Abstract
To improve the outcome of allogeneic stem cell transplantation (allo-SCT) in multiple myeloma as part of first-line treatment, we prospectively investigated the feasibility and efficacy of lenalidomide maintenance. Patients started maintenance 1 to 6 months after nonmyeloablative allo-SCT. Lenalidomide was dosed 10 mg on days 1 to 21 of a 28-day schedule for a total of 24 cycles. Peripheral blood samples were taken to evaluate immune modulating effects. Thirty-five eligible patients were enrolled, and 30 started with lenalidomide. After 2 cycles, 14 patients (47%) had to stop treatment, mainly because of the development of acute graft versus host disease (GVHD). In total, 13 patients (43%) stopped treatment because of development of GVHD, 5 patients (17%) because of other adverse events, and 5 patients (17%) because of progression. Responses improved in 37% of patients, and the estimated 1-year progression-free survival from start of maintenance was 69% (90% confidence interval, 53%-81%). Lenalidomide increased the frequency of human leukocyte antigen-DR+ T cells and regulatory T cells, without correlation with clinical parameters. In conclusion, lenalidomide maintenance 10 mg daily after nonmyeloablative allo-SCT with unmanipulated graft in multiple myeloma patients is not feasible, mainly because of the induction of acute GVHD. This trial was registered at www.trialregister.nl as #NTR1645.
Introduction
The role of allogeneic stem cell transplantation (allo-SCT) in first-line treatment of multiple myeloma (MM) is still undefined.1 Although the introduction of a nonmyeloablative (NMA) regimen has lowered the treatment-related mortality, in “genetically randomized” phase 3 studies, results in terms of event-free survival (EFS) and overall survival (OS) have been conflicting. Whereas some trials have demonstrated a better outcome of NMA allo-SCT, others could not confirm these results, possibly resulting from the use of different conditioning regimens and differences of the nontransplant comparator.2-4 The novel antimyeloma agents may be of special therapeutic interest to improve the graft-versus-myeloma effect and outcome because of their immune-modulating properties.5 Both thalidomide and bortezomib appear to be effective in relapsed patients after allo-SCT, apparently without excessive stimulation of graft-versus-host disease (GVHD).5,6 Impressive results were also obtained with lenalidomide as salvage therapy in a group of patients with progressive symptomatic disease after allo-SCT.7,8
To determine the efficacy and safety of post allo-SCT maintenance treatment with lenalidomide as part of first-line therapy, we designed the HOVON 76 prospective phase 2 study. Lenalidomide was administered after tandem autologous and NMA allo-SCT at a dose of 10 mg daily for 21 days of a 28-day cycle for a maximum of 24 cycles. In addition, we measured T- and NK-cell subsets and activity in the peripheral blood to examine the immune-modulating effects of lenalidomide.
Methods
Patient selection
Newly diagnosed MM patients, 18 to 66 years of age inclusive, in first-line therapy, who had a human leukocyte antigen (HLA) identical sibling donor and a World Health Organization performance status 0 to 2 could be included. Patients had received 3 cycles of induction treatment, stem cell mobilization with cyclophosphamide, and 1 cycle of high-dose melphalan 200 mg/m2 followed by autologous SCT. Additional inclusion criteria for start of lenalidomide were an absolute neutrophil count > 1.0 × 109/L, platelet count > 75 × 109/L, a negative pregnancy test for females of childbearing potential, and the use of adequate contraceptive methods.
Exclusion criteria were progressive disease or relapse before or after autologous SCT, creatinine clearance < 50 m/min, or significant cardiac of hepatic dysfunction. Exclusion criteria for starting lenalidomide were progressive MM, acute GVHD ≥ grade 2 at the time of registration, prior use of lenalidomide, or use of any other anticancer drugs after the NMA allo-SCT.
All patients provided written informed consent before entering the study. The clinical trial was approved by the ethics committee of all participating centers and was conducted in accordance with the Declaration of Helsinki.
Treatment plan and dose modifications
Allo-SCT was performed 2-6 months after autologous SCT using a nonmyeloablative conditioning regimen of only 2 Gy total body irradiation for patients with a 10 of 10 HLA-matched donor. Patients received an unmanipulated graft. Immunosuppressive therapy consisted of cyclosporine A 4.5 mg/kg twice daily until day 120 and tapered afterward with a 10% dose reduction per week in the absence of GVHD. Cyclosporine A was combined with mycophenolate mofetil 15 mg/kg, twice daily until day 84, and thereafter tapered and stopped in 2 weeks if there was no GVHD. All patients received antibiotic prophylaxis, including co-trimoxazole 480 mg twice a day and valacyclovir 500 mg twice a day.
Lenalidomide maintenance therapy 10 mg daily for 21 days in a 28-day cycle was started between 1 and 6 months after the allo-SCT, with a preference to start between 1 and 3 months. During the first 2 cycles of lenalidomide, immunosuppressive drugs were not tapered. Maintenance continued for a planned 24 cycles.
If acute GVHD grade 2 developed, lenalidomide was stopped and restarted at a lower dose level once GVHD had resolved to < grade 2 within 2 months. Lenalidomide was stopped permanently if grade 3 or 4 acute GVHD developed. Before every new cycle, the absolute neutrophil count should be > 1.0 × 109/L, platelets > 50 × 109/L, and any other lenalidomide-related adverse event (AE) resolved to ≤ grade 2 severity. The lenalidomide dose could be lowered to 5 mg. When a patient experienced side effects that precluded the use of this dose, treatment was discontinued permanently.
Definition of endpoints and response criteria
The primary objective of the trial was to estimate progression-free survival (PFS) after start of lenalidomide maintenance, defined as time from maintenance until progression or relapse. The primary endpoint was PFS at 1 year from the start of maintenance (PFS1y).
The secondary objectives were the estimation of overall survival (OS) defined as time from start of maintenance until death from any cause, the ongoing response defined as the achievement of new very good partial response (VGPR) and complete response (CR) after the start of lenalidomide, the incidence and severity of AEs according to the Common Terminology Criteria for Adverse Events criteria, the incidence and severity of acute and chronic GVHD in relation to lenalidomide treatment, PFS, and OS from NMA allo-SCT, and to analyze relevant immunomodulating effects of lenalidomide in MM in vivo.
Clinical response was assessed according to the response criteria based on the European Group for Blood and Marrow Transplantation, International Bone Marrow Transplant Registry, and Autologous Bone Marrow Transplantation criteria9 with the addition of a VGPR category (≥ 90% reduction of M-protein) according to the uniform response criteria.10
GVHD was classified according to the National Institutes of Health working group report that includes the category of acute late-onset GVHD with features of acute GVHD beyond day 100.11
Immunomonitoring
Peripheral blood samples were collected at day 1 of cycles 1, 2, 9, and 10 and day 15 of cycles 1 and 9 of lenalidomide treatment. PBMCs were isolated by Ficoll density centrifugation and preserved in liquid nitrogen until use. The effect of lenalidomide on circulating CD4+ and CD8+ T-cell subsets, B cells, and NK cells was measured by FACS analysis after labeling of PBMCs with the indicated lineage-specific, fluorescently conjugated antibodies. The frequency of circulating T regulatory cells (Tregs) was determined by intracellular staining for FoxP3 and surface staining for CD4. Th1 and Th17 cell subsets capable of producing IFN-γ, but not IL-17, and IL-17, but not IFN-γ, respectively were enumerated by intracellular staining for these cytokines after 48 hours of stimulation of PBMCs with anti-CD3/CD28–coated immunostimulatory beads.
Statistical analysis
A PFS1y of at least 75% was considered sufficiently high to warrant further investigation in clinical trials, whereas a PFS1y of 59% or less was considered too low. Both α (the accepted probability of recommending for further investigation an inactive regimen) and β (the accepted probability to reject for further investigation an active regimen) were set at 0.10. Therefore, the required number of eligible patients was 80, and an interim analysis was planned as soon as 12 events for PFS had been reported. PFS and OS were estimated by the Kaplan-Meier method, and 95% confidence intervals (CIs) were constructed. Curves were generated to illustrate survival. Safety was analyzed using descriptive statistics to summarize the incidence of AE with Common Terminology Criteria grade 2 or more. AEs were scored using the National Cancer Institute Common Terminology Criteria for Adverse Events, Common Terminology Criteria for Adverse Events Version 3.0. The immunomonitoring data were analyzed using Wilcoxon matched-pairs tests. The results were expressed as 2-tailed P values, and P values < .05 were considered significant.
Results
Patients characteristics
From January 2008 until March 2010, 38 patients from 5 Dutch allogeneic transplant centers were included. Three patients were not eligible (1 progression, 2 no first-line allo-SCT) and therefore excluded from the analysis. Two patients did not start with lenalidomide because of development of acute GVHD grade 2 of the skin and increasing liver enzymes after registration, 1 patient because of progression, and 2 patients never started because the study was put on hold and later inclusion stopped prematurely.
Table 1 summarizes the characteristics of the remaining 30 patients who started with lenalidomide maintenance. Two patients had modifications in the induction regimen for the allo-SCT with the addition of fludarabine in one and fludarabine and antithymocyte globulin in the other because of one HLA-mismatched sibling donor. The majority of patients (77%) had received induction and intensification chemotherapy as part of the HOVON 65 trial.12
Patient characteristics of patients who started with lenalidomide
| Patient characteristic . | Value . |
|---|---|
| Total no. | 30 |
| Male/female | 19/10 |
| Median age, y (range) | 53 (37-65) |
| WHO performance status 0/1/2 | 20/9/1 |
| ISS stage at diagnosis I/II/III | 9/11/10 |
| Induction therapy before HDM | |
| VAD/TAD/PAD | 17/4/9 |
| Conditioning NMA allo-SCT | |
| TBI/TBI + fluda/TBI + fluda + ATG | 28/1/1 |
| Median CD34+-infused stem cells, × 106/kg (range) | 5.9 (2.0-10.9) |
| Median time between allo-SCT and start of lenalidomide, wk (range) | 12 (4-27) |
| Patient characteristic . | Value . |
|---|---|
| Total no. | 30 |
| Male/female | 19/10 |
| Median age, y (range) | 53 (37-65) |
| WHO performance status 0/1/2 | 20/9/1 |
| ISS stage at diagnosis I/II/III | 9/11/10 |
| Induction therapy before HDM | |
| VAD/TAD/PAD | 17/4/9 |
| Conditioning NMA allo-SCT | |
| TBI/TBI + fluda/TBI + fluda + ATG | 28/1/1 |
| Median CD34+-infused stem cells, × 106/kg (range) | 5.9 (2.0-10.9) |
| Median time between allo-SCT and start of lenalidomide, wk (range) | 12 (4-27) |
WHO indicates World Health Organization; ISS, International Staging System; HDM, high-dose melphalan; VAD, vincristine, adriamycin, and dexamethasone; TAD, thalidomide, adriamycin, and dexamethasone; PAD, bortezomib, adriamycin, and dexamethasone; TBI total body irradiation; and fluda, fludarabine.
Treatment compliance
The median time from allo-SCT to the start of lenalidomide maintenance was 12 weeks (range, 4-27 weeks). In 12 patients (40%), the dose was reduced to 5 mg because of GVHD in 2 patients, hematologic toxicity in 4, dermatologic toxicity in 2, other toxicities in 3, and patient error in 1. Dose reductions were necessary between the first cycle and fifth cycle. The median number of cycles given was 3 (range, 1-24 cycles).
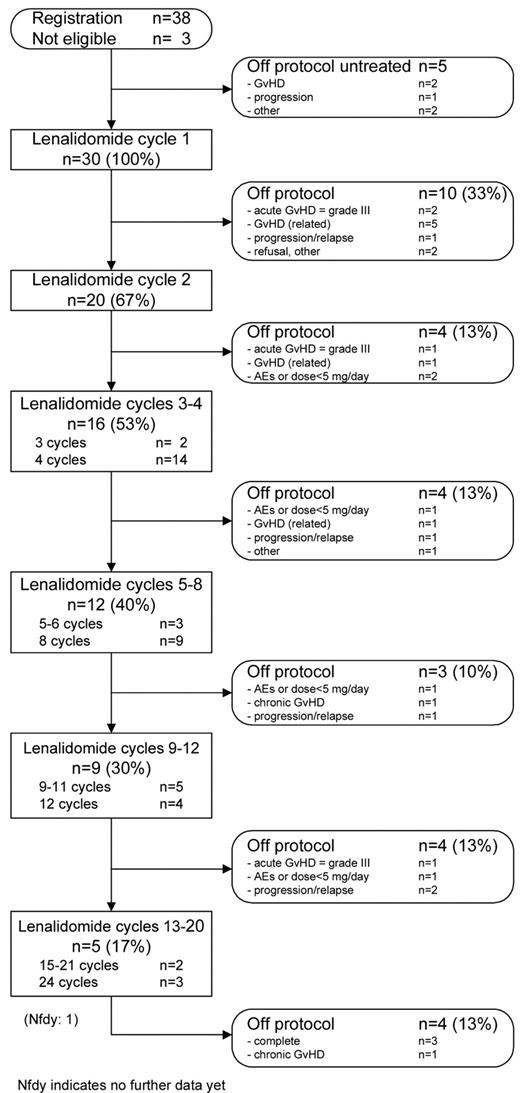
Of 30 patients who started, 10 discontinued treatment during the first cycle and 4 after the second cycle; hence, 14 patients (47%) stopped treatment after only 1 or 2 cycles (Figure 1).
Nine patients stopped treatment because of the occurrence of acute GVHD ≥ grade 2 and 2 because of continued hematologic toxicity after dose reduction. One patient had progressive disease, 1 patient refused further participation, and 1 patient stopped at the advice of the treating physician.
Of 16 patients that started with cycle 3, another 12 patients went off protocol prematurely. Four patients stopped treatment because of acute GVHD ≥ grade 2 and 3 because of other miscellaneous toxicities. Four patients were progressive and one patient developed pancytopenia with reduced chimerism.
Altogether, only 3 patients (10%) completed all 24 cycles, and 1 patient has received 21 cycles and is still on protocol treatment. Twenty-six patients (87%) abrogated treatment prematurely. The main reasons were development of GVHD in 13 patients (43%), other AEs in 5 patients (17%), and progression in 5 patients (17%).
GVHD
Acute GVHD ≥ grade 2 (11 patients, 37%) or chronic extensive GVHD (5 patients, 11%) developed in 16 patients (53%) during therapy. GVHD occurred at a median of 18 days (range, 4-217 days) after start of lenalidomide. Nine patients (56% of all GVHD) developed GVHD during the first 2 cycles. These patients started with lenalidomide a median of 18 weeks (range, 6-27 weeks) after allo-SCT, which was comparable to the start of the other patients (median, 12 weeks; range, 4-26 weeks; P = .11). In all 3 patients who already experienced transient GVHD before the start of lenalidomide, GVHD reactivated, 1 patient developed acute late-onset grade 2, and the other 2 chronic extensive GVHD. No relation was found between type of induction treatment before autologous SCT and GVHD. The GVHD data are summarized in Table 2.
Proportion of patients with AE grade 3 and 4 during cycles 1 to 12 and GVHD and secondary malignancies during follow-up
| . | Grade 3 . | Grade 4 . |
|---|---|---|
| Any grade 3/4 AE, % | 53 | 23 |
| Blood/bone marrow, % | 27 | 17 |
| Metabolic/laboratory, % | 23 | 7 |
| Infection, % | 17 | — |
| GVHD, patients (%) | 16 (53) | — |
| Acute GVHD ≥ grade 2, patients (%) | 11 (37) | — |
| Skin/skin + liver/liver/gut, patients | 5/3/2/1 | — |
| Grade 2/3/4, patients | 5/6/0 | — |
| Classic/late onset, patients | 4/7 | — |
| Chronic extensive GVHD, patients (%) | 5 (17) | — |
| Secondary malignancy, patients (%) | — | 2 (6.7)* |
| . | Grade 3 . | Grade 4 . |
|---|---|---|
| Any grade 3/4 AE, % | 53 | 23 |
| Blood/bone marrow, % | 27 | 17 |
| Metabolic/laboratory, % | 23 | 7 |
| Infection, % | 17 | — |
| GVHD, patients (%) | 16 (53) | — |
| Acute GVHD ≥ grade 2, patients (%) | 11 (37) | — |
| Skin/skin + liver/liver/gut, patients | 5/3/2/1 | — |
| Grade 2/3/4, patients | 5/6/0 | — |
| Classic/late onset, patients | 4/7 | — |
| Chronic extensive GVHD, patients (%) | 5 (17) | — |
| Secondary malignancy, patients (%) | — | 2 (6.7)* |
— indicates none (ie, no patients).
Two solid tumors were reported during follow-up: one ovarian tumor and one breast carcinoma.
AEs
Table 2 summarizes the AE for the first 12 cycles. The incidence of Common Terminology Criteria for Adverse Events grade 3 and 4 toxicity reported was 53% and 23%, respectively. Ten serious AEs were reported in 8 patients, 2 of which were qualified as unrelated (1 Epstein-Barr virus reactivation, 1 rectal bleeding) and 8 as possible or definitely related to lenalidomide and consisted of infection and development of acute GVHD grade 3. Two secondary malignancies have been reported during follow-up. One patient was diagnosed with breast cancer 5 months after completing 24 cycles of lenalidomide; the other patient completed only 4 cycles, discontinued because of progression and was diagnosed with a borderline ovarian tumor 9 months later. Both patients are alive.
Response
Remission status after allo-SCT before start of maintenance was CR in 6, VGPR in 13, and partial response in 11 patients. In 5 patients (20%), ongoing responses were observed during lenalidomide maintenance; from partial response to VGPR in 1, from partial response to CR in 1, and from VGPR to CR in 3. Five patients had progressive disease and discontinued treatment.
During follow-up, another 6 patients improved their response without further therapy; hence, the total response rate was 37%. In the 24 patients not in CR when starting lenalidomide, the response rate was 46%. Six patients had progressive disease during follow-up. Two patients have died: one because of graft failure with neutropenic fever and one because of progression.
PFS and OS
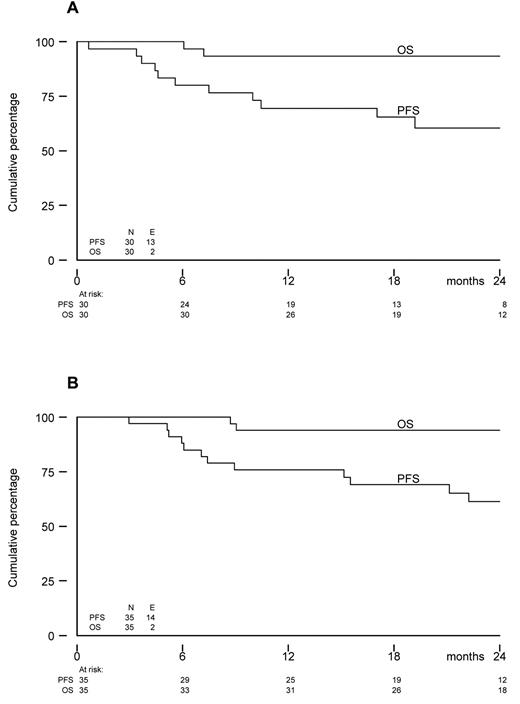
The survival endpoints are based on follow-up data available as of February 2011. The median follow-up of 28 patients still alive is 22 months (range, 8-34 months).
The estimated PFS1y from start of lenalidomide was 69% (90% CI, 53%-81%). At 2 years, PFS was 60% (95% CI, 39%-76%) and OS was 93% (95% CI, 76%-98%; Figure 2). Among the 5 patients who did not start with maintenance, 1 patient had progressive disease 6 months after allo-SCT, whereas the others were without progression and alive after 3, 4, 15, and 35 months. At 2 years from allo-SCT, PFS was 61% (95% CI, 42%-76%), and OS was 94% (95% CI, 78%-98%) for all 35 eligible patients.
Kaplan-Meier survival curves. (A) OS and PFS from the start of lenalidomide maintenance (N = 30). (B) OS and PFS from registration (N = 35).
Kaplan-Meier survival curves. (A) OS and PFS from the start of lenalidomide maintenance (N = 30). (B) OS and PFS from registration (N = 35).
Immunomonitoring
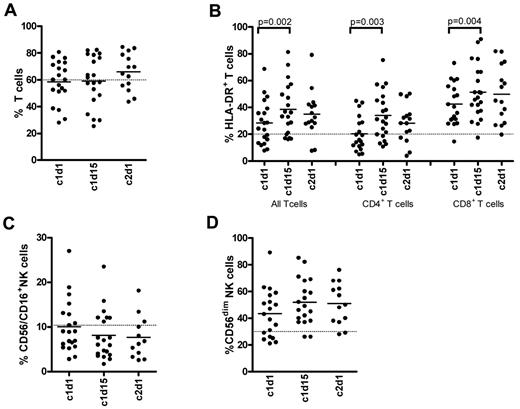
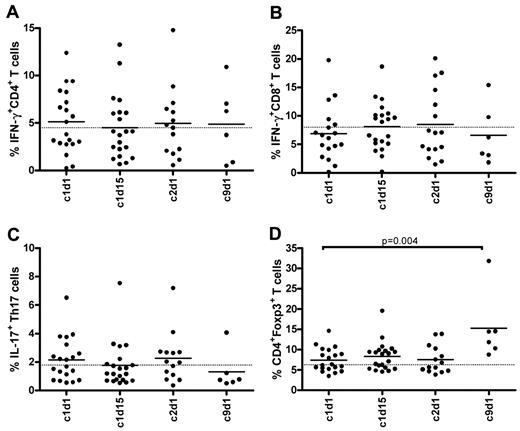
Patients were sampled during the first cycle, and 6 patients could be sampled as planned during cycle 9. In the first cycle, in which 9 patients developed acute GVHD ≥ grade 2, no significant changes were observed in the frequencies of circulating T, NK (Figure 3A,C), or B cells (data not shown), and these frequencies also remained unaffected over time (data not shown). However, there was a significant increase in HLA-DR expression on CD4+ and CD8+ T cells at day 15 of the first cycle, indicating T-cell activation (Figure 3B). In addition, a substantially high proportion of NK cells displayed an activated phenotype with diminished (dim) levels of CD56 at the start of treatment (Figure 3D). Despite the increase in activation status of T cells, no significant changes were detected in the frequencies of IFN-γ–producing CD4 or CD8 T cells (Figure 4A-B) during the first cycle. The frequencies also remained unchanged over time in the patients who completed the ninth cycle. Lenalidomide had no significant influence on the frequencies of IL-17–producing Th17 cells (Figure 4C). The frequency of Tregs showed a slight and temporary increase on day 15 of cycle 1 and reached significantly higher levels on day 15 of the ninth cycle. In these 6 patients, the frequencies were almost doubled at the ninth cycle, reaching a mean value of 13%. None of the analyzed cell subsets, however, showed a clear association with either GVHD or PFS (data not shown).
Frequencies of T- and NK-cell subsets during the first cycle of lenalidomide maintenance. Indicated PBMC samples in the x-axes (c indicates cycle; and d, day) were stained with fluorescent-conjugated antibodies to determine the frequencies of CD3+ T cells (A), HLA-DR+ T-cell subsets (B), CD3−CD56/CD16+ NK cells (C), and the CD56dim NK cells (D) by fluorescence-activated cell sorter. Solid lines in the scatter plots indicate the mean values; and dotted lines in the graphs, the mean value found in healthy persons (n = 7).
Frequencies of T- and NK-cell subsets during the first cycle of lenalidomide maintenance. Indicated PBMC samples in the x-axes (c indicates cycle; and d, day) were stained with fluorescent-conjugated antibodies to determine the frequencies of CD3+ T cells (A), HLA-DR+ T-cell subsets (B), CD3−CD56/CD16+ NK cells (C), and the CD56dim NK cells (D) by fluorescence-activated cell sorter. Solid lines in the scatter plots indicate the mean values; and dotted lines in the graphs, the mean value found in healthy persons (n = 7).
Frequencies of IFN-γ, IL-17–producing T cells, and CD4+Foxp3+ T cells during lenalidomide maintenance. Indicated PBMC samples in the x-axes (c indicates cycle; and d, day) were intracellular stained with fluorescent-conjugated antibodies to determine the frequencies of CD4+ and CD8+ T cells capable of producing IFN-γ (A-B), CD4+ T cells capable of producing IL-17 (C), and CD4+ T cells expressing Foxp3 (D). To stimulate T cells for cytokine production, PBMCs are incubated for 48 hours with anti-CD3/CD28-coated beads. Solid lines in the scatter plots indicate the mean values; and dotted lines in the graphs, the mean value found in healthy persons (n = 7).
Frequencies of IFN-γ, IL-17–producing T cells, and CD4+Foxp3+ T cells during lenalidomide maintenance. Indicated PBMC samples in the x-axes (c indicates cycle; and d, day) were intracellular stained with fluorescent-conjugated antibodies to determine the frequencies of CD4+ and CD8+ T cells capable of producing IFN-γ (A-B), CD4+ T cells capable of producing IL-17 (C), and CD4+ T cells expressing Foxp3 (D). To stimulate T cells for cytokine production, PBMCs are incubated for 48 hours with anti-CD3/CD28-coated beads. Solid lines in the scatter plots indicate the mean values; and dotted lines in the graphs, the mean value found in healthy persons (n = 7).
Discussion
This study prospectively investigated the feasibility and efficacy of lenalidomide maintenance treatment after allo-SCT. We found that early lenalidomide treatment, after an NMA allo-SCT with unmanipulated graft, is not feasible mainly because of the development of GVHD, which was the reason to stop treatment prematurely in 43% of patients.
After our previous experience with the in vivo immunostimulatory effects of lenalidomide after allo-SCT or donor lymphocyte infusions and the occurrence of acute GVHD, we installed strict stopping rules in this study.7 More than half of the patients developed acute GVHD at a median of 18 days after the start of lenalidomide. Notably, patients were not allowed to taper the cyclosporine A or mycophenolate mofetil while starting maintenance. Although late-onset acute GVHD is frequently observed after NMA allo-SCT, this usually occurred after tapering of the immune suppression. Our observation with a rapid development of GVHD after the start of lenalidomide strongly indicates that lenalidomide contributed to the induction of GVHD.
The total incidence of acute GVHD ≥ grade 2 was 37%, which is actually comparable to the incidence of approximately 40% reported in other studies with a similar allo-SCT strategy.2,13 A possible explanation for these comparable incidences might be that lenalidomide induced GVHD only in those patients who were already prone to this and who would otherwise develop GVHD after tapering the immunosuppressive drugs. In addition, our study might be biased to include a population with a possible lower GVHD incidence, as the presence of acute GVHD ≥ grade 2 was an exclusion criterion for the study. For this reason, 8 patients who were transplanted in the same time period could not be included.
The organs most commonly affected by GVHD were the skin and liver, and only one patient had GVHD grade 3 of the gut (Table 2). All cases of gut and skin GVHD were proven by biopsy, but none of the 5 livers was separately biopsied. They were classified as GVHD on the basis of the clinical picture and, if present, the simultaneous occurrence of biopsy proven GVHD of the skin.
With additional laboratory sampling, the immune-modulating effects of lenalidomide after allo-SCT were evaluated. The rapid development of GVHD after the start of lenalidomide suggested a role for the immune-modulatory effect, which was confirmed by the significant increase in HLA-DR+ T cells. In contrast, neither HLA-DR+ T cells nor any other cell subset such as Th1 cells, the T-cell subset, which is frequently associated with acute GVHD, was increased. A possible explanation for this discrepancy could be the rapid migration of cytokine-producing cells from the circulation into the GVHD-affected organs. Further analysis of biopsies seems necessary to further elucidate this. In parallel with previous studies,7,14 lenalidomide appeared to cause an increase in the frequency of Tregs. Whereas in those studies with a dose of 25 mg the Treg elevation was already visible and more profound during the first cycle, we now observed the significant Treg elevation only in cycle 9. Because we have not found a direct stimulatory effect of lenalidomide on Tregs in our in vitro assays (M.S. van der Veer, E.K., M.C.M., H.M.L., T.M., unpublished observations, August 2010), it seems probable that the elevation occurs as a reaction against immune-stimulating effects. Others have demonstrated a small increase in the absolute numbers of NK cells in an elderly population treated with a dose of 15 mg lenalidomide combined with dexamethasone, but we could not confirm this.14
Besides GVHD, the most common AEs were blood and bone marrow toxicities, mainly consisting of neutropenia and thrombocytopenia, infections, and other metabolic/laboratory AEs. A total of 17% of patients stopped treatment because of AEs. In a small dose-finding study by Wolschke et al,15 a dose of 5 mg daily, days 1 to 21, started 100 to 180 days after allo-SCT, caused Common Terminology Criteria for Adverse Events grade 3 toxicity in 4 of 9 patients, indicating that this dose might also be too toxic early after allo-SCT. Future trial designs should therefore focus on other transplantation induction schemes or even dosages < 5 mg a day.
Because most patients stopped treatment prematurely, responses resulting from lenalidomide maintenance therapy were difficult to assess. During treatment and follow-up, there was an improvement of the response rate in 37% of patients. However, 37% of patients also relapsed. PFS at 1 year after the start of lenalidomide was 69% (90% CI, 53%-81%) which would have been considered sufficiently promising for further investigation in clinical trials had this regimen been found to be otherwise feasible. In conclusion, in this phase 2 trial, we found that lenalidomide maintenance 10 mg daily after an NMA allo-SCT with unmanipulated graft is not feasible mainly because of the rapid induction of acute GVHD.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the local and central data managers for assisting in collecting the data and Mrs T. Aarts for technical assistance with the laboratory assays.
This work was supported by the Dutch Cancer Society (CKTO 2007-11) and in part by Celgene Corporation to HOVON (research funding).
Authorship
Contribution: E.K. performed the research, collected, analyzed, and interpreted data, performed statistical analysis, and wrote the manuscript; B.v.d.H. designed research, performed statistical analysis, and wrote the manuscript; M.-J.K., S.Z., E.M., G.H., J.J.C., J.J.J., C.H., and P.S. performed research and reviewed the manuscript; P.B.C. was the coordinating data manager of the trial; C.P.B. and M.E. performed research and analyzed and interpreted data; H.M.L. designed and performed research, analyzed and interpreted data, and wrote the manuscript; T.M. designed and performed research, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; and M.C.M. designed and performed research, collected, analyzed, and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: P.S. has served on advisory boards of Johnson & Johnson and Millennium Pharmaceuticals. H.M.L. has received honoraria as speakers fee from Celgene Corporation. M.C.M. has received honoraria as speakers fee from Johnson & Johnson. The remaining authors declare no competing financial interests.
Correspondence: Monique C. Minnema, Department of Hematology, University Medical Center Utrecht, PO Box 85500, 3508 GA, Utrecht, The Netherlands; e-mail: m.c.minnema@umcutrecht.nl.