Figure 6B). Surprisingly, FlnA-null MKs had normal surface expression of all VWFR subunits compared with controls.
Protein expression in FlnA-null platelets and MKs. FlnA-null and control blood platelets (A) or MKs from day 3 cultures (B) were probed with fluorescein isothiocyanate-labeled antibodies for surface expression of GPIbα, GPIbβ, GPIX, GPV, and CD61 and analyzed by flow cytometry. Results are expressed as mean fluorescence intensity (MFI) and are mean plus or minus SD (n = 4). *P < .05. Expression of VWFR subunits is decreased on FlnA-null blood platelets but normal on FlnA-null MKs. Lysates of control and FlnA-null blood platelets (C) and MKs from day 3 cultures (D) were subjected to SDS-PAGE and probed with antibodies directed against FlnA, GPIbα, CD61, MMP9, and ADAM17; GAPDH was used as loading control. Blots shown are representative of 4 independent experiments. The increased degradation of GPIbα in the FlnA-null platelets correlated with high expression levels of MMP9 and ADAM17. (E) Lysates of mouse FLCs (day 0), immature (day 2), and mature (day 3) MKs and blood platelets (mPlt) were probed for FlnA, GPIbα, GPIbβ, and actin; GAPDH was used as loading control. The anti-GPIbβ antibody detects GPIbβ bound to GPIbα and the membrane-anchored truncated remainder of GPIbα (t-GPIbα) in nonreducing conditions. Maturation of MKs leads to increased expression of FlnA, GPIbα, GPIbβ, and actin. (F) Linkage of GPIb to the MK and platelet cytoskeleton. Triton X-100 soluble (S) and insoluble (P) fractions were collected by centrifugation of control MK and platelet lysates at 100 000g for 30 minutes at 4°C and probed for FlnA, GPIbα, and GPIbβ (in nonreducing conditions) and actin. At day 3, FlnA, GPIbα, and GPIbβ are tethered to F-actin in control MKs and platelets. GPIb is not tethered to the actin cytoskeleton of FlnA-null MKs (G) or platelets (H). Blots shown are representative of 3 independent experiments.
Protein expression in FlnA-null platelets and MKs. FlnA-null and control blood platelets (A) or MKs from day 3 cultures (B) were probed with fluorescein isothiocyanate-labeled antibodies for surface expression of GPIbα, GPIbβ, GPIX, GPV, and CD61 and analyzed by flow cytometry. Results are expressed as mean fluorescence intensity (MFI) and are mean plus or minus SD (n = 4). *P < .05. Expression of VWFR subunits is decreased on FlnA-null blood platelets but normal on FlnA-null MKs. Lysates of control and FlnA-null blood platelets (C) and MKs from day 3 cultures (D) were subjected to SDS-PAGE and probed with antibodies directed against FlnA, GPIbα, CD61, MMP9, and ADAM17; GAPDH was used as loading control. Blots shown are representative of 4 independent experiments. The increased degradation of GPIbα in the FlnA-null platelets correlated with high expression levels of MMP9 and ADAM17. (E) Lysates of mouse FLCs (day 0), immature (day 2), and mature (day 3) MKs and blood platelets (mPlt) were probed for FlnA, GPIbα, GPIbβ, and actin; GAPDH was used as loading control. The anti-GPIbβ antibody detects GPIbβ bound to GPIbα and the membrane-anchored truncated remainder of GPIbα (t-GPIbα) in nonreducing conditions. Maturation of MKs leads to increased expression of FlnA, GPIbα, GPIbβ, and actin. (F) Linkage of GPIb to the MK and platelet cytoskeleton. Triton X-100 soluble (S) and insoluble (P) fractions were collected by centrifugation of control MK and platelet lysates at 100 000g for 30 minutes at 4°C and probed for FlnA, GPIbα, and GPIbβ (in nonreducing conditions) and actin. At day 3, FlnA, GPIbα, and GPIbβ are tethered to F-actin in control MKs and platelets. GPIb is not tethered to the actin cytoskeleton of FlnA-null MKs (G) or platelets (H). Blots shown are representative of 3 independent experiments.
In FlnA-null platelet lysates, total expression of GPIbα was reduced and additional bands of 65, 50, 45, and 30 kDa were observed (Figure 6C), indicative of increased degradation. On the other hand, CD61 levels were similar in control and FlnA-null platelets. In MK lysates, no difference between control and FlnA-null cells was detected for total GPIbα and CD61 expression (Figure 6D). The normal expression of GPIbα in FlnA-null MKs followed by its degradation in platelets indicates that FlnA stabilizes GPIbα in platelets but is not required for its surface expression in MKs.
Because ADAM17 cleaves GPIbα in platelets20 and FlnA absence leads to MMP9 overexpression in human melanoma cells,21 the expression of these 2 metalloproteinases was investigated in platelets and MKs. Expression of both ADAM17 and MMP9 was increased in FlnA-null platelets, compared with controls (Figure 6C), but normal in FlnA-null MKs (Figure 6D).
Formation of the FlnA-GPIb linkage in megakaryocytes
In platelets FlnA crosslinks GPIbα to underlying actin filaments. We investigated when this linkage is made in cultured MKs. FLCs (day 0) were cultured to obtain MKs at different stages of maturation. Expression of GPIb was analyzed by immunoblot as a single subunit by using an anti-GPIbα antibody in reducing conditions or as part of the VWFR complex by using an anti-GPIbβ antibody in nonreducing conditions.22 FlnA, GPIbα, GPIbβ, and actin expression increased dramatically as MKs matured, with maximal expression at day 3 (Figure 6E), when MKs are of large size with a 2 to 32N ploidy, just before making proplatelets.18
At day 2, when MKs are of intermediate size with a 2 to 16N ploidy, both FlnA and GPIb were present mostly in the detergent-soluble fraction of cultured control MKs, indicating no association with the actin cytoskeleton (Figure 6F). At day 3, both FlnA and GPIb tethered with F-actin, as in control blood platelets. In similar conditions, GPIb failed to associate to the MK and platelet actin cytoskeletons in the absence of FlnA (Figure 6G-H). However, analysis in nonreducing conditions revealed that GPIb subunits were capable of forming a complex in MKs and platelets, independently of FlnA presence. Distribution of G- and F-actin was similar in control and FlnA-null MKs in all stages studied.
Microvesiculation of FlnA-null platelets
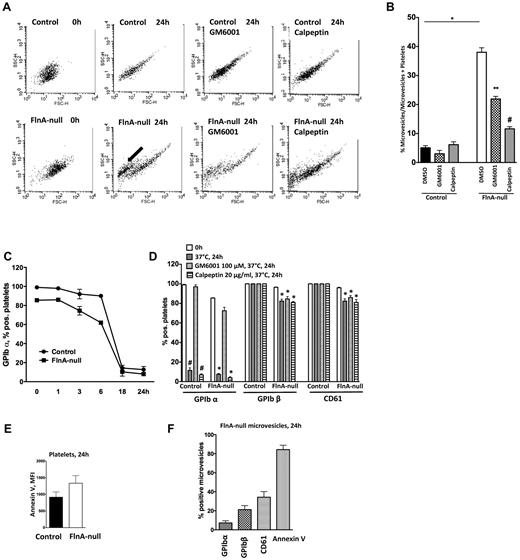
The emergence of microvesicles in the circulation of FlnAloxP PF4-Cre mice after clodronate treatment prompted us to investigate the membrane stability of FlnA-null platelets. Washed control and FlnA-null platelets were incubated at 37°C for 24 hours and samples were analyzed by flow cytometry at different time points (Figure 7A-B). Microvesicles appeared in FlnA-null samples, composing 38% ± 6% of all events after 24 hours of incubation at 37°C, compared with 5.1% ± 2.6% in control samples (P < .001; n = 10).
Microvesiculation of FlnA-null platelets. (A) Control or FlnA-null platelets were stored at 37°C for 24 hours with or without metalloproteinase inhibitor GM6001 (100μM), calpain inhibitor calpeptin (20 μg/mL), or DMSO as control, and analyzed by flow cytometry. Forward/side scatter dot plots shown are representative of 4 independent experiments. FlnA-null platelets release microvesicles after 24 hours of storage at 37°C (arrow). (B) Quantification of microvesicle formation. Results represent the percentage of microvesicle events over all events (microvesicles and platelets) and are mean ± SD (n = 4). *P < .05. (C) Expression of GPIbα on the surface of control and FlnA-null platelets stored for 24 hours at 37°C was analyzed by flow cytometry. GPIbα expression decreased on both control and FlnA-null platelets. (D) Effect of GM6001 and calpeptin on the surface expression of GPIbα on control and FlnA-null platelets. Data are expressed as percentage of positive platelets and are mean ± SD (n = 4). #P < .05 versus 0 hour control platelets. *P < .05 versus 0 hour FlnA-null platelets. (E) PS exposure was evaluated on control and FlnA-null platelets stored for 24 hours at 37°C by annexin V binding assay in flow cytometry. Results are expressed as MFI and are mean ± SD (n = 4). (F) FlnA-null microvesicles were analyzed for glycoprotein expression (GPIbα, GPIbβ, and CD61) and PS exposure (annexin V) at 24 hours. Results are expressed as percentage of positive microvesicles and are mean ± SD (n = 4).
Microvesiculation of FlnA-null platelets. (A) Control or FlnA-null platelets were stored at 37°C for 24 hours with or without metalloproteinase inhibitor GM6001 (100μM), calpain inhibitor calpeptin (20 μg/mL), or DMSO as control, and analyzed by flow cytometry. Forward/side scatter dot plots shown are representative of 4 independent experiments. FlnA-null platelets release microvesicles after 24 hours of storage at 37°C (arrow). (B) Quantification of microvesicle formation. Results represent the percentage of microvesicle events over all events (microvesicles and platelets) and are mean ± SD (n = 4). *P < .05. (C) Expression of GPIbα on the surface of control and FlnA-null platelets stored for 24 hours at 37°C was analyzed by flow cytometry. GPIbα expression decreased on both control and FlnA-null platelets. (D) Effect of GM6001 and calpeptin on the surface expression of GPIbα on control and FlnA-null platelets. Data are expressed as percentage of positive platelets and are mean ± SD (n = 4). #P < .05 versus 0 hour control platelets. *P < .05 versus 0 hour FlnA-null platelets. (E) PS exposure was evaluated on control and FlnA-null platelets stored for 24 hours at 37°C by annexin V binding assay in flow cytometry. Results are expressed as MFI and are mean ± SD (n = 4). (F) FlnA-null microvesicles were analyzed for glycoprotein expression (GPIbα, GPIbβ, and CD61) and PS exposure (annexin V) at 24 hours. Results are expressed as percentage of positive microvesicles and are mean ± SD (n = 4).
Because ADAM17 cleaves GPIbα during platelet storage20 and calpain is a major protease involved in microvesiculation,23 we used a broad-spectrum inhibitor of metalloproteinases (GM6001, 100μM) and calpeptin (20 μg/mL) to investigate the role of these proteases in the microvesiculation of FlnA-null platelets (Figure 7A-B). The percentage of microvesicles in FlnA-null samples was reduced to 21.2% ± 2.2% (P < .05; n = 5) and 11.6% ± 1.2% (P < .001; n = 3) of all events by GM6001 and calpeptin, respectively. Together, the results show that FlnA-null platelets have increased membrane instability and spontaneously microvesiculate, a process that depends partially on metalloproteinases and principally on calpain.
Glycoprotein expression and phosphatidylserine exposure on FlnA-null microvesicles
The expression of major glycoproteins on the surface of platelets and microvesicles 24 hours after storage at 37°C was further analyzed by flow cytometry. Although all control platelets expressed GPIbα at rest, 15% of FlnA-null platelets were GPIbα− (Figure 7C-D). GPIbα surface expression decreased with similar kinetics and was similarly rescued by GM6001 in control and FlnA-null platelets. Although all control platelets preserved GPIbβ and CD61 on their surface 24 hours after storage at 37°C, expression of these 2 glycoproteins was decreased in FlnA-null samples to 82.5% ± 2.0% (P < .05; n = 4) and 82.3% ± 4.0% (P < .05; n = 4), respectively, and GM6001 had little effect. In similar conditions, calpeptin had no effect on the loss of GPIbα, GPIbβ, and CD61 expression.
Both control and FlnA-null platelets were annexin-V+, a marker of PS exposure 24 hours after storage at 37°C (Figure 7E). Whereas 84.2% ± 9.1% (mean ± SD; n = 4) of FlnA-null microvesicles were annexin V+ (Figure 7F), only 7.2% ± 4.4% were GPIbα+, 21.2% ± 8.3% were GPIbβ+, and 34.2% ± 11.8% were CD61+, Thus, the formation of microvesicles in FlnA-null platelet samples parallels with the loss of surface GPIbα, GPIbβ, and CD61, and the appearance of surface PS, events, which are further amplified on produced microvesicles.
Discussion
FlnAloxP PF4-Cre mice have a severe macrothrombocytopenia that results from the formation of large and fragile platelets that are rapidly removed from the circulation by macrophages. FlnAloxP PF4-Cre mice compensate for these deficiencies by increasing bone marrow and spleen megakaryopoiesis and by prematurely releasing enlarged platelets.
FlnA deficiency is lethal in mice by embryonic day 14.5.24 Here we used the PF4-Cre transgenic mouse to specifically inactivate the FLNA gene in the MK lineage.15 FlnAloxP PF4-Cre mice were viable and fertile, and their platelets and MKs did not contain FlnA. Although an increased lethality in late embryogenesis was observed, the high numbers of FlnAloxP PF4-Cre animals obtained strikingly contrasted with our previous GATA1-Cre model, in which only few FlnAloxP GATA1-Cre mice survived gestational stage.10 Recent studies have shown that the embryonic lymphatic vascular development is regulated by the platelet C-type lectin-like receptor 2 signaling cascade,25 a cascade that is impaired in FlnA-null platelets.10 It is probable that low counts of poorly C-type lectin-like receptor 2-responsive FlnA-null platelets contribute to the increased lethality associated with FlnA deficiency.
FlnAloxP PF4-Cre mice had a severe macrothrombocytopenia, similar to Bernard-Soulier syndrome patients or to GPIbα-null, GPIbβ-null, or FlnAloxP GATA1-Cre mice.10,26,27 Large platelets and low platelet counts have been described in some, but not all, periventricular heterotopia patients with FLNA mutations.28-30 It is probable that only mutations that cause loss of the FlnA-GPIbα linkage lead to morphologic platelet defects. However, a significant platelet phenotype may be hindered in females with only one mutant allele, the vast majority of periventricular heterotopia patients with FLNA mutations, as FLNA is X-linked and platelets expressing full-length FlnA are expected to have a selective advantage in vivo. This notion is supported by our previous findings that female mice carriers for FlnA deficiency only have a mild thrombocytopenia with 95% of their circulating platelets expressing FlnA, significantly higher than the expected 50%.10 Thus, careful analysis is required to determine whether large platelets and low platelet counts are a common feature or rarely occur in patients with defective FlnA.
The thrombocytopenia of FlnAloxP PF4-Cre mice resulted from the increased removal of FlnA-null platelets by macrophages, as in vivo macrophage ablation by clodronate-encapsulated liposomes increased their blood platelet counts. Microvesicles also appeared in the intravascular circulation of FlnAloxP PF4-Cre mice following clodronate treatment or during the storage of FlnA-null platelets at 37°C for 24 hours. Together, the data show that FlnA-null platelets are fragile and spontaneously microvesiculate, resulting in their rapid elimination in vivo. The data are consistent with previous observations, as platelets isolated from Bernard-Soulier syndrome patients are fragile and more readily form microvesicles than controls.31,32 Further, loss of the FlnA-GPIbα connection leads to disintegration of the platelet cell body under shear.33 Thus, the FlnA-GPIbα connection stabilizes platelets by preventing microvesiculation.
FlnAloxP PF4-Cre mice had increased megakaryopoiesis in their bone marrow and spleen, consistent with other thrombocytopenic models, such as the GPIbβ-null or WASp-null mice,34,35 and in agreement with an increased demand in platelet production because of low blood platelet counts. Cultured FlnA-null MKs had slightly decreased ploidy and prematurely released enlarged platelets, compared with controls. The experiments compared proplatelet production in nonadherent MKs derived from fetal liver cells. Whether interactions with the matrix environment could alter the kinetics of proplatelet production in FlnA-null MKs derived from bone marrow cells remains to be investigated.36 Identical numbers of CD61+ cells developed from control and FlnA-null fetal liver cells in vitro. The results point that FlnA is required for late thrombopoieisis but is dispensable for MK differentiation from embryonic stem cells. Other filamin isoforms, such as FlnB, may compensate for FlnA absence in early megakaryopoiesis.37
Control proplatelets released platelets that were larger than blood platelets, consistent with the model that platelet formation is finalized in the circulation.38 An intermediate stage between proplatelet and platelet, called preplatelet, was recently defined in vitro.39 Preplatelets can reversibly transform to barbell shapes that fission into platelets in the circulation. FlnA-null cultured and blood platelets were of similar size, but larger in diameter, than control platelets. Further, FlnAloxP PF4-Cre mice had very few barbell-shaped preplatelets in their circulation (data not shown). Thus, enlarged FlnA-null platelets resemble preplatelets that are unable to form barbells and do not undergo the final fission reaction that shrinks the platelet volume and doubles the platelet number.
FlnA-null platelets had increased microtubular mass, a phenotype observed in giant platelets isolated from patients with May-Hegglin anomaly, Epstein syndrome, and gray platelet syndrome or from GPIbβ-null mice.34,40 Similar to GPIbβ-null platelets, the majority of FlnA-null platelets exhibited a discoid shape and had 2- to 3-fold increased number of micotubules in their coils, compared with 10- to 20-fold in May-Hegglin anomaly and Epstein syndrome, in which the majority of platelets are spherical.34,40 The marginal band provides major structural support for the platelet discoid shape.41,42 Increased microtubules in FlnA-null platelets may represent a compensation mechanism by which defective platelets fortify their delicate structure or more likely may represent a condensed microtubule motor that would normally be dispersed into 2 or more platelets.
One of the key interactions that provide stability to the platelet membrane is the FlnA-GPIbα linkage to the membrane skeleton. Normal platelets have approximately 12 500 of these FlnA-GPIbα connections.43 Our studies are the first to address the assembly of this connection in MKs, in addition to further demonstrating its importance in stabilizing the resting platelet. Production of both FlnA and components of the VWFR began in earnest on day 2 in culture and increased dramatically just before proplatelet elaboration. GPIbα became linked to the cytoskeleton on day 3, albeit at levels somewhat lower than in platelets, indicating that the FlnA-GPIbα linkage has been consummated at this time point. As expected, incorporation of the VWFR into the cytoskeleton was dependent on FlnA because no association was observed in FlnA-null cells. In platelets, FlnA anchors the cytoplasmic tail of GPIbα to the actin cytoskeleton and FlnA deficiency leads to disorganized GPIbα topology and macrothombocytopenia.10
Surface expression of all VWFR subunits was reduced in FlnA-null platelets, and surprisingly 15% of them did not have detectable GPIbα, although FlnA-null MKs had normal surface VWFR expression. The continuous synthesis of VWFR subunits in MKs with multiple gene copies may explain this difference in VWFR surface expression between platelets and MKs, as VWFR synthesis is reduced and its removal is increased in platelets because it is not stabilized by the FlnA-actin linkage. Alternatively, post-translational modifications of GPIbα, such as its glycosylation, could occur at later stages, in proplatelets or platelets. Defects of post-translational modifications in the absence of FlnA could lead to decreased VWFR stability. Normal GPIbα expression in MKs demonstrates that FlnA is not necessary for surface expression of GPIbα in MKs, in agreement with previous studies in CHO cells.44 In this system, a portion of GPIbα unbound to FlnA was directed to the cell surface but was more prone to proteolysis.
The increased degradation of the VWFR in FlnA-null platelets is probably related to increased expression of the ADAM17 and MMP9 metalloproteinases. ADAM17 is a major sheddase for GPIbα,20 and the cytoplasm and α-granules of platelets contain MMP9.45 The broad-range metalloproteinase inhibitor GM6001 preserved GPIbα expression on the surface of FlnA-null platelets stored 24 hours at 37°C, as did a selective MMP9 inhibitor (data not shown). How FlnA-null platelets increase ADAM17 and MMP9 expression and GPIbα cleavage is unclear. ADAM17 is activated via a p38 MAPK-dependent pathway during platelet storage and FlnA down-regulates MMP9 transcription by negatively modulating the ERK pathway in melanoma cells.21,46 Whether increased ADAM17 and MMP9 expression is the result of potentiated p38 MAPK and ERK pathways in FlnA-null cells remains to be determined.
It appears that loss of surface receptors is continued once FlnA-null platelets microvesiculate because only some microvesicles were CD61+ and GPIbβ+, although all had exposed PS. These processes were not because of increased platelet activation, as only a small percentage of platelets activated their αIIbβ3 integrin or exposed P-selectin (data not shown). Calpain inhibition almost completely blocked microvesiculation of FlnA-null platelets while metalloproteinase inhibition was partially effective. How exactly calpain mediates microvesiculation in the absence of FlnA in platelets remains to be established.
In conclusion, the macrothrombocytopenia associated with FlnA deficiency results from decreased survival and ineffective platelet production because of platelet instability. FlnA-null proplatelets rapidly release large platelets that further fragment and are removed from circulation by macrophages.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sarah Weber and Terese Jönsson for technical help and Drs Joseph Italiano and François Maignen for helpful discussion.
This work was supported by the National Institutes of Health (grant HL-056252, J.H.H.; grant HL-089224, K.M.H.; grant HL-056949, J.H.H. and K.M.H.; and grant HL-059561, J.H.H. and H.F.).
National Institutes of Health
Authorship
Contribution: A.J.B. designed and performed research, analyzed data, and drafted the manuscript; K.M.H., J.H.H., and H.F. designed and performed research and analyzed data; and all authors contributed to the writing of the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hervé Falet, Division of Translational Medicine, Brigham and Women's Hospital, One Blackfan Circle, Karp 6, Boston, MA 02115; e-mail: hfalet@rics.bwh.harvard.edu.