Abstract
Microparticles (MPs) are shed from activated and dying cells. They can transmit signals from cell to cell, locally or at a distance through the circulation. Monocytic MPs are elevated in different diseases, including bacterial infections. Here, we investigated how monocytic MPs activate endothelial cells. We found that MPs from lipopolysaccharide (LPS)–treated THP-1 monocytic cells bind to and are internalized by human endothelial cells. MPs from LPS-treated THP-1 cells, but not untreated cells, induced phosphorylation of ERK1/2, activation of the nuclear factor-κB pathway and expression of cell adhesion molecules intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin. Similar results were observed using MPs from LPS-treated peripheral blood mononuclear cells. We next investigated the mechanism by which monocytic MPs activated endothelial cells and found that they contain IL-1β and components of the inflammasome, including apoptosis-associated speck-like protein containing a CARD, caspase-1, and NLRP3. Importantly, knockdown of NLRP3 in THP-1 cells reduced the activity of the MPs and blockade of the IL-1 receptor on endothelial cells decreased MP-dependent induction of cell adhesion molecules. Therefore, monocytic MPs contain IL-1β and may amplify inflammation by enhancing the activation of the endothelium.
Introduction
Microparticles (MPs) are small (100-1000 nm) membrane-bound bodies that are released from cells during activation or cell death.1 A crucial step in the MP formation is the loss of plasma membrane asymmetry leading to the exposure of phosphatidylserine (PS).1 PS on the MPs allows their detection by flow cytometry using annexin V. In addition, flow cytometry can be used to determine the cell type that released the MPs because MPs possess cell surface markers of their cell origin. In addition to membrane-bound cell surface receptors, MPs can also contain mRNA, microRNA, cytokines, and growth factors.2 Indeed, it was shown that endothelial cells incubated with MPs derived from cells expressing mRNA encoding green fluorescent protein subsequently expressed green fluorescent protein.3 Thus, MPs can act as mediators of cell to cell communication, either locally or at a distance via the circulation.
Although platelets are the primary source of MPs in the circulation under normal conditions, MPs released by monocytes are increased during experimental human and mouse endotoxemia4,5 and systemic bacterial infections,6 as well as other diseases.7-11 It is thought that these monocyte MPs may contribute to disseminated intravascular coagulation, which often occurs during sepsis. The highly procoagulant nature of MPs is probably the result of the exposure of PS on the MP surface and the expression of tissue factor, the primary activator of the extrinsic coagulation cascade. Interestingly, elevated numbers of CD14-positive, tissue factor-positive MPs were found in a septic patient with disseminated intravascular coagulation.6
Elevated proinflammatory cytokine production also occurs during endotoxemia and sepsis. One important proinflammatory cytokine up-regulated in response to bacterial infection and lipopolysaccharide (LPS) stimulation is IL-1β. IL-1β is unusual because it does not contain an N-terminal signal sequence for secretion and therefore must be released from the cell via an alternative mechanism. In addition, it is synthesized as a larger precursor protein that must be cleaved into the active cytokine. Cleavage is mediated by an active inflammasome. The LPS-activated inflammasome contains the nucleotide-binding domain, leucine rich repeat containing protein (NLR) NLRP3, an adaptor molecule known as apoptosis-associated speck-like protein containing a CARD (ASC), and caspase-1.12 The current model suggests that LPS induces a conformational change in NLRP3 that allows interaction with ASC, via homotypic pyrin domain interactions.13 Importantly, it has previously been shown that IL-1β can be packaged and released in MPs and that this process requires adenosine triphosphate activation of P2 × 7, a receptor required for inflammasome activation and IL-1β release.14
Proinflammatory cytokines, such as IL-1β and TNF-α, induce the expression of cell adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin, on the endothelium. The expression of these cell adhesion molecules facilitates binding of leukocytes to activated endothelium, which is critical for a functional immune response.15
Previous studies have examined the role of both platelet MPs and leukocyte MPs in the activation of endothelial cells. Platelet MPs generated by shear stress induce the expression of ICAM-1 on endothelial cells, but the underlying mechanism is unclear.16 In addition, MPs from collagen-stimulated platelets have been shown to contain IL-1β and these MPs amplify inflammation in an arthritis model.17 Mesri and Altieri18,19 have shown that blood-derived MPs, probably neutrophil-derived MPs, activate endothelial cells and induce the expression of tissue factor and cytokines, such as IL-6 and MCP-1. Aharon et al20 found that monocyte MPs, which were generated by costimulation with LPS and calcium ionophore, could interact with endothelial cells, induce apoptosis, and induce tissue factor expression. Importantly, the mechanism of endothelial cell activation by MPs is not fully understood.
In this study, we investigated how monocytic MPs activate human umbilical vein endothelial cells (HUVECs). We found that LPS stimulation increased the amount of MPs released by human THP-1 monocytic cells and that these monocytic MPs interacted with endothelial cells. Importantly, we demonstrated that monocytic MPs from LPS-treated cells, but not MPs from untreated cells, induced phosphorylation of intracellular signaling molecules and expression of cell adhesion molecules by endothelial cells. Similar results were observed using MPs derived from LPS-treated peripheral blood mononuclear cells (PBMCs). We also demonstrated that pro- and active-IL-1β, as well as inflammasome components, ASC, caspase-1, and NLRP3, were found in MPs derived from LPS-treated THP-1 cells. Finally, we showed that knockdown of NLRP3 in THP-1 cells reduced the activity of the MPs and inhibition of the IL-1 receptor on endothelial cells partially blocked the MP-dependent induction of cell adhesion molecules.
Methods
Reagents
LPS (Escherichia coli serotype O111:B4), MG-132, and Bay11–7082 were obtained from Sigma-Aldrich. Polymyxin B sulfate was obtained from Fluka. A mouse monoclonal antibody against phospho-ERK1/2 (sc-7383), rabbit polyclonal antibodies against human E-selectin (sc-14 011), ICAM-1 (sc-7891), VCAM-1 (sc-8304), IL-1β (sc-7884), caspase-1 (sc-514), HDAC2 (sc-7899), p65 (sc-372), and goat polyclonal antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH; sc-48167) were obtained from Santa Cruz Biotechnology. Rabbit polyclonal antibodies against ERK1/2 (#9102), phospho-p65 (#3031), and IκB-α (#9242) were from Cell Signaling. A rabbit polyclonal antibody (ALX-210–905) against ASC was obtained from Enzo Diagnostics. A mouse monoclonal antibody (AG-20B-0014-C100) against NLRP-3 was obtained from Axxora Life Sciences. A human IL-1 receptor antagonist (Kineret) was purchased from Amgen. A small-molecule inhibitor of Toll-like receptor-4–mediated signaling, TAK-242 (CLI-095), was obtained from InvivoGen. Recombinant human TNF-α was purchased from R&D Systems.
Cell culture
HUVECs were purchased from VEC Technologies and cultured as previously described21 and used at passage ≤ 5. The human monocytic leukemia cell line (THP-1) was purchased from ATCC (TIB-202) and cultured as previously described.22 Generation of shNLRP3 THP-1 and empty vector cells has been previously described.23 PBMCs were isolated as previously described.24
Microparticle isolation
THP-1 cells and PBMCs (1 × 106/mL) were treated with or without 5 μg/mL of LPS for 24 hours in culture media as described in “Cell culture.” The cells were centrifuged at 500g for 5 minutes at room temperature. The supernatant was collected and centrifuged at 1500g for 15 minutes to pellet the cellular debris. The supernatant containing MPs was centrifuged at 20 000g for 15 minutes at 4°C to pellet the MPs. MPs were washed twice with PBS and resuspended in PBS and stored at −20°C until use. The amount of MPs was quantified by the total protein concentration measured using a Dc protein Assay kit (Bio-Rad).
Endotoxin detection in the MP samples
Levels of endotoxin in MPs were detected using a ToxinSensor Chromogenic Limulus Amebocyte Lysate Endotoxin Assay Kit obtained from GenScript.
IL-1β and TNF-α enzyme-linked immunosorbent assay
Levels of human IL-1β and TNF-α in MP lysate or cell culture medium were measured using Duoset ELISA Kits (DY201and DY210, R&D Systems).
Flow cytometry
THP-1 cells (1 × 106/mL) were stimulated with or without LPS (5 μg/mL) in a 24-well plate. MPs were isolated as described in “Microparticle isolation” and stained with allophycocyanin annexin V (BD Biosciences PharMingen) in Apop Buffer (5mM KCl, 1mM MgCl2, 136mM NaCl, 2mM CaCl2, 1% BSA, pH 7.4) or without CaCl2 as a negative control for annexin V binding for 30 minutes at room temperature in the dark. A total of 400 μL of Apop Buffer was added to each sample, and then samples were centrifuged at 20 000g for 15 minutes at 4°C. The supernatant was removed, and MPs were resuspended in 400 μL of Apop Buffer containing 10 μL of Flow Count Beads (Beckman Coulter). Forward scatter by side scatter MP gate was determined using mega-mix beads per the International Society on Thrombosis and Hemastasis protocol.25 Samples were analyzed using a LSRII flow cytometer (BD Biosciences) and FlowJo (Version 7.6.4) software.
Western blot
HUVECs were cultured in 6-well plates. Confluent HUVECs were serum-starved for 4 hours and then incubated with MPs (0-300 μg/mL of total protein), TNF-α (10 ng/mL), or LPS (1 or 10 μg/mL) for the indicated time in a serum-free M199 medium. For inhibitor experiments, HUVECs were preincubated with MG-132 (5 or 10μM), Bay11–7082 (5 or 10μM), polymyxin B (10 or 20 μg/mL), TAK-242 (1 μg/mL), or Kineret (1-100 ng/mL) for 30 minutes before the addition of MPs. HUVECs were then washed twice with ice-cold PBS, and 100 μL of cell lysis buffer (20mM Tris-HCl, 150mM NaCl, 1mM EDTA, 1mM ethyleneglycoltetraacetic acid, 1% Triton, 2.5mM sodium pyrophosphate, 1mM β-glycerophosphate, 1mM Na3VO4, and 1 mg/mL leupeptin, pH 7.5) was added. HUVEC nuclear or cytosolic protein was prepared using a nuclear extraction kit (Affymetric-Panomics). HUVECs or MP lysates were mixed with 6× Laemmli buffer and boiled for 5 minutes, then separated on Novex 4% to 10% or 4% to 20% Tris-glycine gels and transferred to polyvinylidene difluoride membranes (Millipore). Membranes were blocked for 1 hour at room temperature with Odyssey blocking buffer (LI-COR Biosciences). Primary antibodies were incubated overnight at 4°C. After washing 3 times with PBS containing 0.1% Tween-20, membranes were incubated with fluorescence-labeled secondary antibodies (1:15 000 dilution) for 1 hour at room temperature. Membranes were then washed 3 times and scanned using an Odyssey Infrared Imaging System (LI-COR Biosciences).
Binding of microparticles with endothelial cells
HUVECs were cultured in 96-well plates and serum-starved for 4 hours in M199 media. MPs were isolated from LPS-treated THP-1 cells as described in “Microparticle isolation.” Isolated MPs were stained with 1μM calcein AM (Invitrogen) in PBS for 40 minutes at 37°C, then washed twice with PBS. MPs were resuspended in PBS and incubated with HUVECs in M199 media for 0.5 to 4 hours at 37°C. After washing once with PBS, fluorescence was measured at 490 nm using a SpectraMax M5 plate reader (Molecular Devices). Background fluorescence of cells alone was subtracted. In addition, HUVECs were stained with calcein Orange (Invitrogen) and grown on culture slides (BD Biosciences). Calcein AM-labeled MPs were incubated with HUVECs for 4 hours at 37°C. After washing 4 times with PBS, HUVECs were stained with 4,6 diamidino-2-phenylindole. Images were captured by confocal microscopy using an Olympus Fluoview 1000 microscope and analyzed using Olympus Fluoview Version 2.0 viewer and Image J (Version 1.43) software.
Real-time PCR
HUVECs were stimulated as described in “Western blot.” Cells were then lysed in RLT buffer (QIAGEN), and RNA was isolated using RNeasy plus mini kit (QIAGEN). RNA was quantified, and equal amounts were reverse-transcribed into cDNA using the RETROscript Kit (Applied Biosystems) according to the manufacturer's instructions. Levels of mRNAs were analyzed by real-time PCR using RealMasterMix and realplex2 Mastercycler (Eppendorf). Real-time primers and probes for human Icam-1, Vcam-1, and E-selectin were obtained from IDT. Amplification of 18S rRNA was used to correct for variations in input RNA levels and efficiencies of the reactions. All experiments are expressed as relative expression normalized to untreated HUVECs.
Statistical analysis
Data are expressed as mean ± SD. Differences between different groups were analyzed using a Student t test, 1- or 2-way ANOVA, (Dunnett post-test) where appropriate as indicated in the figure legend. P < .05 was considered statistically significant.
Results
MPs from LPS-treated THP-1 cells bind to endothelial cells
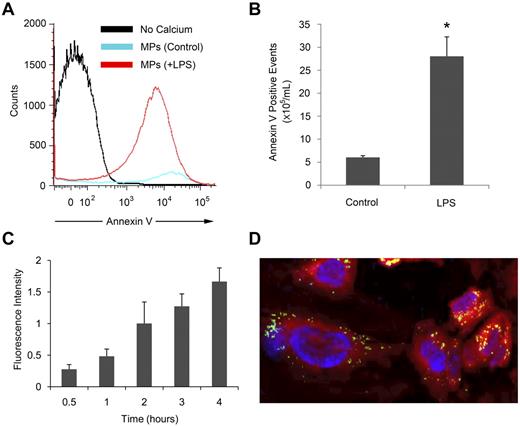
We used a monocytic cell line, THP-1, to generate MPs in vitro. Flow cytometry analysis was performed to measure the number of annexin V-positive MPs in the cell culture medium from untreated or LPS-treated THP-1 cells (Figure 1A). We observed a 6-fold increase in annexin V–positive MPs released from LPS-treated compared with untreated THP-1 cells (Figure 1B). To explore the biologic function of these MPs, MPs from LPS-treated THP-1 cells were labeled with calcein AM, and binding to HUVECs was quantified using a fluorescent plate reader. The fluorescence intensity increased in a time-dependent manner (Figure 1C), which indicates increasing numbers of MPs binding to the cells. A similar increase in binding of MPs from untreated THP-1 cells was observed (data not shown). Confocal microscopy showed that after MPs bind to HUVECs they are internalized and appear in the cytoplasm in the same 1-μm section as the nucleus at 4 hours (Figure 1D).
MPs released by LPS-treated THP-1 cells bind to endothelial cells. MPs were isolated from THP-1 cells left untreated or treated with 5 μg/mL LPS for 24 hours. (A) MPs were stained with annexin V with or without calcium and detected by flow cytometry. (B) Annexin V-positive events per milliliter were calculated using a known concentration of flow count beads. (C) MPs from LPS-treated THP-1 cells were labeled with calcein AM and incubated with HUVECs for the indicated times. Fluorescence intensity was measured using a fluorescent plate reader and shown after the subtraction of the background signal of HUVECs alone. (D) Calcein AM-labeled MPs (green) from LPS-treated THP-1 cells were incubated with calcein orange and 4,6 diamidino-2-phenylindole (blue nuclei)-labeled HUVECs for 4 hours and observed in a 1-μm section by confocal microscopy at room temperature using an Olympus Fluoview 1000 with 40× lens. The image was acquired using Olympus Fluoview Version 2.0 viewer and processed using Image J software. Results are representative of at least 3 independent experiments (n = 3 for panels A and B; n = 6 for panel C). *P < .05 (Student t test).
MPs released by LPS-treated THP-1 cells bind to endothelial cells. MPs were isolated from THP-1 cells left untreated or treated with 5 μg/mL LPS for 24 hours. (A) MPs were stained with annexin V with or without calcium and detected by flow cytometry. (B) Annexin V-positive events per milliliter were calculated using a known concentration of flow count beads. (C) MPs from LPS-treated THP-1 cells were labeled with calcein AM and incubated with HUVECs for the indicated times. Fluorescence intensity was measured using a fluorescent plate reader and shown after the subtraction of the background signal of HUVECs alone. (D) Calcein AM-labeled MPs (green) from LPS-treated THP-1 cells were incubated with calcein orange and 4,6 diamidino-2-phenylindole (blue nuclei)-labeled HUVECs for 4 hours and observed in a 1-μm section by confocal microscopy at room temperature using an Olympus Fluoview 1000 with 40× lens. The image was acquired using Olympus Fluoview Version 2.0 viewer and processed using Image J software. Results are representative of at least 3 independent experiments (n = 3 for panels A and B; n = 6 for panel C). *P < .05 (Student t test).
MPs from LPS-treated monocytic cells activate endothelial cells
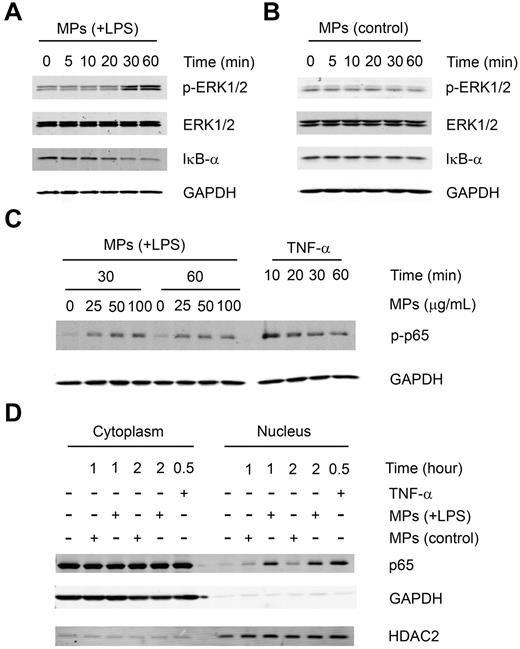
MPs from either untreated or LPS-treated THP-1 cells were incubated with serum-starved HUVECs for the different times. Western blot analysis was performed to detect the activation of ERK1/2 and NF-κB signaling pathways in HUVECs. We found that MPs from LPS-treated THP-1 cells but not MPs from untreated cells induced phosphorylation of ERK1/2 at 30 and 60 minutes and the degradation of IκB-α (Figure 2A-B). Consistent with this observation, the phosphorylation of p65 was also induced by MPs from LPS-treated THP-1 cells at both 30 and 60 minutes (Figure 2C). Furthermore, we found that MPs from LPS-treated THP-1 cells, but not from untreated THP-1 cells, induced translocation of p65 from the cytoplasm to the nucleus at 1 and 2 hours (Figure 2D). TNF-α was used as a positive control in these experiments (Figure 2C-D). We further explored the effect of both types of MPs on the expression of 3 NF-κB-dependent genes: ICAM-1, VCAM-1, and E-SELECTIN. We found that under serum-free conditions, MPs from LPS-treated THP-1 cells induced ICAM-1, VCAM-1, and E-selectin mRNA expression at a dose of 100 μg/mL (Figure 3A). In addition, we observed a dose-dependent increase in protein levels of all 3 cell adhesion molecules in HUVECs (Figure 3B). In contrast, MPs from untreated THP-1 cells had no significant effect on expression of the adhesion molecules at a dose of 100 μg/mL (Figure 3A-B) or even up to a 300 μg/mL dose (data not shown). We also observed a dose-dependent increase in VCAM-1 protein by HUVECs stimulated with increasing concentrations of MPs from LPS-treated PBMCs (Figure 3C). Of note, the positive control, 10 ng/mL of TNF-α, induced higher levels of the adhesion molecules than MPs from LPS-stimulated cells (Figure 3B-C). Finally, the NF-κB pathway inhibitors MG-132 and Bay11–7082 abolished VCAM-1 protein expression induced by MPs from LPS-treated THP-1(Figure 3D).
MPs released by LPS-treated THP-1 cells stimulate intracellular signaling in HUVECs. A total of 100 μg/mL of MPs from LPS-treated (A) or untreated (B) THP-1 cells was incubated in serum-free conditions with HUVECs. Phosphorylation of ERK1/2 and degradation of IκB-α were observed by Western blot at the indicated times. (C) Indicated amounts of MPs from LPS-treated THP-1 cells or 10 ng/mL of TNF-α were incubated in serum-free conditions with HUVECs for the indicated time. Phosphorylation of p65 was detected by Western blot. (D) Subcellular localization of p65 was examined in HUVECs after 1 or 2 hours of stimulation with MPs from untreated or LPS-treated THP-1 cells or 10 ng/mL of TNF-α. HDAC2 served as the nuclear loading control, and GAPDH served as a cytoplasmic loading control. Results are representative of 2 independent experiments.
MPs released by LPS-treated THP-1 cells stimulate intracellular signaling in HUVECs. A total of 100 μg/mL of MPs from LPS-treated (A) or untreated (B) THP-1 cells was incubated in serum-free conditions with HUVECs. Phosphorylation of ERK1/2 and degradation of IκB-α were observed by Western blot at the indicated times. (C) Indicated amounts of MPs from LPS-treated THP-1 cells or 10 ng/mL of TNF-α were incubated in serum-free conditions with HUVECs for the indicated time. Phosphorylation of p65 was detected by Western blot. (D) Subcellular localization of p65 was examined in HUVECs after 1 or 2 hours of stimulation with MPs from untreated or LPS-treated THP-1 cells or 10 ng/mL of TNF-α. HDAC2 served as the nuclear loading control, and GAPDH served as a cytoplasmic loading control. Results are representative of 2 independent experiments.
MPs released by LPS-treated THP-1 cells induce the expression of cell adhesion molecules by HUVECs. (A) HUVECs were incubated for 1 or 2 hours in serum-free conditions with MPs from untreated or LPS-treated THP-1 cells or 10 ng/mL TNF-α. ICAM-1, VCAM-1, and E-selectin mRNA expression was measured by real-time polymerase chain reaction. (B) HUVECs were incubated for 4 hours in serum-free conditions with MPs derived from LPS-treated or untreated THP-1 cells or 10 ng/mL TNF-α. Expression of VCAM-1, E-selectin, and ICAM-1 by HUVECs was detected by Western blot. (C) HUVECs were incubated for 4 hours in serum-free conditions with MPs from LPS-treated PBMCs or 10 ng/mL TNF-α. VCAM-1 was detected by Western blot. (D) HUVECs were treated with 5 or 10μM of the NF-κB pathway inhibitors MG-132, Bay11–7082, or vehicle control (dimethyl sulfoxide [DMSO]) and then stimulated with MPs from LPS-treated THP-1 cells. VCAM-1 was detected by Western blot. GAPDH served as a loading control. Results are representative of 3 independent experiments.
MPs released by LPS-treated THP-1 cells induce the expression of cell adhesion molecules by HUVECs. (A) HUVECs were incubated for 1 or 2 hours in serum-free conditions with MPs from untreated or LPS-treated THP-1 cells or 10 ng/mL TNF-α. ICAM-1, VCAM-1, and E-selectin mRNA expression was measured by real-time polymerase chain reaction. (B) HUVECs were incubated for 4 hours in serum-free conditions with MPs derived from LPS-treated or untreated THP-1 cells or 10 ng/mL TNF-α. Expression of VCAM-1, E-selectin, and ICAM-1 by HUVECs was detected by Western blot. (C) HUVECs were incubated for 4 hours in serum-free conditions with MPs from LPS-treated PBMCs or 10 ng/mL TNF-α. VCAM-1 was detected by Western blot. (D) HUVECs were treated with 5 or 10μM of the NF-κB pathway inhibitors MG-132, Bay11–7082, or vehicle control (dimethyl sulfoxide [DMSO]) and then stimulated with MPs from LPS-treated THP-1 cells. VCAM-1 was detected by Western blot. GAPDH served as a loading control. Results are representative of 3 independent experiments.
The activity of the MPs is not the result of LPS contamination
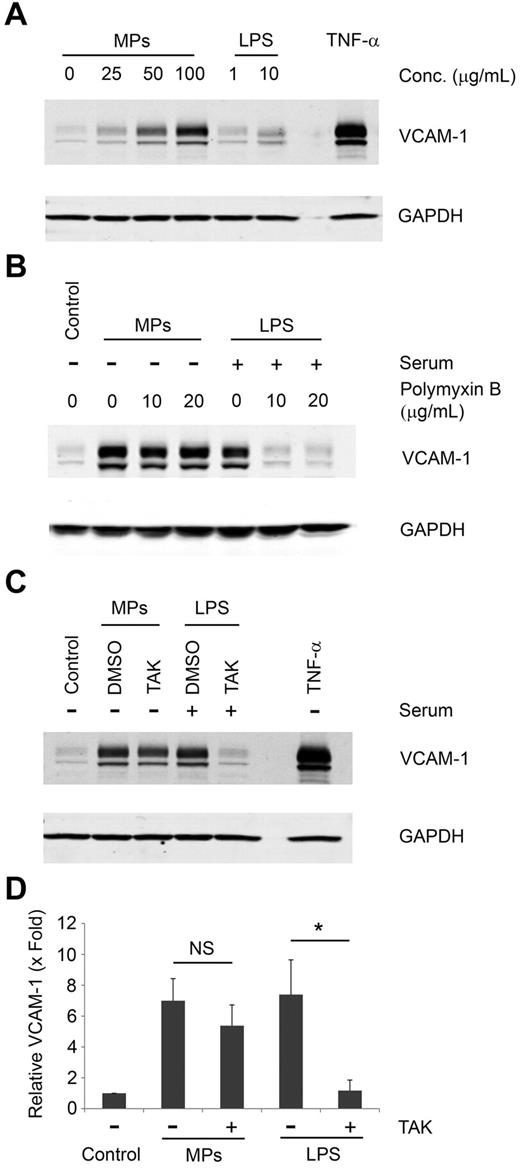
Although the MPs generated from LPS-treated THP-1 cells were washed twice with PBS, it is possible that LPS was not completely removed from the MPs. We measured the levels of LPS in MP samples from LPS-treated THP-1 cells using a ToxinSensor Endotoxin Assay Kit and found that they contained a very small residual amount of LPS (18 ng of LPS per milligram MP protein). It is well known that LPS activation of HUVECs requires a complex containing LPS binding protein, CD14, myeloid differentiation protein-2, and Toll like receptor-4.26,27 However, LPS is a very weak agonist in serum-free culture conditions because of the lack of LPS binding protein and soluble CD14. Indeed, 10 μg/mL of LPS only induced a small increase of VCAM-1 expression in HUVECs in serum-free culture conditions (Figure 4A). In contrast, VCAM-1 expression was strongly induced by MPs from LPS-treated THP-1 (Figure 4A). We further tested the effect of the LPS inhibitor, polymyxin B, and a small-molecule inhibitor of Toll like receptor-4–mediated signaling (TAK-242) on VCAM-1 induction by MPs. Polymyxin B and TAK-242 abolished VCAM-1 induction on HUVECs by LPS in culture conditions with serum (Figure 4B-D). However, neither inhibitors affected VCAM-1 expression induced by MPs from LPS-treated THP-1 cells (Figure 4B-D). Therefore, our data indicate that the small amount of LPS in the washed MPs does not explain their ability to activate the endothelial cells in serum-free culture conditions.
Assessment of LPS contamination in MPs. (A) HUVECs were incubated in serum-free conditions with the indicated amounts of MPs from LPS-treated THP-1 cells, LPS, or 10 ng/mL of TNF-α. Expression of VCAM-1 was detected by Western blot. (B) HUVECs were incubated with increasing concentrations of polymyxin B and then stimulated in serum-free conditions with MPs from LPS-treated THP-1 cells or in serum-containing media with LPS. VCAM-1 was detected by Western blot. (C) HUVECs were pretreated with the Toll like receptor-4 inhibitor TAK-242 or vehicle control (DMSO) and then stimulated with either 100 μg/mL of MPs derived from LPS-treated THP-1 cells in serum-free conditions or with 10 μg/mL of LPS in serum-containing media. HUVECs incubated with 10 ng/mL of TNF-α or left untreated served as controls. VCAM-1 expression in HUVECs was detected by Western blot. GAPDH served as a loading control. (D) Densitometry of Western blots shown in panel C. Data are mean ± SD; n = 4. *P < .01 (2-way ANOVA post-test).
Assessment of LPS contamination in MPs. (A) HUVECs were incubated in serum-free conditions with the indicated amounts of MPs from LPS-treated THP-1 cells, LPS, or 10 ng/mL of TNF-α. Expression of VCAM-1 was detected by Western blot. (B) HUVECs were incubated with increasing concentrations of polymyxin B and then stimulated in serum-free conditions with MPs from LPS-treated THP-1 cells or in serum-containing media with LPS. VCAM-1 was detected by Western blot. (C) HUVECs were pretreated with the Toll like receptor-4 inhibitor TAK-242 or vehicle control (DMSO) and then stimulated with either 100 μg/mL of MPs derived from LPS-treated THP-1 cells in serum-free conditions or with 10 μg/mL of LPS in serum-containing media. HUVECs incubated with 10 ng/mL of TNF-α or left untreated served as controls. VCAM-1 expression in HUVECs was detected by Western blot. GAPDH served as a loading control. (D) Densitometry of Western blots shown in panel C. Data are mean ± SD; n = 4. *P < .01 (2-way ANOVA post-test).
MPs from LPS-treated THP-1 cells contain IL-1β and inflammasome components
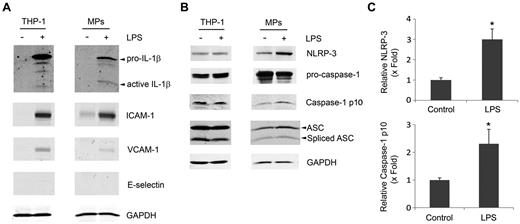
To explore how MPs activate intracellular signaling molecules and induce the expression of cell adhesion molecules, we analyzed the content of the MPs. Because IL-1β is known to activate HUVECs and is also known to be released within MPs,14 we measured levels of IL-1β in MP lysates by ELISA. We observed very low levels of IL-1β (1.0 ± 0.4 pg/mg protein, n = 3) in MPs from untreated THP-1 cells and significantly higher levels of IL-1β (1016 ± 407 pg/mg protein, n = 4) in MPs from LPS-treated THP-1 cells. In addition, we found an even greater amount of IL-1β (1780.6 ± 627 pg/mg protein, n = 5) in MPs from LPS-treated PBMCs. Western blot analysis was performed to measure levels of pro-IL-1β and mature-IL-1β in both THP-1 cells and MPs. As shown in Figure 5A, no detectable levels of pro- or mature-IL-1β were observed in untreated THP-1 cells or MPs from these cells. LPS treatment induced a dramatic increase of IL-1β (Figure 5A). Of note, active-IL-1β was enriched in MPs from LPS-treated THP-1 cells. Interestingly, we also observed an increase of levels of ICAM-1 and VCAM-1 in both LPS-treated THP-1 cells and MPs from these cells while E-selectin was undetectable (Figure 5A).
MPs from LPS-treated THP-1 cells contain IL-1β and inflammasome components. MPs were isolated from THP-1 cells treated with or without LPS. Lysates of MPs and their parental THP-1 cells were subjected to Western blot for (A) IL-1β and ICAM-1, VCAM-1, and E-selectin or (B) NLRP3, caspase-1, and ASC. GAPDH served as a loading control. Western blots are representative of 3 independent experiments. (C) Densitometry from representative MP blots in panel B. Data are mean ± SD; n = 3. *P < .05 (Student t test).
MPs from LPS-treated THP-1 cells contain IL-1β and inflammasome components. MPs were isolated from THP-1 cells treated with or without LPS. Lysates of MPs and their parental THP-1 cells were subjected to Western blot for (A) IL-1β and ICAM-1, VCAM-1, and E-selectin or (B) NLRP3, caspase-1, and ASC. GAPDH served as a loading control. Western blots are representative of 3 independent experiments. (C) Densitometry from representative MP blots in panel B. Data are mean ± SD; n = 3. *P < .05 (Student t test).
The generation of active-IL-1β from pro-IL-1β from LPS-stimulated cells requires an active inflammasome consisting of NLRP3, ASC, and, caspase-1.28 We found similar levels of NLRP3, pro-caspase-1, cleaved-caspase-1 (p10), and ASC in control and LPS-treated THP-1 cells (Figure 5B). In contrast, MPs from LPS-treated THP-1 cells contain significantly more NLRP3 (3.0 ± 0.51-fold, n = 3) and caspase-1 p10 (2.31 ± 0.52-fold, n = 3) than MPs from control THP-1 cells (Figure 5B-C). We also observed an increase in the inflammasome adaptor molecule ASC in MPs from LPS-treated THP-1 cells compared with MPs from control THP-1 cells (1.62 ± 0.28-fold, n = 3; Figure 5B). These results indicate the MPs from LPS-treated THP-1 cells contain IL-1β and components of the inflammasome.
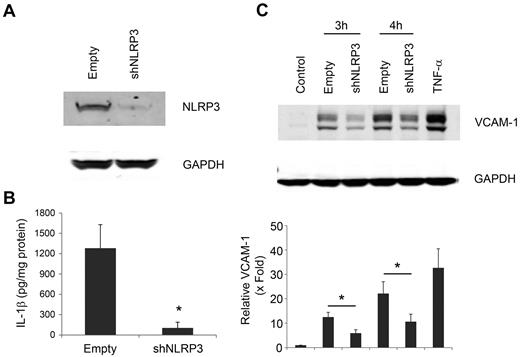
NLRP3 knockdown in THP-1 cells reduces levels of IL-1β in MPs
NLRP3 is required for the generation of mature IL-1β. To determine whether the inflammasome was required for the packaging of IL-1β in MPs, we used THP-1 cells with a stable knockdown of NLRP3 and control cells expressing an empty vector. As shown in Figure 6A, we confirmed the NLRP3 protein expression was reduced 80% in shNLRP3 THP-1 cells and MPs (data not shown). As expected, MPs from LPS-treated shNLRP3 THP-1 cells had significantly less IL-1β compared with MPs from LPS-treated empty vector THP-1 cells (Figure 6B). HUVECs stimulated with MPs from LPS-treated shNLRP3 THP-1 cells showed less VCAM-1 expression compared with HUVECs treated with MPs from LPS-treated THP-1 cells containing the empty vector (Figure 6C). Densitometry analysis showed a 50% reduction in VCAM-1 expression at both 3 and 4 hours using shNLRP3 knockdown MPs (Figure 6C). Thus, both NLRP3 and IL-1β are required for MP induction of VCAM-1 expression by HUVECs.
NLRP3 is required for IL-1β expression in MPs from LPS-treated THP-1 cells. (A) NLRP3 protein was detected by Western blot in untreated stable THP-1 cells containing shNLRP3 or empty vector. (B) IL-1β was measured by enzyme-linked immunosorbent assay in MPs from LPS-treated shNLRP3 or empty vector THP-1 cells. (C) HUVECs were treated for 3 or 4 hours with 50 μg/mL of MPs from LPS-treated shNLRP3 or empty vector THP-1 cells or 10 ng/mL of TNF-α for 4 hours. VCAM-1 was detected by Western blot, and the relative level of VCAM-1 expression was shown by densitometry. GAPDH served as a loading control. Data are mean ± SD; n = 4. *P < .01 (2-way ANOVA post-test analysis).
NLRP3 is required for IL-1β expression in MPs from LPS-treated THP-1 cells. (A) NLRP3 protein was detected by Western blot in untreated stable THP-1 cells containing shNLRP3 or empty vector. (B) IL-1β was measured by enzyme-linked immunosorbent assay in MPs from LPS-treated shNLRP3 or empty vector THP-1 cells. (C) HUVECs were treated for 3 or 4 hours with 50 μg/mL of MPs from LPS-treated shNLRP3 or empty vector THP-1 cells or 10 ng/mL of TNF-α for 4 hours. VCAM-1 was detected by Western blot, and the relative level of VCAM-1 expression was shown by densitometry. GAPDH served as a loading control. Data are mean ± SD; n = 4. *P < .01 (2-way ANOVA post-test analysis).
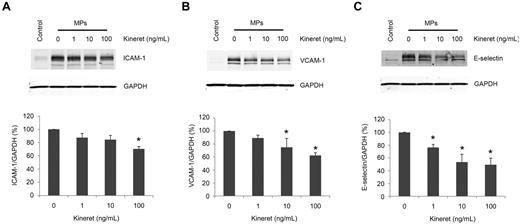
An IL-1 receptor antagonist attenuates the induction of VCAM-1 and E-selectin by MPs from LPS-treated THP-1 cells
To independently determine whether IL-1β was responsible for the effects of the MPs on HUVECs, we tested the effect of an IL-1 receptor antagonist (Kineret) on the induction of ICAM-1, VCAM-1, and E-selectin by MPs from LPS-treated THP-1 cells. Kineret (1-100 ng/mL) pretreatment of HUVECs inhibited the MP-induced expression of all 3 adhesion molecules in a dose-dependent manner. Densitometry analysis showed that 100 ng/mL Kineret produced a maximal inhibition of ICAM-1 expression of 20% (Figure 7A), which is less than that observed for VCAM-1 (40%; Figure 7B) and for E-selectin (50%; Figure 7C). Higher doses of Kineret (1 and 10 μg/mL) had no further inhibitory effect (data not shown).
IL-1 receptor antagonist inhibits the induction of adhesion molecules on HUVECs by MPs. HUVECs were preincubated with the indicated amounts of the IL-1R antagonist (Kineret) for 30 minutes before a 4-hour incubation with 100 μg/mL of MPs from LPS-treated THP-1 cells in serum-free conditions. Lysates were then subjected to Western blot for (A) ICAM-1, (B) VCAM-1, or (C) E-selectin (representative of 3 experiments). Densitometry measurements of these experiments: Data are mean ± SD; n = 3. *P < .05 compared with no Kineret sample (1-way ANOVA post-test analysis).
IL-1 receptor antagonist inhibits the induction of adhesion molecules on HUVECs by MPs. HUVECs were preincubated with the indicated amounts of the IL-1R antagonist (Kineret) for 30 minutes before a 4-hour incubation with 100 μg/mL of MPs from LPS-treated THP-1 cells in serum-free conditions. Lysates were then subjected to Western blot for (A) ICAM-1, (B) VCAM-1, or (C) E-selectin (representative of 3 experiments). Densitometry measurements of these experiments: Data are mean ± SD; n = 3. *P < .05 compared with no Kineret sample (1-way ANOVA post-test analysis).
Discussion
In this study, we found that monocytic MPs bind and are internalized by HUVECs. Importantly, MPs from LPS-treated monocytic cells, but not untreated cells, activate intracellular signaling pathways, such as ERK1/2 and NF-κB, which in turn induce the expression of the cell adhesion molecules ICAM-1, VCAM-1, and E-selectin in endothelial cells. We also demonstrate that the MPs contain IL-1β and that activation of the endothelial cells is the result in part of signaling through the IL-1R.
Although we demonstrate binding of monocytic MPs to endothelial cells, the molecules that mediate this interaction were not analyzed. It is probable that cell surface ligands and/or PS on the MP are interacting with receptors on the endothelial cell. Indeed, MPs from LPS-treated THP-1 cells express PSGL-1 and LFA-1 (data not shown).29 However, Rautou et al30 found that an interaction between PSGL-1 and P-selectin was not required for atherosclerosis plaque-derived MPs to transfer ICAM-1 to endothelial cells. Several groups have reported that blocking PS prevents binding of MPs to endothelial cells.30,31 It has been suggested that high PS expression on the MPs enhances fusion to cell membranes.30,32,33 This may be mediated by the expression of the PS receptors Axl and αvβ3 by endothelial cells. Importantly, Terrisse et al34 found that internalization of MPs by HUVECs was inhibited by antibodies to αvβ3 or annexin V. Further investigation is required to determine how monocytic MPs bind to HUVECs in our model, and the role of these interactions in the activation of the endothelial cells.
It has previously been described that MPs may act to transfer cell surface receptors from one cell to another.35-38 Most relevant here, some MPs from atherosclerotic plaques were able to fuse with the endothelial cells and transfer ICAM-1 from the MPs to the endothelial cells.30 Similarly, we found that MPs derived from LPS-treated THP-1 cells contained ICAM-1. Interestingly, inhibition of the IL-1R on the endothelial cells only reduced the expression of ICAM-1 by 20%, which is consistent with the notion that a portion of ICAM-1 found in the HUVEC lysate after stimulation with monocytic MPs may be derived from the MPs themselves.
We demonstrated that full-length (pro) and mature (active) IL-1β are found in lysates from LPS-treated THP-1 cells and MPs derived from these cells. Interestingly, we found that mature IL-1β is enriched in the MPs compared with the cells. It is still unclear how IL-1β within an MP would be exposed to IL-1R on the surface of the endothelial cell. It is possible that some MPs lyse after binding to the endothelial cell, thus releasing IL-1β. This may be the result of an increase in the levels of adenosine triphosphate at the cell surface, which may activate P2 × 7 receptors on MPs, in a manner similar to IL-1β release from cells.39 Another possibility is that, once the MPs are internalized by the endothelial cells, the MP membrane becomes compromised, leaking its contents into the endosome, which may contain IL-1R.40 Indeed, it has been demonstrated that endosomal localization of IL-1R via MyD88-dependent signaling is required for a fully active receptor complex.41
Similar to our findings, Brown and McIntyre previously demonstrated that IL-1β is found within MPs from activated platelets, and these MPs induce the expression of VCAM-1 on endothelial cells in an IL-1R–dependent manner.42 This group showed complete inhibition of platelet MP-dependent VCAM-1 expression by HUVECs using an IL-1R antagonist. Conversely, using both pharmacologic and genetic approaches, we found partial inhibition of MP induction of cell adhesion molecule expression by HUVECs. One possible explanation is that the MPs from LPS-treated monocytic cells express other membrane-associated cytokines, such as TNF-α,43 which is not expressed by platelets. Although TNF-α is recognized to be a soluble cytokine, it is first presented in a membrane-tethered form on the expressing cell and is then cleaved by the metalloprotease, TNF-α converting enzyme, to release the soluble cytokine. Indeed, a recent study showed that the membrane-bound form of TNF-α is functional and even more potent than the soluble form of the cytokine.43 Importantly, we detected TNF-α in lysates of MPs from LPS-treated THP-1 cells and PBMCs (data not shown). Thus, membrane-bound TNF-α on the MPs could interact with receptors on the endothelial cell and contribute to their activation.
We observed IL-1β in lysates of LPS-treated THP-1 cells and MPs derived from these cells but not untreated cells. In contrast, NLRP3, ASC, and capsase-1 are in lysates from both LPS-treated and untreated THP-1 cells, as well as MPs derived from these cells. Importantly, levels of NLRP3 and cleaved caspase-1 were enriched in MPs from LPS-treated THP-1 cells compared with MPs from untreated cells. Although it has been previously demonstrated that caspase-1 can be packaged into MPs,44 to our knowledge, this is the first demonstration of the inflammasome components NLRP3 and ASC being packaged into MPs. Because MPs can fuse with endothelial cells, it is also possible that MPs can deliver functional inflammasomes to the endothelial cell. In this scenario, delivery of an active inflammasome to a resting endothelial cell could amplify the inflammatory response. Indeed, one group found that platelet MPs delivered the cytokine Regulated on Activation, Normal T-cell Expressed and Secreted (RANTES) to endothelial cells, which resulted in increased monocyte adhesion.45
Normal human plasma contains 2 to 4 μg/mL of MP protein,46,47 but leukocyte-derived MPs are < 10% of the total.48 The lowest MP dose we used (25 μg/mL for THP-1 and 5 μg/mL for PBMCs) may be higher than the pathophysiologic levels in the circulation. However, levels of circulating monocyte MPs are dramatically increased in sepsis, although the protein concentration of MPs under these conditions is unknown.4 In addition, the contribution of MPs to inflammatory signaling in vivo remains to be determined. One mitigating factor is the presence of the phospholipid hydrolyzing enzyme, secretory phosphlipase A2, which is up-regulated during inflammatory conditions.49 It is possible that secretory phosphlipase A2 contributes to the degradation of MPs in vivo, thus diluting the potential effects of IL-1β–loaded MPs. Nonetheless, IL-1β levels in our MPs are probably underestimated because a portion of IL-1β within the MPs may be released into the supernatant during isolation procedures using centrifugation.14 Moreover, we found that the amount of IL-1β in MPs from isolated LPS-treated PBMCs was greater than that found in MPs from LPS-treated THP-1 cells. We propose that monocytic MPs provide a vehicle for the transport of IL-1β in blood and they deliver IL-1β to sites of inflammation where the cytokine contributes to the activation of the endothelium.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Noppacharn Uaprasert for technical assistance.
This work was supported by the National Institutes of Health HL-066350 (N.M.).
National Institutes of Health
Authorship
Contribution: J.-G.W. and J.C.W. designed and preformed experiments, analyzed results, and wrote the paper; B.K.D. and J.P.-Y.T. provided essential reagents and expertise; C.M.D. provided scientific input; K.J. provided vital expertise and reagents; and N.M. supervised experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nigel Mackman, 98 Manning Dr, Chapel Hill, NC 27599-7035; e-mail: nmackman@med.unc.edu.
References
Author notes
J.-G.W. and J.C.W. contributed equally to this study.

![Figure 3. MPs released by LPS-treated THP-1 cells induce the expression of cell adhesion molecules by HUVECs. (A) HUVECs were incubated for 1 or 2 hours in serum-free conditions with MPs from untreated or LPS-treated THP-1 cells or 10 ng/mL TNF-α. ICAM-1, VCAM-1, and E-selectin mRNA expression was measured by real-time polymerase chain reaction. (B) HUVECs were incubated for 4 hours in serum-free conditions with MPs derived from LPS-treated or untreated THP-1 cells or 10 ng/mL TNF-α. Expression of VCAM-1, E-selectin, and ICAM-1 by HUVECs was detected by Western blot. (C) HUVECs were incubated for 4 hours in serum-free conditions with MPs from LPS-treated PBMCs or 10 ng/mL TNF-α. VCAM-1 was detected by Western blot. (D) HUVECs were treated with 5 or 10μM of the NF-κB pathway inhibitors MG-132, Bay11–7082, or vehicle control (dimethyl sulfoxide [DMSO]) and then stimulated with MPs from LPS-treated THP-1 cells. VCAM-1 was detected by Western blot. GAPDH served as a loading control. Results are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/8/10.1182_blood-2011-01-330878/4/m_zh89991176420003.jpeg?Expires=1767881396&Signature=hsBlauIe2j7h9LvGACjoa3kNmrmbkMcXHAuF~2zqrsxwdEFHEPHdtxDEdzRtSscmh8EKm35tFNTA1FJFhIZbmgEwwz8tjRUGuh292RjUnhshk3VDkXsfJzcPj-o7ew29WSG1GbDi96AKE-LGxswsKveyWA7P3BHp5AsQCBqVqfZFsQDusAKxPnHE-gbBUaMUFe69SV-DXP6YWdDZ~Dz7S4eEAHUSBuoJnFT8p34oFCC9zlZ7vrIdxgQkkajEVDGq3HnP1PKqQJ5nPq2TPS6TRKv-zYwkfw53flA27spNH40JS3sPHWt8AK9E5DlxmbbCj89KMRUvtyTgPoSo5sIbGg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal