Abstract
Mature dendritic cells (DCs) are stimulators of T-cell immune response, whereas immature DCs support T-cell tolerance. Murine B cells can inhibit the production of IL-12 by DCs and thereby hinder the inflammatory response. Notwithstanding the importance of this modulation, only a few studies are available in humans. Here, we have developed an in vitro model of cocultures to assess its significance. We establish that human activated B cells restrained the development of monocytes into immature DCs and their differentiation into mature DCs. In addition, they decreased the density of HLA-DR from mature DCs, the expression of CD80 and CD86 coactivation molecules, the production of IL-12p70 required for antigen presentation and Th1 differentiation, and inhibited the DC-induced T-cell proliferation. These modulations were mediated by CD19+IgDlowCD38+CD24lowCD27− B cells and needed direct cell-to-cell contacts that involved CD62L for the control of CD80 and CD86 expression and a soluble factor for the control of IL-12 production. Moreover, mature DCs from patients with systemic lupus erythematosus displayed insensitivity to the regulation of IL-12. Overall, it appears that human B cells can regulate DC maturation and function and that inefficient B-cell regulation may influence an improper balance between an effector inflammatory response and tolerance induction.
Introduction
Dendritic cells (DCs) are potent professional APCs that sensitize T cells to switch on appropriate immune responses to Ag danger signals. For this to occur, they take up Ags in peripheral tissues and transport them to lymphoid organs, where they interact with T cells to prime a specific response.1 This property of DCs implies particular characteristics, including high expression of HLA class II molecules for efficient Ag presentation, high expression of CD80 and CD86 costimulatory molecules, and production of T-cell activating cytokines, such as IL-12, to trigger differentiation of T cells into efficient Th1 effector cells.2 To acquire these characteristics, DC precursors differentiate into immature (iDC) and then to mature DCs (mDCs)3 as a result of activation mediated by GM-CSF and IL-4 and by TLR-activating products such as lipopolysaccharides (LPS). However, the functional properties of DCs vary depending on the stage of maturation. Thus, mDCs stimulate T-cell immunity, whereas iDCs favor T-cell tolerance rather than immunity.4 Furthermore, autoimmune diseases such as systemic lupus erythematosus (SLE) might result from break-in peripheral tolerance because of the activation of DCs.5 If so, the control of the maturation and function of DCs should provide important tools to modulate aberrant autoimmune and inflammatory responses.6,7
B cells were first recognized as immune cells committed to Ab production, ie, defined as effector cells of the humoral immune response. However, the authors of numerous studies have since discovered that B cells are regulators of immunity.8 They have the ability to synthesize cytokines,9 to act as APCs for T cells,10 and to govern the architecture of secondary lymphoid organs by encouraging the development of DCs.11 Moreover, murine models of autoimmune and inflammatory diseases have suggested that B cells also may regulate pathologic processes.
After the release of one or several mediators and/or direct cell-to-cell contacts, B cells can modulate DC functions and consequently immune responses. Thus, in mice with lupus, autoreactive B cells contribute to the severity of the disorder once activated through their TLR9 but also exert a negative role on DC function if they secrete considerable amount of IL-10.12 In the μMT mouse model of arthritis, B cells are lacking and hence, DCs produce high quantities of proinflammatory IL-12p70 and do not secrete IL-4, compared with wild-type mice. This DC-mediated exacerbation of the inflammatory response is caused by the absence of IL-10high–secreting B cells.13
The results of these experiments suggest that B cells have the ability to regulate DC functions and thereby to control the induction of immune responses. However, much evidence indicates that B cells can also modulate the immune reactions by controlling T-cell responses. For example, in the TCRα−/− Igμ−/− murine model of inflammatory bowel disease,14 the development of spontaneous chronic colitis is down-regulated by the transfer of B cells from TCRα−/− mice. B cells impair the severity of disease by interactions of CD40 and B7.2 with T-cell CD40L and CD28, respectively.15 However, excessive Th1 immunity in nonobese diabetic mice can be suppressed by the transfer of LPS-activated B cells that bind to Th1 cells and produce large amounts of TGF-β1. This action inhibits APC activity, restricts T-cell proliferation, and induces Th1 cells into apoptosis.16 Overall, these murine models strongly support a pivotal role for B cells in the final outcome of the immune responses, even in pathologic conditions, and on the basis of various molecular mechanisms.
To date, few data are available regarding the role of regulatory B cells in humans. It has been shown recently that the CD19+CD24highCD38high cells suppress the differentiation of Th1 cells by the secretion of IL-10 and cell-to-cell contacts through CD80 and CD86.17 Furthermore, we have demonstrated that T cells use CD40L-CD40 contact with the B cells to induce their own B-cell–dependent regulation.18 In addition, we distinguished control of T-cell proliferation from control of the Th1 differentiation. T-cell differentiation depends on IL-10 production and CD86 interactions, whereas proliferation depends on CD40 interactions and does not require any soluble factor. Interestingly, this regulation was defective in human SLE.
In this study, we asked whether human DCs can be controlled by B lymphocytes and evaluated the efficiency of the B-cell regulation in autoimmunity. We have demonstrated that, on activation by CD40L and CpG-oligodeoxynucleotides (ODN), human B cells inhibit the maturation and function of DCs. They modulate the maturation of monocytes into iDCs and slow down their differentiation into mDCs. They also reduce the expression of HLA-DR, CD80, and CD86 coactivation molecules and the production of IL-12p70 from mDCs. The DC-induced T-cell proliferation was therefore restrained. Cell-to-cell contacts without provision of soluble factors were sufficient to regulate the expression of cell-surface molecules. Among them was CD62L, which participated into the regulation of CD80 and CD86, whereas cell-to-cell contacts in association with an unidentified soluble factor regulated the production of IL-12.
Our data suggest that human B cells can be potent regulators of inflammation induced by DCs. In addition, mDCs from patients with SLE were refractory to the regulation of IL-12p70 by either SLE or control B cells, suggesting that the lack of B-cell regulation in SLE may be because of inefficient response of mDCs. The insensitivity of mDCs to these regulatory B cells may influence the balance between effector inflammatory responses and tolerance induction in SLE and may contribute to the pathogenesis of this autoimmune disorder.
Methods
Isolation of cells
Peripheral blood from healthy control subjects was donated by healthy laboratory staff members. Blood samples from patients with SLE and rheumatoid arthritis (RA) were collected after we obtained their informed consent in accordance with the Declaration of Helsinki. Ethical approval was provided by the Institutional Review Board of the Brest University Hospital. All patients fulfilled the criteria for the respective diseases.19,20
Tonsils were minced up and filtered to remove tissue fragments and clumps. Blood samples and tonsillar cell suspensions were layered onto Ficoll-Hypaque and centrifuged. Mononuclear cells were incubated with neuraminidase-treated sheep red blood cells and T cells depleted by a second 30-minute round of centrifugation. B cells were further purified by negative selection with a B-cell enrichment kit (StemCell Technologies) and monocytes purified by a positive CD14 selection kit (StemCell Technologies). All preparations were > 95% pure CD19+ B cells and > 94% pure monocytes, respectively.
B cells were cultured for 5 days in 24-well plates in RPMI-1640 medium supplemented with 10% inactivated FCS, 2mM L-glutamine (Invitrogen Life Technologies), 100 μg/mL streptomycin, and 200 U/mL penicillin (RPMI-1640 complete medium). They were seeded at 5.105 cells/mL and stimulated on 5.103 NIH-3T3 fibroblasts transfected with or without human CD40L gene and treated with mitomycin C in the presence of 0.25μM CpG-ODN 2006 (Cayla-InvivoGen).
Monocytes were seeded at 5.105 cells/mL in 24-well plates in RPMI-1640 complete medium in the presence of 1000 U/mL recombinant human (rh) GM-CSF and 500 U/mL rhIL-4 for 6 days. At day 3, one-half of the medium was renewed with fresh RPMI-1640 complete medium supplemented with cytokines. After 6 days, differentiated iDCs were harvested, and 4.104 cells/well were cultured for 3 days in 96-well plates in RPMI-1640 complete medium in the presence of 1000 U/mL rhIFN-γ and 100 ng/mL LPS to obtain mDCs.
Coculture assays
Monocytes were purified and seeded at 4.104 cells in 96-well plates in complete RPMI-1640 medium in the presence of 1.6 × 105 activated B cells (ratio 1 monocyte/4 B cells). They were stimulated with GM-CSF and IL-4 and analyzed by flow cytometry after 2-6 days.
After a 6-day culture, differentiated iDCs were harvested and seeded at 4.104 iDCs in 96-well plates in RPMI-1640 complete medium in the presence of 1.6 × 105 activated B cells (ratio 1 iDC/4 B cells). They were stimulated with IFN-γ and LPS, and cells were analyzed by flow cytometry after 2 days.
At the beginning of the 8th day of DC cultures, one-half of the medium was removed and substituted by activated B cells in their supernatant or resting B cells in RPMI-1640 complete medium at a ratio 1 mDC/4 B cells, unless otherwise indicated. Cell analyses were then performed after 2 days by flow cytometry.
For the blocking experiments, mDCs or activated B cells were preincubated for 1 hour before coculture with anti-CD40L (R&D Systems) or anti-CD54 (R&D Systems) Abs, or with anti–IL-10 (BD Biosciences), anti–TGF-β (Abcam), anti-CD80 (ImmunoTools), anti-CD86 (ImmunoTools), anti-CD18 (Beckman Coulter), or anti-CD62L (R&D Systems) Abs, respectively, as previously determined.18 Isotype-matched Abs were also used as controls.
For the experiments in Transwells, one-half of the medium of DC cultures was removed at the beginning of the 8th day, mDCs were left in the lower chamber, and activated B cells in their supernatant added in the upper chamber. Cells were analyzed by flow cytometry after 2 days. For the experiments with T cells, 2 × 105 CFSE-labeled T cells were cultured in the presence of mDCs for 5 days. With flow cytometry, we evaluated T-cell proliferation by measuring the decrease in the mean fluorescence intensity (MFI) of CFSE. Activated B cells were added at the onset of the mixed lymphocytic reaction.
Flow cytometry
All mAbs were purchased from Bekman-Coulter, unless otherwise specified. We used FITC-conjugated anti-CD5, anti-CD11b, anti-CD14, anti-CD18, anti-CD19, and anti-CD25 (Dako), anti-CD27 (BD Biosciences), anti-CD54, anti-CD58, anti-CD62L, anti-CD80, anti-CD83, and anti-IgD (BD Biosciences), PE-conjugated anti-CD3, anti-CD5, anti-CD11c, anti-CD19, anti-CD24, anti-CD27, anti-CD40, anti-CD45RO, and anti-CD56 (Dako), anti-CD86 and anti-IgD (BD Biosciences); and PE-linked to cyanin (PC) 5-conjugated anti-CD38, anti-HLA-DR, and PC7-conjugated anti-CD19.
Intracellular staining for IL-12 was performed after permeabilization of the cells by the use of cytofix/cytoperm permeabilization kit (BD Biosciences) with PE-conjugated anti–IL-12p70 mAb (BD Biosciences). In coculture assays, PC7-conjugated anti-CD19 enabled the distinguishing of CD19-positive B cells from CD19-negative monocytes, DCs, or T cells. Cells were examined on an Epics XL or FC500 flow cytometer (Beckman-Coulter) and the results analyzed with EXPO32 analysis software (Beckman-Coulter).
ELISA
IL-10 and IL-12p70 in the supernatants of cultured and cocultured cells were measured at different dilutions with commercial ELISA kits with the use of paired Abs from BD Biosciences and Beckman-Coulter, respectively. RPMI-1640 complete medium was used as negative control.
Statistical analysis
Statistical analyses were performed with Prism 4 Graphpad software. Comparisons were performed with the Wilcoxon test for paired data. P < .05 was considered as significant.
Results
B cells regulate DC maturation
To obtain mDCs, purified monocytes were stimulated with GM-CSF and IL-4 to differentiate them into iDCs and then with LPS and IFN-γ to ensure their complete maturation into mDCs. The expression of CD83 was thus induced and increased, confirming the differentiation of monocytes into DCs (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). A 6-day stimulation with GM-CSF and IL-4 triggered the differentiation into iDCs that up-regulated CD54, CD40, HLA-DR, CD80, and CD86 molecules on their surface without synthesis of IL-12. The LPS and IFN-γ costimulation gave rise to an induction of intracellular IL-12p70 production, which characterized their full maturation at day 7. IL-12 synthesis was further enhanced at day 9 and associated with a significant secretion of the cytokine (122.8 ± 67.9 μg/mL). All molecules examined were further elevated as well, which substantiated the complete and homogeneous maturation of monocytes into mDCs.
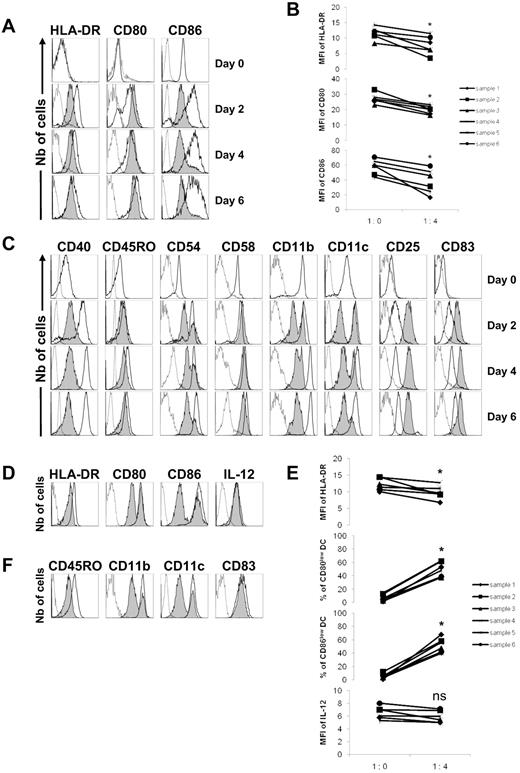
Activated tonsillar B cells slow down the differentiation of monocytes into iDCs and delay the complete maturation of iDCs into mDCs. Tonsillar B cells were purified and stimulated for 5 days on CD40L-transfected NIH3T3 murine fibroblasts in the presence of 0.25μM CpG-ODN 2006. Peripheral blood monocytes (A-C) were purified from healthy donors (day 0), cultured alone (1:0), or with activated B cells added with their supernatant (ratio 1 monocyte/4 B cells) in the presence of GM-CSF (1000 U/mL) and IL-4 (500 U/mL) for 6 days. (A) Expression of HLA-DR, CD80, and CD86 were evaluated by flow cytometry on CD19-negative cells at days 2, 4, and 6. A representative experiment is shown with dotted lines corresponding to isotype controls, open histograms to monocytes cultured alone, and gray histograms to monocytes cocultured with B cells. (B) MFI of HLA-DR, CD80, and CD86. *P < .05. (C) Representative example of the expression of CD40, CD45RO, CD54, CD58, CD11b, CD11c, CD25, and CD83 evaluated by flow cytometry. In other experiments, monocytes were purified from the peripheral blood of healthy donors and stimulated with GM-CSF and IL-4 for 6 days. (D-F) Differentiated iDCs were then cultured alone (1:0) or with activated B cells added with their supernatant (ratio 1 iDC/4 B cells), in the presence of LPS (100 ng/mL) and IFN-γ (1000 U/mL) for 48 hours. (D) Expression of HLA-DR, CD80, CD86, and IL-12 were evaluated by flow cytometry on CD19-negative cells. A representative experiment is shown with dotted lines corresponding to isotype controls, open histograms to iDCs cultured alone, and gray histograms to iDCs cocultured with B cells. (E) MFI of HLA-DR, percentages of CD80low and CD86low iDCs, and MFI of IL-12 are shown. *P < .05; ns, nonsignificant. (F) Representative example of the expression of CD45RO, CD11b, CD11c, and CD83 evaluated by flow cytometry.
Activated tonsillar B cells slow down the differentiation of monocytes into iDCs and delay the complete maturation of iDCs into mDCs. Tonsillar B cells were purified and stimulated for 5 days on CD40L-transfected NIH3T3 murine fibroblasts in the presence of 0.25μM CpG-ODN 2006. Peripheral blood monocytes (A-C) were purified from healthy donors (day 0), cultured alone (1:0), or with activated B cells added with their supernatant (ratio 1 monocyte/4 B cells) in the presence of GM-CSF (1000 U/mL) and IL-4 (500 U/mL) for 6 days. (A) Expression of HLA-DR, CD80, and CD86 were evaluated by flow cytometry on CD19-negative cells at days 2, 4, and 6. A representative experiment is shown with dotted lines corresponding to isotype controls, open histograms to monocytes cultured alone, and gray histograms to monocytes cocultured with B cells. (B) MFI of HLA-DR, CD80, and CD86. *P < .05. (C) Representative example of the expression of CD40, CD45RO, CD54, CD58, CD11b, CD11c, CD25, and CD83 evaluated by flow cytometry. In other experiments, monocytes were purified from the peripheral blood of healthy donors and stimulated with GM-CSF and IL-4 for 6 days. (D-F) Differentiated iDCs were then cultured alone (1:0) or with activated B cells added with their supernatant (ratio 1 iDC/4 B cells), in the presence of LPS (100 ng/mL) and IFN-γ (1000 U/mL) for 48 hours. (D) Expression of HLA-DR, CD80, CD86, and IL-12 were evaluated by flow cytometry on CD19-negative cells. A representative experiment is shown with dotted lines corresponding to isotype controls, open histograms to iDCs cultured alone, and gray histograms to iDCs cocultured with B cells. (E) MFI of HLA-DR, percentages of CD80low and CD86low iDCs, and MFI of IL-12 are shown. *P < .05; ns, nonsignificant. (F) Representative example of the expression of CD45RO, CD11b, CD11c, and CD83 evaluated by flow cytometry.
Most murine B cells require activation before the acquisition of regulatory function. Therefore, to facilitate the development of regulatory capacities, human B cells were polyclonally stimulated on CD40L-transfected fibroblasts with CpG-ODN 2006. They were stained with propidium iodide and FITC-conjugated annexin V in preliminary experiments. Activated B cells were found viable with 96% ± 3% of propidium iodide-negative/annexin V–negative cells, thus indicating a high rate of survival and the absence of apoptotic cells.
We first asked whether B cells had a potential effect on the maturation process, ie, at precursors level to retard monocyte differentiation into iDCs and then, at iDCs level, to prevent their complete maturation into mDCs. When monocytes were stimulated in the presence of activated tonsillar B cells, their differentiation into iDCs was contained because the GM-CSF and IL-4–dependent up-regulation of the markers was lower (Figure 1A). Thus, the MFIs of HLA-DR, CD80, and CD86 were significantly diminished (P < .05) on day 6 (Figure 1B). The whole monocyte population was also affected as seen by CD40, CD45RO, CD58, and CD11b expression that decreased homogeneously, leading to a unique population of dimly positive cells (Figure 1C). However, reduction of CD54 and CD11c expression was observed, but not on all iDCs. Furthermore, the expression of CD25 and CD83 was increased, implying that regulatory effects were specific. These results suggest that human B cells have a regulatory influence over monocytes to prevent their optimal differentiation into iDCs.
Differentiated iDCs were then stimulated at day 6 with LPS and IFN-γ in the presence of activated B cells for another 2 days. Expression of HLA-DR decreased on the whole iDC population, and subpopulations of CD80low and CD86low iDCs appeared, whereas the production of IL-12p70 was unaffected (Figure 1D). Thus, B cells generated a unique population of iDCs with significantly less HLA-DR expression (P < .05), and also high numbers of cells (P < .05) that weakly expressed CD80 and CD86 (Figure 1E). Singularly, CD45RO was down-regulated on all iDCs, and the B-cell regulation on CD11b and CD11c was restricted to a subpopulation of iDCs, whereas CD83 expression remained unchanged (Figure 1F). Overall, these data indicate that B cells have the capacity to slow-down the differentiation of iDCs into mDCs.
B cells regulate mDC functions
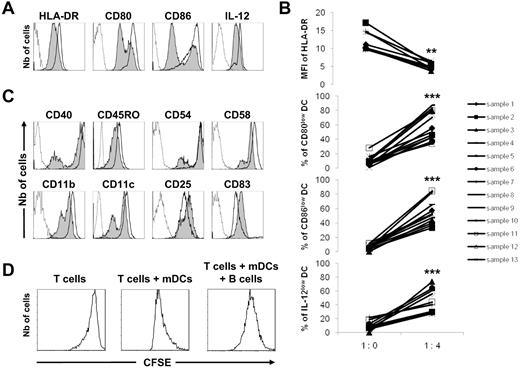
Having established the regulatory capacity of B cells over DC maturation, we asked the question as to whether it could affect mDC functions. To this end, mDCs at day 7 were further stimulated with LPS and IFN-γ for 2 days in the presence of activated B cells. At day 9, mDCs revealed a diminished surface expression of HLA-DR, CD80, and CD86 molecules as well as reduced intracellular production of IL-12p70, compared with mDCs cultured alone (Figure 2A). The density of HLA-DR molecules was significantly and homogeneously decreased (P < .01) and was associated with a larger proportion (P < .001) of CD80low, CD86low, and IL-12p70low mDCs (Figure 2B). Moreover, we identified 2 populations of mDCs with lower densities of CD40, CD45RO, CD54, CD58, CD11b, and CD11c in the presence of B cells, whereas CD25 and CD83 were up-regulated (Figure 2C). These experiments indicate that activated B cells have the ability to regulate mDC functions by modulating the expression of functional markers.
Activated tonsillar B cells have negative regulatory effects on mDC functions. Monocytes were purified from the peripheral blood of healthy donors and stimulated with GM-CSF (1000 U/mL) and IL-4 (500 U/mL) for 6 days. The differentiated immature DCs were stimulated with LPS (100 ng/mL) and IFN-γ (1000 U/mL) for 24 hours to generate mDCs. Tonsillar B cells were purified and stimulated for 5 days on CD40L-transfected NIH3T3 murine fibroblasts in the presence of 0.25μM CpG-ODN 2006. (A) mDCs were cultured alone (1:0) or in the presence of activated B cells with their supernatant (ratio 1 DC/4 B cells) for a further 48 hours. Expression of HLA-DR, CD80, CD86, and IL-12 was evaluated by flow cytometry on CD19-negative cells. A representative experiment is shown with dotted lines corresponding to isotype controls, open histograms to mDCs cultured alone, and gray histograms to mDCs cocultured with B cells. (B) MFI of HLA-DR (9 samples), and percentages of CD80low (13 samples), CD86low (11 samples), and IL-12low (11 samples) mDCs are shown. **P < .01; ***P < .001. (C) Representative example of the expression of CD40, CD45RO, CD54, CD58, CD11b, CD11c, CD25, and CD83 evaluated by flow cytometry. (D) CFSE-labeled T cells were cultured alone or in the presence of mDCs for 5 days. The T-cell proliferative response was evaluated by the dilution of CFSE expression by flow cytometry. Activated B cells were also added at the onset of the coculture. A representative experiment is shown.
Activated tonsillar B cells have negative regulatory effects on mDC functions. Monocytes were purified from the peripheral blood of healthy donors and stimulated with GM-CSF (1000 U/mL) and IL-4 (500 U/mL) for 6 days. The differentiated immature DCs were stimulated with LPS (100 ng/mL) and IFN-γ (1000 U/mL) for 24 hours to generate mDCs. Tonsillar B cells were purified and stimulated for 5 days on CD40L-transfected NIH3T3 murine fibroblasts in the presence of 0.25μM CpG-ODN 2006. (A) mDCs were cultured alone (1:0) or in the presence of activated B cells with their supernatant (ratio 1 DC/4 B cells) for a further 48 hours. Expression of HLA-DR, CD80, CD86, and IL-12 was evaluated by flow cytometry on CD19-negative cells. A representative experiment is shown with dotted lines corresponding to isotype controls, open histograms to mDCs cultured alone, and gray histograms to mDCs cocultured with B cells. (B) MFI of HLA-DR (9 samples), and percentages of CD80low (13 samples), CD86low (11 samples), and IL-12low (11 samples) mDCs are shown. **P < .01; ***P < .001. (C) Representative example of the expression of CD40, CD45RO, CD54, CD58, CD11b, CD11c, CD25, and CD83 evaluated by flow cytometry. (D) CFSE-labeled T cells were cultured alone or in the presence of mDCs for 5 days. The T-cell proliferative response was evaluated by the dilution of CFSE expression by flow cytometry. Activated B cells were also added at the onset of the coculture. A representative experiment is shown.
To prove the physiologic significance of these findings, ex vivo–sorted DCs were stimulated with LPS and IFN-γ for 3 days in the presence of activated B cells. The surface expression of HLA-DR, CD80, and CD86 molecules as well as intracellular IL-12p70 production were reduced, compared with DCs cultured alone (supplemental Figure 2). These data manifest that B cells can exert their regulatory effect on in vitro–generated as well as ex vivo–sorted DCs.
Although the intracellular synthesis of IL-12 in mDCs was significantly decreased in the presence of B cells, the IL-12p70 concentration was increased in the supernatant of cocultured cells (178.7 ± 74.2 μg/mL). This result can be explained by the fact that activated B cells alone also secreted IL-12p70 (40.8 ± 29.3 μg/mL). Similar observations were made regarding the secretion of IL-6 (not shown). We therefore concluded that the B-cell–dependent regulation could not be assessed at the secretion level in vitro.
To substantiate the regulatory function of activated B cells, their effect on mDC-dependent control of the T-cell proliferation was assessed. To this end, mDCs at day 9 were cultured with CFSE-labeled T cells for 5 days. The support of the T-cell proliferation was then evaluated in the presence of activated B cells. The proliferative response of T cells was increased by mDCs, compared with T cells cultured alone, and the mDC-dependent T-cell proliferation was dampened in the presence of activated B cells (Figure 2D). These experiments illustrate the functional deregulation of mDCs in the presence of activated B cells.
B-cell regulation requires cell-to-cell contacts
Coactivation of CD40 and TLR9 on B cells induced the secretion of IL-10 (1073.9 ± 815.6 μg/mL) that has been suspected to play a key regulatory role in several models.12,13,17,18 Furthermore, IL-10 has been identified as a key cytokine to control the phenotype of monocytes21,22 and of iDCs.23 We have therefore sought ways to measure the importance of IL-10 in the regulatory effect of human B cells on mDCs.
On the one hand, mDCs were cultured in the supernatant of activated B cells with or without anti–IL-10–blocking Ab. After 48 hours, the level of HLA-DR and proportions of CD80low and CD86low cells were similar in the mDCs cultured in the B-cell supernatant to those cultured in their medium (Figure 3A). However, there was a significant increase in the proportion of IL-12p70low cells (P < .05), indicating that the production of IL-12, but not the density of surface markers, was regulated by a soluble factor. Anti–IL-10–blocking Abs impaired neither the expression of HLA-DR, CD80, and CD86 nor the synthesis of IL-12. These results suggest that IL-10 from activated B cells does not exert modulatory effects on cell surface molecules expression or on production of IL-12p70 by mDCs.
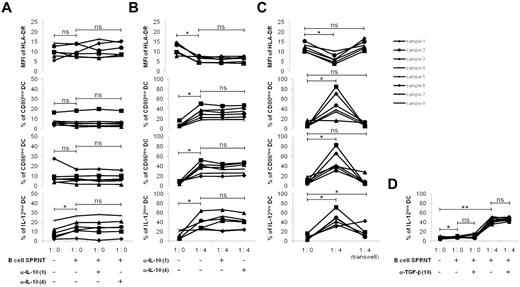
The regulation of DC functions is independent of IL-10 but requires cell-to-cell contact. Monocytes were purified from the peripheral blood of healthy donors and stimulated with GM-CSF (1000 U/mL) and IL-4 (500 U/mL) for 6 days. The differentiated immature DCs were stimulated with LPS (100 ng/mL) and IFN-γ (1000 U/mL) for 24 hours to generate mDCs. Tonsillar B cells were purified and stimulated for 5 days on CD40L-transfected NIH3T3 murine fibroblasts in the presence of 0.25μM CpG-ODN 2006. mDCs were cultured for 48 hours with or without anti–IL-10–blocking Abs (α-IL-10), at 1 or 4 μg/mL final concentration (A) in the supernatant of activated B cells (1:0), or (B) with activated B cells in their supernatant (ratio 1 DC/4 B cells). (C) Experiments were repeated in Transwells with mDCs in the lower chamber and B cells with their supernatant in the upper chamber. Cocultures without Transwells were used as controls. MFI of HLA-DR and percentages of CD80low, CD86low, and IL-12low mDCs were evaluated by flow cytometry on CD19-negative cells. (D) mDCs were cocultured with or without 10 μg/mL of anti–TGF-β–blocking Ab (α-TGF-β) in the supernatant of activated B cells (1:0) or in the presence of activated B cells with their supernatant (ratio 1 DC/4 B cells). The percentage of IL-12lowmDCs was evaluated by flow cytometry. *P < .05; **P < .01; ns, nonsignificant.
The regulation of DC functions is independent of IL-10 but requires cell-to-cell contact. Monocytes were purified from the peripheral blood of healthy donors and stimulated with GM-CSF (1000 U/mL) and IL-4 (500 U/mL) for 6 days. The differentiated immature DCs were stimulated with LPS (100 ng/mL) and IFN-γ (1000 U/mL) for 24 hours to generate mDCs. Tonsillar B cells were purified and stimulated for 5 days on CD40L-transfected NIH3T3 murine fibroblasts in the presence of 0.25μM CpG-ODN 2006. mDCs were cultured for 48 hours with or without anti–IL-10–blocking Abs (α-IL-10), at 1 or 4 μg/mL final concentration (A) in the supernatant of activated B cells (1:0), or (B) with activated B cells in their supernatant (ratio 1 DC/4 B cells). (C) Experiments were repeated in Transwells with mDCs in the lower chamber and B cells with their supernatant in the upper chamber. Cocultures without Transwells were used as controls. MFI of HLA-DR and percentages of CD80low, CD86low, and IL-12low mDCs were evaluated by flow cytometry on CD19-negative cells. (D) mDCs were cocultured with or without 10 μg/mL of anti–TGF-β–blocking Ab (α-TGF-β) in the supernatant of activated B cells (1:0) or in the presence of activated B cells with their supernatant (ratio 1 DC/4 B cells). The percentage of IL-12lowmDCs was evaluated by flow cytometry. *P < .05; **P < .01; ns, nonsignificant.
On the other hand, the possibility exists that IL-10 acted in cooperation with a direct contact between the membrane of mDCs and that of B cells. To test this hypothesis, mDCs were cultured with activated B cells kept in their supernatant, with or without anti–IL-10–blocking Abs (Figure 3B). As already observed in the presence of B cells, the MFI of HLA-DR decreased on mDCs (P < .05), and the number of CD80low, CD86low, and IL-12p70low mDCs increased (P < .05). This regulatory effect was not altered by the addition of the anti–IL-10–blocking Abs because the lower MFI of HLA-DR and the greater frequencies of CD80low, CD86low, and IL-12p70low mDCs remained constant. Thus, these experiments indicated that IL-10 did not affect mDCs, even in presence of B cells.
In addition, soluble factor(s) other than IL-10 could act synergistically with cell-to-cell contacts. To determine their importance, cultures were performed in a Transwell system with mDCs in the lower chamber and activated B cells in their supernatant in the upper chamber (Figure 3C). B cells diminished the HLA-DR MFI (P < .05) and favored the appearance of CD80low, CD86low, and IL-12p70low mDCs (P < .05), provided cell-to-cell contacts were possible. However, when B cells did not interact with mDCs the MFI of HLA-DR and the percentages of CD80low and CD86low mDCs were similar to that of mDCs alone, whereas the percentages of IL-12p70low cells remained significantly greater (P < .05). These results demonstrate that B cells require only direct cell-to-cell contacts with mDCs to regulate HLA-DR, CD80, and CD86 expressions, and this strengthens the idea that B-cell–soluble factor(s) that are involved in the regulation of IL-12p70 are likely to act synergistically with cell-to-cell contacts for their effects.
On the basis of our finding that IL-10 does not play a role here, we wondered whether TGF-β could be the regulator of IL-12 production by mDCs. Addition of anti–TGF-β–blocking Abs to B-cell supernatant did not affect the frequency of IL-12p70low mDCs, even in the presence of B cells (Figure 3D). These data indicate that TGF-β is not the B cell–derived cytokine that regulates the production of IL-12p70 by mDCs in our model.
The phenotype of the regulatory B cells
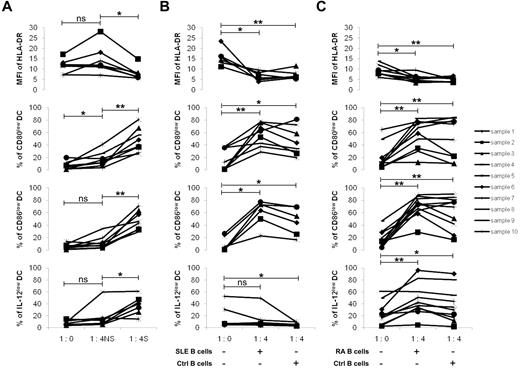
We then asked the question as to whether nonstimulated B cells could possess intrinsic regulatory capacities. In the presence of freshly isolated B cells, a decrease in the MFI of HLA-DR was not observed, whereas the populations of CD80low (P < .01), CD86low (P < .02), and IL12p70low (P < .01) mDCs were slightly increased (Figure 4A). Furthermore, activated B cells induced stronger regulatory effects that were statistically more important on the 3 mDC functions than those with the nonstimulated B cells (P < .05 for HLA-DR, P < .001 for the proportions of CD80low and CD86low mDCs, and P < .01 for the proportion of IL-12p70low mDCs). Although a limited regulation was observed with nonstimulated B cells (likely because of a preliminary in vivo activation within tonsils), these results indicate that stimulation of B cells is necessary to trigger potent regulatory activities on mDCs.
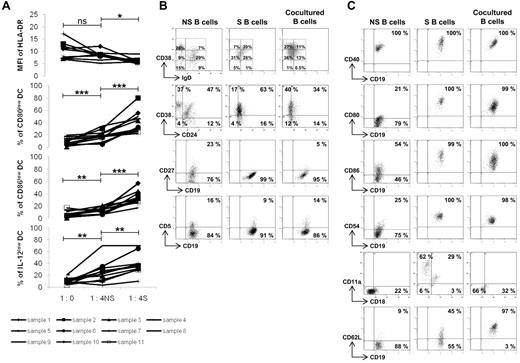
Characterization of the regulatory B cells. Monocytes were purified from the peripheral blood of healthy donors and stimulated with GM-CSF (1000 U/mL) and IL-4 (500 U/mL) for 6 days. The differentiated immature DCs were stimulated with LPS (100 ng/mL) and IFN-γ (1000 U/mL) for 24 hours to generate mature DCs. Tonsillar B cells were purified and stimulated for 5 days on CD40L-transfected NIH3T3 murine fibroblasts in the presence of 0.25μM CpG-ODN 2006. Mature DCs were cultured for 48 hours alone (ratio 1:0) or in the presence of nonstimulated (NS) or stimulated (S) B cells in their supernatant at a ratio (1 DC/4 B cells). (A) MFI of HLA-DR (9 samples), and percentages of CD80low, CD86low, and IL-12low DCs (11 samples) were evaluated by flow cytometry on CD19-negative cells. *P < .05; **P < .01; ***P < .001; ns, nonsignificant. (B) Expression of IgD/CD38, CD24/CD38, CD19/CD27, and CD19/CD5 was evaluated by flow cytometry on CD19-positive B cells before stimulation (NS), after stimulation (S) and after a 48-hour coculture period (cocultured). (C) Expression of CD19/CD40, CD19/CD80, CD19/CD86, CD19/CD54, CD18/CD11a, and CD19/CD62L was similarly analyzed. Representative results are shown of 3 experiments.
Characterization of the regulatory B cells. Monocytes were purified from the peripheral blood of healthy donors and stimulated with GM-CSF (1000 U/mL) and IL-4 (500 U/mL) for 6 days. The differentiated immature DCs were stimulated with LPS (100 ng/mL) and IFN-γ (1000 U/mL) for 24 hours to generate mature DCs. Tonsillar B cells were purified and stimulated for 5 days on CD40L-transfected NIH3T3 murine fibroblasts in the presence of 0.25μM CpG-ODN 2006. Mature DCs were cultured for 48 hours alone (ratio 1:0) or in the presence of nonstimulated (NS) or stimulated (S) B cells in their supernatant at a ratio (1 DC/4 B cells). (A) MFI of HLA-DR (9 samples), and percentages of CD80low, CD86low, and IL-12low DCs (11 samples) were evaluated by flow cytometry on CD19-negative cells. *P < .05; **P < .01; ***P < .001; ns, nonsignificant. (B) Expression of IgD/CD38, CD24/CD38, CD19/CD27, and CD19/CD5 was evaluated by flow cytometry on CD19-positive B cells before stimulation (NS), after stimulation (S) and after a 48-hour coculture period (cocultured). (C) Expression of CD19/CD40, CD19/CD80, CD19/CD86, CD19/CD54, CD18/CD11a, and CD19/CD62L was similarly analyzed. Representative results are shown of 3 experiments.
Therefore, to identify the B-cell subsets and the contact molecules involved in this regulation, B cells were phenotyped before and after activation by CD40L/CpG-ODN, and after a 48-hour delay in culture with mDCs. Activation of B cells and interaction with mDCs led to the emergence of a homogeneous population of IgDlowCD38+ B cells (Figure 4B). Furthermore, there was a shift to a CD38+CD24low population. There was the disappearance of CD27, suggesting that memory B cells did not have regulatory effects. The CD5+ B cell population, which is increased and involved in the regulation of T cells,18 remained stable. Overall, B cells having a regulatory effect on mDCs were defined as CD19+IgDlowCD38+CD24lowCD27− and CD5+/−.
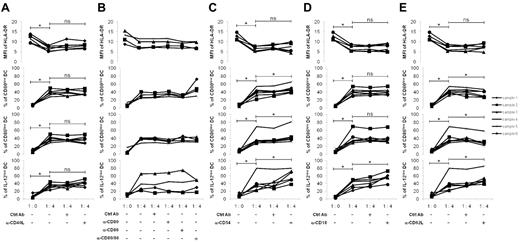
The expression of cell-interaction molecules and those involved in cellular adhesion were also measured (Figure 4C). Nonstimulated B cells were CD40+ and remained so after CD40L/CpG-ODN activation. In coculture, CD40 density increased in B cells. Because DCs can express CD40L,24 CD40 appeared to be a viable candidate in terms of having a role in the regulatory process. mDCs and activated B cells were cultured in the presence of anti-CD40L–blocking Ab (Figure 5A). Their regulatory effect on HLA-DR, CD80, CD86, and IL-12p70 expressions still persisted (P < .05) and was not statistically modified. It is therefore unlikely that CD40-CD40L interactions had a role in the regulatory effects of B cells in our model.
Characteristic of the intercellular contact involved in regulation. Monocytes were purified from the peripheral blood of healthy donors and stimulated with GM-CSF (1000 U/mL) and IL-4 (500 U/mL) for 6 days. The differentiated immature DCs, were stimulated with LPS (100 ng/mL) and IFN-γ (1000 U/mL) for 24 hours to generate mature DCs. Tonsillar B cells were purified and stimulated for 5 days on CD40L-transfected NIH3T3 murine fibroblasts in the presence of 0.25μM CpG-ODN 2006. Mature DCs were cultured for 48 hours alone (1:0) or with activated B cells in their supernatant (ratio 1 DC/4 B cells) in the presence of blocking Ab for (A) CD40 (α-CD40L), (B) CD80 and CD86 (α-CD80 and α-CD86), (C) CD54 (α-CD54), (D) CD18 (α-CD18), and (E) CD62L (α-CD62L) at 10 μg/mL final concentration. A specific isotype control for each blocking Ab was used. Six samples were evaluated except for α-CD80 and α-CD86 Ab (4 samples). MFI of HLA-DR, and percentages of CD80low, CD86low, and IL-12low DCs were evaluated by flow cytometry on CD19-negative cells. *P < .05; ns, nonsignificant.
Characteristic of the intercellular contact involved in regulation. Monocytes were purified from the peripheral blood of healthy donors and stimulated with GM-CSF (1000 U/mL) and IL-4 (500 U/mL) for 6 days. The differentiated immature DCs, were stimulated with LPS (100 ng/mL) and IFN-γ (1000 U/mL) for 24 hours to generate mature DCs. Tonsillar B cells were purified and stimulated for 5 days on CD40L-transfected NIH3T3 murine fibroblasts in the presence of 0.25μM CpG-ODN 2006. Mature DCs were cultured for 48 hours alone (1:0) or with activated B cells in their supernatant (ratio 1 DC/4 B cells) in the presence of blocking Ab for (A) CD40 (α-CD40L), (B) CD80 and CD86 (α-CD80 and α-CD86), (C) CD54 (α-CD54), (D) CD18 (α-CD18), and (E) CD62L (α-CD62L) at 10 μg/mL final concentration. A specific isotype control for each blocking Ab was used. Six samples were evaluated except for α-CD80 and α-CD86 Ab (4 samples). MFI of HLA-DR, and percentages of CD80low, CD86low, and IL-12low DCs were evaluated by flow cytometry on CD19-negative cells. *P < .05; ns, nonsignificant.
B cells needed to be stimulated to express high levels of CD80 and CD86 (Figure 4C). These molecules have already been shown to have a role in murine B-cell regulation of the T-cell response15 and, although the MFI of both markers was diminished on cocultured B cells, they could still potentially play a similar role in their interaction with DCs. However, this does not seem to be the case in our model because blocking either CD80 or CD86, or both at the same time, did not interfere with B-cell regulation (Figure 5B). Moreover, the expression level of both CD54 and the ligand CD11a/CD18 molecules increased on the B cells after activation and slightly diminished in the coculture (Figure 4C). Coexpression on DCs makes them credible as candidates. Preincubation of mDCs with anti-CD54–blocking Ab did not alter the B-cell regulatory effect (Figure 5C). The decreased expression of HLA-DR molecules was maintained, and percentages of CD80low, CD86low, and IL-12p70low mDCs populations were even further increased (P < .05). It is thus unlikely that the CD54 molecule is important in the interactions of B cells with DCs responsible for regulation. This finding was confirmed by the preincubation of B cells with anti-CD18–blocking Ab (Figure 5D). The decrease of HLA-DR as well as the increase in CD80low and CD86low mDCs persisted, whereas the percentages of IL-12p70low mDCs were even up-regulated (P < .05).
Finally, CD62L-selectin, almost absent on nonstimulated B cells, was induced on activated B cells, and the percentage of CD62L+ B cells was even more increased after culture with mDCs (Figure 4C), suggesting a role for CD62L in the regulation process. The addition of anti-CD62L–blocking Abs had various effects (Figure 5E). The regulation of HLA-DR molecules was unaffected, and the decreased MFI did not change. However, the percentages of CD80low and CD86low mDCs were diminished compared with the absence of Ab (P < .05), whereas the population of IL12p70low mDCs increased (P < .05). These results suggested that CD62L might be partly involved in the cell contacts required for the regulation, at least with regard to CD80 and CD86 molecules.
B-cell regulation in autoimmune diseases
Having demonstrated the regulatory capacities of normal tonsillar B cells, we wished to analyze the regulatory efficiencies of circulating B cells from patients with autoimmune diseases. We have hypothesized that abnormal regulation of B cells could contribute to the development of exacerbated responses. To test this hypothesis, we set up cocultures of mDCs with peripheral blood B cells from healthy donors and from patients with SLE and RA. Monocytes were differentiated into mDCs and then cultured with autologous B cells. Tonsillar B cells cultured with patient's mDCs were used as controls.
Freshly isolated B cells from healthy donors first were cultured with autologous mDCs. The decreasing MFI of HLA-DR was not observed, the population of CD86low mDCs not up-regulated, and the population of IL12-p70low mDCs not increased (Figure 6A). In contrast, stimulated B cells induced a potent regulation of the 3 signals compared with resting cells (P < .05 for the decrease HLA-DR MFI, P < .01 for the increase percentages of CD80low and CD86low mDCs, and P < .05 for that of IL-12p70low mDCs). These results strongly suggest that activation of circulating B cells was necessary to trigger an efficient regulatory function of DCs.
Regulatory effect of peripheral blood B cells. Monocytes were purified from the peripheral blood of (A) healthy donors, (B) patients with SLE, or (C) patients with RA and stimulated with GM-CSF (1000 U/mL) and IL-4 (500 U/mL) for 6 days. The differentiated immature DCs were stimulated with LPS (100 ng/mL) and IFN-γ (1000 U/mL) for 24 hours to generate mature DCs. Peripheral blood B cells were stimulated for 5 days on CD40L-transfected NIH-3T3 murine fibroblasts in the presence of 0.25μM CpG-ODN 2006. (A) B cells from healthy donors were added to their autologous DCs (ratio 1 DC/4 B cells) either nonstimulated (NS) or stimulated (S) with their supernatant. MFI of HLA-DR (7 samples), percentages of CD80low (8 samples), CD86low (8 samples), and IL-12low (7 samples) DCs were evaluated by flow cytometry on CD19-negative cells. (B) B cells from patients with SLE or (C) from patients with RA were activated as described previously and added with their supernatant to autologous DCs. MFI of HLA-DR (6 and 8 samples for SLE and RA patients, respectively), percentages of CD80low (8 and 10 samples for SLE and RA patients, respectively), CD86low (6 and 10 samples for SLE and RA patients, respectively), and IL-12low (7 and 10 samples for SLE and RA patients, respectively) DCs were evaluated by flow cytometry on CD19-negative cells. Activated tonsillar B cells were used as controls (Ctrl). *P < .05; **P < .01; ns, nonsignificant.
Regulatory effect of peripheral blood B cells. Monocytes were purified from the peripheral blood of (A) healthy donors, (B) patients with SLE, or (C) patients with RA and stimulated with GM-CSF (1000 U/mL) and IL-4 (500 U/mL) for 6 days. The differentiated immature DCs were stimulated with LPS (100 ng/mL) and IFN-γ (1000 U/mL) for 24 hours to generate mature DCs. Peripheral blood B cells were stimulated for 5 days on CD40L-transfected NIH-3T3 murine fibroblasts in the presence of 0.25μM CpG-ODN 2006. (A) B cells from healthy donors were added to their autologous DCs (ratio 1 DC/4 B cells) either nonstimulated (NS) or stimulated (S) with their supernatant. MFI of HLA-DR (7 samples), percentages of CD80low (8 samples), CD86low (8 samples), and IL-12low (7 samples) DCs were evaluated by flow cytometry on CD19-negative cells. (B) B cells from patients with SLE or (C) from patients with RA were activated as described previously and added with their supernatant to autologous DCs. MFI of HLA-DR (6 and 8 samples for SLE and RA patients, respectively), percentages of CD80low (8 and 10 samples for SLE and RA patients, respectively), CD86low (6 and 10 samples for SLE and RA patients, respectively), and IL-12low (7 and 10 samples for SLE and RA patients, respectively) DCs were evaluated by flow cytometry on CD19-negative cells. Activated tonsillar B cells were used as controls (Ctrl). *P < .05; **P < .01; ns, nonsignificant.
The regulatory capacity of peripheral blood B cells from patients with SLE was therefore evaluated after CD40L and CpG-ODN activation (Figure 6B). They decreased the expression of HLA-DR (P < .05) and increased the percentages of CD80low (P < .01) and of CD86low (P < .05) autologous SLE mDCs. However, the regulation of IL-12p70 production was lacking because the percentages of IL-12p70low mDCs did not increase. Interestingly, this regulation was not observed with control B cells either, where the percentages of IL-12p70low mDCs were even diminished (P < .05).
In contrast, circulating B cells from patients with RA (Figure 6C) did not exhibit defective regulation. Thus, HLA-DR (P < .05), CD80 (P < .01), and CD86 (P < .05) molecules, as well as IL-12p70 production (P < .05) by autologous RA mDCs, were normally regulated. Furthermore, this was also the case in the presence of control B cells. The MFI of HLA-DR molecules (P < .01) decreased, and the percentages of CD80low (P < .01), CD86low (P < .01), and IL-12p70low (P < .05) mDCs increased, as expected.
Discussion
In this study, we have shown that human B cells can present a novel negative regulatory effect on monocyte-derived DCs. First, B cells stimulated via CD40 and TLR9 pathways inhibited the maturation of monocytes into iDCs and slowed-down the final differentiation into mDCs. In addition, there was a significant down-regulation in HLA-DR, CD80, CD86, and IL-12p70 expression on terminally differentiated mDCs, and subsequently a decrease of the DC-dependent T-cell proliferative response.
Reports from several investigators indicate clearly that IL-10 is principally involved in B-cell–mediated regulation of at least T-cell responses.12,14,17,18 Furthermore, murine DCs may also be the target of IL-10–derived B cells.13 Thus, through the provision of IL-10, stimulated B cells down-regulated IL-12 secreted by DCs.25 This B cell–dependent negative feedback leads to Th2 polarization by orientation of the differentiation of IL-4–producing T cells and the decrease of IFN-γ producing T cells. Likewise, DCs from μMT mice produced greater levels of IL-12 than wild-type mice, and consequently, T cells produced IFN-γ but were unable to secrete IL-4.13 This Th1/Th2 balance appears to be regulated by IL-10–producing B cells because the transfer of DCs from IL-10−/− mice into control mice resulted in reduced IL-4 production and increased IFN-γ production. However, our observations differ from these conclusions. Experiments with B cell–derived supernatant, with anti–IL-10 Abs, anti–TGF-β Abs, or with Transwells indicated that neither IL-10 nor TGF-β, secreted as the result of the CD40L/CpG-ODN stimulation of the B cells, was required for human B cell–dependent regulation of mDCs. Yet, the production of IL-12p70 seems to be regulated by an unidentified soluble factor in association with intercellular contact. Moreover, cell-to-cell contacts were required for the regulation of cell-surface marker expression, with CD62L involved in the control of CD80 and CD86 coactivation molecules. This regulatory mechanism contrasts also with those responsible for the control of the human T-cell responses that uses CD40 and CD86.18 It is clear at this point that B-cell regulation of immune response results from a complex process requiring specific signals depending on the type of the target cells. Overall, our results strongly suggest that a Th1 response polarized by human mDCs could be down-regulated in the presence of activated B cells.
We have recently demonstrated that CD19highIgD+CD38highCD24highCD5high B cells are responsible for the regulation of T-cell proliferation and Th1 differentiation.18 Here, we observed that B cells with functional regulatory effects on mDCs were CD19+IgDlowCD38+CD24lowCD27−CD5+/−. These considerations suggest that either B cells regulating T cells are different from those that control mDCs or that intercellular contacts with T cells on the one hand and with mDCs on the other hand modify the expression of cell surface molecules on the whole B-cell population, explaining the differential phenotypes that arise. We think the latter possibility is unlikely because we found that sorted CD5+ as well as CD5− B cells exhibited the same regulatory capacities over mDCs (not shown) whereas CD5+ but not CD5− B cells regulated T-cell responses.18 Furthermore, B cells need to be stimulated to develop regulatory function because freshly purified circulating B cells were unable to modulate the maturation and function of DCs. It can be concluded that the regulatory capacity of B cells is an “acquired” function. Polyclonal activation after CD40 and TLR9 stimulation indicates that the development of regulatory function is independent of specific Ag activation and can be carried out by any B cell that has been appropriately stimulated.
We have therefore hypothesized that anomalies of B-cell regulation could play a role in the development of exacerbated inflammatory responses such as in autoimmune disorders. The results with B cells from patients with SLE highlighted unexpected deficiencies. B cells control the expression of HLA-DR, CD80, and CD86 molecules but are unable to modulate the production of IL-12p70. Interestingly, the defective regulation of IL-12p70 could be because of mDCs rather than B cells because mDCs from SLE were insensitive to the regulatory effect of control B cells as well. The limited regulatory activities of the B cells and the ensuing defective control of inflammation may thus be the consequences of impaired responses of mDCs in SLE. In contrast, using our model inflammatory responses could not be ascribed to defective B-cell regulation in RA. Thus, RA B cells were as efficient as control B cells in modulating HLA-DR, CD80, and CD86 expression as well as IL-12p70 production from RA mDCs.
It is now evident that DCs are not only considered as APCs that initiate primary immune responses, but are also involved in tolerance induction.26 However, there is almost no evidence that a specific subset of DCs determine immunity or tolerance.27 These properties may be the consequence of activation status28 and more likely might depend on their maturation status.4 Because of diminished expression of costimulatory molecules, iDCs favor the induction of peripheral T-cell tolerance23 whereas mDCs appear to be efficient stimulators of T-cell immune response because of the elevated expression of HLA-DR, CD80, CD86, and production of cytokines. Consequently, through their capacity to inhibit the maturation of DCs, we assume that B cells can operate as inhibitors of T-cell immunity and as instigators of DC-based tolerance induction. Importantly, DCs can contribute to the development of autoimmunity. Thus, when they are deleted in lupus mice, the disease is ameliorated.29
In contrast, systemic autoimmunity occurs when they cannot be eliminated in normal mice.30 mDCs have the ability to break tolerance and to induce autoimmune responses,31 and therefore can contribute to the pathogenesis of autoimmune disorders such as SLE.32,33 The insensitivity of SLE mDCs to the B-cell regulatory effects may take part in this process. The fine comprehension of this novel aspect of the B-cell regulatory network could open new therapeutic approaches to autoimmune conditions by the conversion of immunostimulatory DCs into tolerogenic DCs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Professor Peter Lydyard for editorial assistance and for critically reading the manuscript. They also thank Simone Forest and Geneviève Michel for secretarial assistance.
Authorship
Contribution: A.M., S.L., and A.A. performed the experiments; A.M., C.J., and J.-O.P. analyzed data; and P.Y. and C.J. designed the research and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Pierre Youinou, Laboratory of Immunology, Brest University Medical School Hospital, BP 824, F 29609 Brest, France; e-mail: youinou@univ-brest.fr.