To establish central tolerance in T cells, Aire, a nuclear protein expressed in medullary thymic epithelial cells, not only induces the ectopic expression of various self-antigens but also facilitates the indirect presentation of medullary thymic epithelial cell (mTEC)–derived self-antigens by thymic dendritic cells.
The medullary region of the thymus plays an essential role in the establishment of self-tolerance in developing T cells. The role of mTECs, especially mTECs that express the nuclear protein Aire, has been well documented.1,2 Aire is involved in the ectopic, or promiscuous, expression of many self-antigens, including antigens whose expression is restricted to peripheral tissues. The additional involvement of thymic dendritic cells (tDCs) in imposing self-tolerance in the thymic medulla has also been documented.3,4 However, how mTECs and tDCs cooperate to accomplish their tasks to establish self-tolerance has been unclear. In this issue of Blood, Hubert and colleagues show that Aire regulates the indirect presentation of mTEC-derived self-antigens by tDCs.5
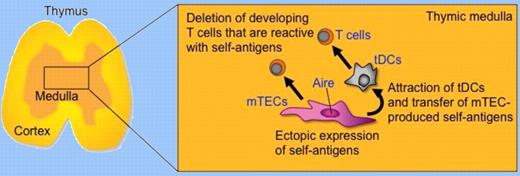
To address the role of Aire in central tolerance, Hubert et al examined the impact of Aire deficiency in the thymus where insulin-promoter-driven transgenic ovalbumin (OVA) that was ectopically expressed by mTECs would delete T cells that expressed OVA-specific transgenic T-cell receptors (TCRs). In a widely used OVA transgenic model where OVA was produced in the membrane-bound form, they found that OVA-specific CD4 and CD8 T cells could be deleted as a consequence of Aire expression, which they show partially regulated OVA expression in mTECs. On the contrary, in another OVA transgenic model in which the secreted form of OVA was expressed under the control of the transgenic insulin promoter, they found that OVA expression was not regulated by Aire and as a consequence deletion of OVA-specific OT-I-TCR-transgenic CD8 T cells was not dependent on Aire expression. Interestingly, however, the deletion of OVA-specific OT-II-TCR-transgenic CD4 T cells was dependent on Aire expression but was not directly mediated by mTEC antigen presentation. Instead of mTECs, bone marrow–derived antigen-presenting cells, presumably tDCs, were required for the deletion. Thus, the requirement for the transfer of mTEC-produced self-antigens to tDCs seems dependent on the form of antigens expressed in mTECs. Some forms of mTEC-produced self-antigens need to be transferred to tDCs for presentation to developing T cells, and Aire regulates such an mTEC-to-tDC transfer of self-antigens. Hubert and colleagues further sought the possibility that Aire regulated the expression of chemokines that might bring tDCs and mTECs together. Microarray analysis using mRNAs from wild-type and Aire-deficient mTECs showed that many chemokines were significantly affected by Aire, although Hubert et al did not examine the functions of individual chemokines in the mTEC-tDC interplay. Nonetheless, their study has revealed that a fraction of self-antigens produced by mTECs are transferred to tDCs for the deletion of developing T cells that are reactive to those self-antigens, and that Aire regulates the transfer of the self-antigens from mTECs to tDCs. They suggested that the chemokine-mediated attraction of tDCs by mTECs may help increase the cooperation between mTECs and tDCs in transferring mTEC-produced self-antigens to antigen-presenting tDCs (see figure).
Aire expressed by mTECs regulates the ectopic expression of self-antigens and molecules, possibly including XCL1, to attract tDCs and transfer mTEC-produced self-antigens. mTECs and tDCs cooperate for the deletion of self-reactive T cells that are positively selected in the thymic cortex and migrate into the thymic medulla.
Aire expressed by mTECs regulates the ectopic expression of self-antigens and molecules, possibly including XCL1, to attract tDCs and transfer mTEC-produced self-antigens. mTECs and tDCs cooperate for the deletion of self-reactive T cells that are positively selected in the thymic cortex and migrate into the thymic medulla.
These results highlight the idea that the role of Aire in the establishment of self-tolerance is not limited to the ectopic expression of self-antigens in mTECs but includes regulation to facilitate the interplay between mTECs and tDCs. Recent papers have also reported that Aire regulates the expression of several chemokines in mTECs.6,7 One of those papers has further shown that mTECs produce the chemokine XCL1 in an Aire-dependent manner and the receptor XCR1 is expressed by tDCs.7 XCL1-deficient mice are defective in the medullary accumulation of tDCs and the thymic generation of naturally occurring regulatory T (nTreg) cells. Thymocytes from XCL1-deficient mice elicit dacryoadenitis in T cell–deficient nude mice. mTEC expression of XCL1, tDC medullary accumulation, and nTreg cell generation are diminished in Aire-deficient mice, indicating that the XCL1-mediated medullary accumulation of tDCs contributes to the establishment of central tolerance and is regulated by Aire. Thus, XCL1 may participate in the Aire-mediated attraction of tDCs for the efficient transfer of mTEC-produced self-antigens. Further studies of the molecular mechanisms that regulate the interplay between mTECs and tDCs will improve our understanding and aid in the future manipulation of autoimmune diseases.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal