Abstract
Monoclonal Ig light chains (LC) can be responsible for pathologic conditions in humans, as in systemic amyloid light amyloidosis. Protean clinical manifestations characterize this disorder with the most varied combination of symptoms generated by different degrees of diverse organ involvement. Kidney and heart are most frequently interested, with major heart involvement as the most relevant prognostic factor. The identification of the underlying mechanism involved in organ targeting is of major relevance for the pathobiology of this disorder. To this aim, we characterized the repertoire of variable region germline genes of λ LC preferentially targeting the heart and compared it with the repertoire of LC that do not in a case-control study. We found that the repertoires were highly restricted, showing preferential use of the same few germline genes but with a different frequency pattern. A single gene, IGVL1-44, was found associated with a 5-fold increase in the odds of dominant heart involvement (after adjusting for confounders in a multivariable logistic model). These results support an involvement of LC genetics in the determination of organ targeting. Study of the characteristics of IGVL1-44-LC with, and of the minority without, heart involvement might lead to identification of LC/tissue interactions.
Introduction
Amyloid light (AL) amyloidosis is an uncommon hematologic protein conformational disorder characterized by systemic stromal deposition of monoclonal Ig light chains (LCs) in form of β-sheet fibrils (amyloid) demonstrating pathognomonic apple-green birefringence with Congo-red staining, leading to damaged organ function.1,2 LCs are largely synthesized unassembled with the heavy chain partner as “free” LC and circulate in the blood as soluble products of a plasma cell clone resident in the BM.3 This clone is usually indolent and of small size (median plasma cell infiltration of 7%).2 However, not uncommonly, plasma cell numbers can be greater, occasionally reaching 30% of the total marrow cellularity, but usually no signs of overt myeloma (anemia, hypercalcemia, bone lesions, progressive increase in plasma cell number) are observed. Circulating, antigen-selected,4-6 preplasma-cell precursors feed the marrow plasma cell clone and are responsible for widespread bone infiltration.7,8 Therapy is primarily directed to eradicating the plasma cell clone.9-12
Free LC production is frequently observed in plasma cell disorders, including monoclonal gammopathy of undetermined significance, multiple myeloma, and Waldenström macroglobulinemia, but only a fraction of free LC can lose solubility and organize into amyloid deposits.13 Research has focused on the properties of amyloid-forming LC, and specifically on the variable (V) region, and this for the following reasons: (1) the V region is the amino terminal portion of the Ig LC deputed to antigen binding and responsible for its sequence variation because it is formed via a somatic rearrangement of one of a multitude of germline gene VL segments to one of few joint (J) segments; (2) amyloid deposits are constituted of fragments of LC, comprising the V region and parts of the constant region; and (3) characteristic of AL amyloidosis is the predominance of the λ isotype over κ (3:1 ratio), suggesting a genetic propensity in Vλ segments.
Indeed, research has shown that the genetics of LC is central to the understanding of the pathogenesis of AL amyloidosis. Investigators have consistently shown restricted, biased germline gene use14-16 that is significantly different from the normal repertoire expressed in polyclonal marrow plasma cells.15 Disease-associated VL gene segments also were found, IGVL6-57 (previously named 6a) and IGVL3-1 (formerly 3r),14-16 and the frequency of their involvement in LC rearrangements (approximately 20% each, compared with < 5% in the normal) was such to give reason for the λ isotype predominance phenomenon.15
The genetics of LC also provided a background to the understanding of the diverse clinical presentation of AL amyloidosis, a typical feature of AL amyloidosis and related to the amyloid organ targeting phenomenon. Despite the systemic deposition of amyloid in all patients, it is usually possible to identify a predominantly involved organ at diagnosis (such as kidney, heart, liver, or peripheral nervous system), and this feature is used to discriminate and categorize patients according to the clinically most relevant amyloid syndrome, ie, nephrosis for kidney or heart failure for cardiac involvement.17
No plausible explanation for amyloid organ targeting was available until Comenzo et al,14 followed by 2 independent studies from Italy and the United States,15,16 found that LCs with the V region derived from rearrangement of IGVL6-57 gene segment were significantly more likely to be observed in patients with predominant or exclusive kidney involvement at diagnosis (P < .01). Along this line of evidence, amyloid targeting to soft tissue and bone was found to be significantly more frequent in LCs belonging to the κI family.6 However, other gene/phenotype associations were more uncertain (not statistically proved or conflicting), such as in the case of the amyloid-associated gene segment IGVL3-1 that was found to be associated with soft tissue,16 major cardiac and multisystem disease,14 or with no specific organ target.15
Major heart involvement is the most important prognostic parameter in AL amyloidosis and directs the therapeutic options used.2,12 Heart involvement can be determined and measured by echocardiography and serum biomarkers such as N-terminal pro-B-type natriuretic peptide (NT-proBNP), BNP, and cardiac troponins.18-20 The repertoire of LCs that cause major heart dysfunction is not fully determined because of limited sampling, and investigators found weak or no association with germline gene use. Then, unlike IGVL6-57 for kidney,14 there is no clear demonstration of specific germline gene association with major heart involvement. Information on the repertoire of LC targeting the heart may provide important clues to the understanding of the mechanism of damage, possibly identifying the target favoring tissue deposition and revealing new pathways to therapeutic interventions.
In the present case-control study, we tested the hypothesis that germline gene use was involved in the determination of major cardiac targeting. As in previous works, we focused on the λ LC population because we considered it more informative, given the typical association of λ isotype with amyloid.
Methods
Study design
This was a single-center case-control study. The patient population (n = 99) constituted a series of patients with λ AL amyloid who were referred to the Italian Amyloid Center in Pavia; who provided written informed consent in accordance with the Declaration of Helsinki for the use of their biologic samples and clinical data for research purposes, according to the institutional review board guidelines; and who underwent a BM study as their initial workup. Patients from all over the country are referred here for diagnosis and the various forms of treatment and therefore constitute a “bona-fide” unbiased population. Amyloid was demonstrated by the typical apple-green birefringence of Congo red-stained abdominal fat aspiration. All patients presented with a monoclonal LC of the λ isotype.
Definition of cases and controls
Patients from this series with dominant cardiac involvement were identified as cases (from now on labeled as “dominant heart”), in accordance with all the previous studies on LC germline gene use and organ tropism in AL amyloidosis.14-16 More specifically, cardiac involvement was defined as interventricular septum thickness > 12 mm at echocardiography in the absence of other causes.21 Cardiac amyloidosis was deemed as “dominant” when it was the major manifestation of the disease at presentation, on the basis of clinical evaluation. According to Comenzo et al, “dominant” heart patients were New York Heart Association class II or greater and had interventricular septum thickness > 12 mm.14,22 Fifty-five cases were enrolled. In 10 of 55 patients (18%), a positive endomyocardial biopsy was also available, most of the times performed in other institutions.
Patients from this series without dominant cardiac involvement were identified as controls (from now on labeled as “no dominant heart”). Forty-four controls were identified. They included patients with predominantly renal amyloid in 27 of 44 (61%), soft-tissue amyloid in 6 (14%), or other amyloid organ involvement (liver in 4, peripheral nervous system in 3, gastroenteric tract, and lung in 2 cases each). Of note, given that, by design, cases only included dominant cardiac involvement, a quota of controls (14 patients, 32%) consisted of amyloid patients with echocardiographic signs of heart involvement but without symptoms (New York Heart Association class I), attesting subclinical heart involvement. Given the systemic nature of amyloid deposition, this feature is expected in a typical AL population. Assignment to case (dominant cardiac) and control groups (no dominant cardiac) was made at first visit by expert clinical investigators, and it was therefore conducted a priori, before sequencing.
Sequencing of monoclonal Ig LC-variable regions and identification of Vλ and Jλ germline segments
Sequencing of the Vλ regions was obtained by a very sensitive universal strategy based on the inverse PCR.4,15,23 Correct identification of the monoclonal sequence is obtained even in the presence of minimal plasma cell infiltrates and is warranted by unbiased amplification (both 5′ and 3′ primers are located on the constant region) and cloning procedures.
To determine the presumed germline genes of Vλ regions, sequence alignment was made with the current releases of EMBL-GenBank, V-BASE (V-BASE Sequence Directory, Tomlinson et al, MRC Center for Protein Engineering), and IMGT sequence directories using the relative search tools. Sequences discussed in this publication have been deposited in GenBank database (http://www.ncbi.nlm.nih.gov/genbank/) under accession numbers AF026919, AF026922, AF026924-6, AF026932, AF462643-89, HQ172109-55.
The multinomial distribution model described by Lossos et al was used to determine the likelihood that the cloned sequence had a distribution of nucleotide substitutions that was suggestive of antigenic selection,24,25 that is, typical of Igs with improved antibody activity. The formula calculates the probability that the amino acid–replacing mutations observed in a gene segment (framework [FR] or complementarity determining regions [CDR]) occurred by chance. The formula predicts the expected number of replacing mutations and is based on the total observed mutations (replacing plus silent mutations), the replacing mutations found in the CDR or FR, the relative lengths of the CDR or FR, and the expected proportion of replacing mutations on the basis of the specific nucleotide composition of each germline gene. Similar to our previous analysis, evidence of antigenic selection was considered when both CDR and FR analysis were statistically significant.4
Statistical analysis
Means and SD or median and quartiles (IQRs) were used to describe continuous variables; they were compared between cases and controls with the unpaired t test or the Mann-Whitney U test. Absolute and relative frequencies were used to describe categorical variables; they were compared by the Fisher exact test. Logistic models were used to compute the odds ratio (OR) and 95% confidence interval (95% CI) for the association of germline and dominant cardiac involvement, without and with adjustment for potential confounders (age, sex, multiorgan involvement, and BM plasma cell infiltration > 7%, a parameter associated with heart involvement in the present series). Kaplan-Meier survival curves were plotted and compared with the log-rank test. Stata 11 (StataCorp) was used for computation. A P value < .05 was considered statistically significant.
Results
The case-control population
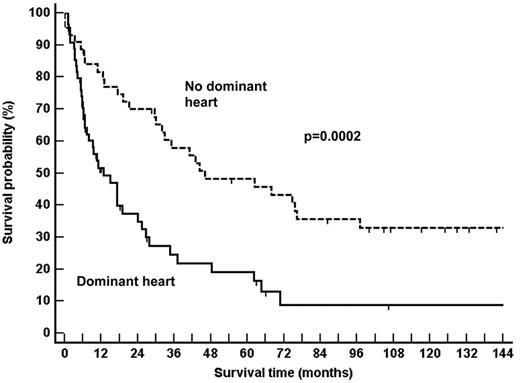
The characteristics of the 2 groups of patients are listed in Table 1. No differences were noted for age, sex, numbers of involved organs at presentation, or the presence of serum-free LC only at immunofixation. The dominant heart population presented increased interventricular septum thickness, lower ejection fraction, and greater NT-proBNP serum concentrations compared with the no-dominant heart population. The dominant heart population was also characterized by a median plasma cell marrow infiltration that was twice that of the controls (Table 1). Indeed, 66% of patients with dominant heart compared with 38% of the control population (Fisher exact, P = .007) had a BM plasma cell infiltration that was greater than 7%, which is the median plasma cell infiltration of a general consecutive population of amyloidosis patients seen at our center.26 As expected for 2 populations that are discriminated according to the major prognostic factor, dominant heart involvement, survival times markedly differed (Figure 1).
Clinical and laboratory findings for the AL amyloidosis patients studied
| . | Dominant heart population (n = 55) . | No-dominant heart population (n = 44) . |
|---|---|---|
| Median age, y (range) | 57 (34-83) | 57 (38-77) |
| Men/women | 35/20 | 29/15 |
| Plasma cell infiltration, %, median (IQR)* | 11 (7-15) | 5.5 (4-11) |
| Serum monoclonal component of the λ isotype, n (%) | ||
| Present | 55 (100) | 40 (91) |
| Light chains only | 29 (53) | 17 (39) |
| Urine monoclonal component of the λ isotype, n (%) | ||
| Present | 52 (95) | 38 (86) |
| Light chains only | 46 (84) | 30 (68) |
| Organs involved, n (%) | ||
| 1 | 19 (35) | 20 (45) |
| 2 | 20 (36) | 16 (36) |
| 3 | 12 (22) | 7 (16) |
| 4 | 2 (4) | 1 (2) |
| 5 | 1 (2) | 0 (0) |
| Heart parameters* | ||
| Interventricular septum thickness, mm, mean (SD) | 16.5 (2.7) | 11.9 (2.5) |
| Ejection fraction, %, mean (SD) | 49.9 (14.7) | 59.1(8.1) |
| NT-proBNP, serum, ng/L, mean (IQR) | 5288.9 (3090.7-13 009.3) | 322.9 (57.6-1049.1) |
| . | Dominant heart population (n = 55) . | No-dominant heart population (n = 44) . |
|---|---|---|
| Median age, y (range) | 57 (34-83) | 57 (38-77) |
| Men/women | 35/20 | 29/15 |
| Plasma cell infiltration, %, median (IQR)* | 11 (7-15) | 5.5 (4-11) |
| Serum monoclonal component of the λ isotype, n (%) | ||
| Present | 55 (100) | 40 (91) |
| Light chains only | 29 (53) | 17 (39) |
| Urine monoclonal component of the λ isotype, n (%) | ||
| Present | 52 (95) | 38 (86) |
| Light chains only | 46 (84) | 30 (68) |
| Organs involved, n (%) | ||
| 1 | 19 (35) | 20 (45) |
| 2 | 20 (36) | 16 (36) |
| 3 | 12 (22) | 7 (16) |
| 4 | 2 (4) | 1 (2) |
| 5 | 1 (2) | 0 (0) |
| Heart parameters* | ||
| Interventricular septum thickness, mm, mean (SD) | 16.5 (2.7) | 11.9 (2.5) |
| Ejection fraction, %, mean (SD) | 49.9 (14.7) | 59.1(8.1) |
| NT-proBNP, serum, ng/L, mean (IQR) | 5288.9 (3090.7-13 009.3) | 322.9 (57.6-1049.1) |
AL indicates amyloid light; IQR, interquartile range; and NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Parameters that were significantly different between the 2 populations: plasma cell infiltration, P = .007; interventricular septum thickness, P < 10−4; ejection fraction, P = .0009; NT-proBNP serum concentrations, P < 10−4
Survival of the AL amyloidosis populations studied. As expected for populations discriminated according to the major prognostic factor, dominant heart involvement, the survival times markedly differed.
Survival of the AL amyloidosis populations studied. As expected for populations discriminated according to the major prognostic factor, dominant heart involvement, the survival times markedly differed.
Family and germline gene use
Identification of the monoclonal amyloid Vλ nucleotide sequences was obtained in all patients. Sequences were complete and potentially functional (no stop codons, frameshifts, or pseudogenes). Database assignment of germline gene segments was unequivocal, and sequences were then grouped into Vλ families.
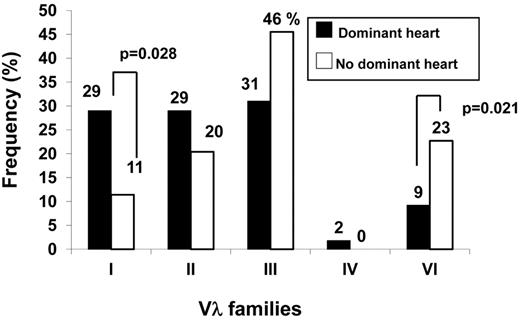
Figure 2 shows that Vλ family use differed in the 2 groups. The control population was dominated by the λIII family, with the λII and λVI families each accounting for approximately 20% and the λI family involved in just 11%. By contrast, the dominant heart group was characterized by the even distribution of the first 3 families (equally contributing to approximately 30% of sequences) and underrepresentation of the λVI family (P = .021, Fisher exact; Figure 2). This latter family is known to be preferentially associated with kidney involvement. The frequency of the VλI family was almost 3 times as much in the dominant heart group, revealing an association with major heart involvement (Figure 2; P = .028, Fisher exact; OR 3.48, 95% CI 1.08-13.17), and suggesting biased expression at the level of germline genes.
Comparison of the Vλ family expression pattern in patients with or without dominant heart involvement. The population showing dominant heart involvement was characterized by increased expression of the VλI family and underrepresentation of the kidney-associated VλVI family.
Comparison of the Vλ family expression pattern in patients with or without dominant heart involvement. The population showing dominant heart involvement was characterized by increased expression of the VλI family and underrepresentation of the kidney-associated VλVI family.
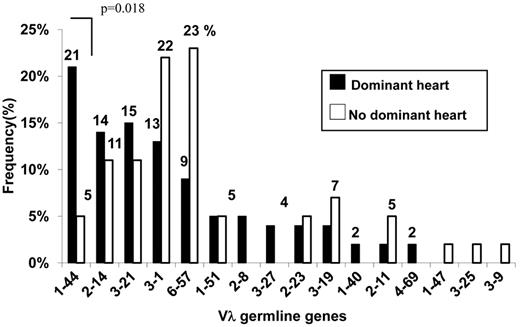
Figure 3 reports the Vλ germline gene expression repertoires of the 2 series of patients. Restriction was apparent in both cases because only a small fraction of the 29-30 functional Vλ segments contributed significantly to the repertoire, with just 5 segments (Figure 3: IGLV1-44, 2-14, 3-21, 3-1, 6-57) being collectively responsible for approximately 70% of amyloid LC. However, the frequency distribution of these same germline genes markedly differed according to major heart involvement. Germline gene IGLV1-44 was predominant among amyloid LC with heart tropism (Figure 3), and its occurrence was 4 times greater compared with the control, thus revealing a clear-cut association with major cardiac involvement (P = .018, Fisher exact; OR 5.86, 95% CI 1.18-56.19). Risk for major heart involvement for LC expressing the IGLV1-44 segment was maintained (P = .045; OR 5.13, CI 1.03-25.47) when we adjusted it in a multivariable logistic model for age, sex, multiorgan involvement, and BM plasma cell infiltration > 7% (a parameter associated with heart involvement in the present series), thus demonstrating its value as an independent predicting variable (Figure 4).
Comparison of the Vλ germline gene repertoires of patients with or without dominant heart involvement. Germline gene use was restricted to the same few segments in both populations. However, the frequency of gene use markedly differed, with the population demonstrating dominant heart involvement characterized by increased expression of the VλI family germline gene IGVL 1-44.
Comparison of the Vλ germline gene repertoires of patients with or without dominant heart involvement. Germline gene use was restricted to the same few segments in both populations. However, the frequency of gene use markedly differed, with the population demonstrating dominant heart involvement characterized by increased expression of the VλI family germline gene IGVL 1-44.
Risks of developing major heart AL amyloidosis according to the involved Ig LC germline gene adjusted for potential confounders (see “Family and germline gene use”) in multivariable logistic model.IGVL1-44 was the only germline gene to be associated with heart involvement, as shown by the OR > 1 and the 95% CI whisker not crossing the no-effect line (OR = 1). Conversely, IGVL6-57 expression was against major heart involvement, being this gene associated with kidney involvement.
Risks of developing major heart AL amyloidosis according to the involved Ig LC germline gene adjusted for potential confounders (see “Family and germline gene use”) in multivariable logistic model.IGVL1-44 was the only germline gene to be associated with heart involvement, as shown by the OR > 1 and the 95% CI whisker not crossing the no-effect line (OR = 1). Conversely, IGVL6-57 expression was against major heart involvement, being this gene associated with kidney involvement.
Somatic mutations were found in all sequences, and the overall frequency of nucleotide substitutions for dominant heart sequences was lower than controls, but this difference was not significant (dominant heart, mean 5.0%, 95% CI, 3.9%-6.1%; no-dominant heart, 6.2% mean, 95% CI, 4.8%-7.0%, P = .087). Figure 5 reports the somatic mutation rates of individual Vλ families in the dominant heart sequences. The greatest number of somatic mutations was found for the VλIII family, whereas the lowest for the VλVI, a feature already observed in a previous series of a general population of amyloid patients.15 There was no difference in the VλIII family mutation rate between the 2 groups of patients analyzed in the present paper.
Somatic mutation rates in the population with dominant heart involvement. The most frequently mutated was the VλIII family.
Somatic mutation rates in the population with dominant heart involvement. The most frequently mutated was the VλIII family.
Characteristics of IGVL1-44 sequences: relation with heart parameters
Although the present database of IGVL1-44 sequences in heart patients (n = 12) warrants for caution in the interpretation of the following analyses, we wished to investigate whether these LC specifically targeting the heart presented distinctive features.
Plasma cell numbers of IGVL1-44 clones were not dissimilar to other germlines, which is in agreement with the multivariate analysis demonstrating that IGVL1-44 use was an independent risk factor with marrow infiltration as confounding variable (see Figure 4). However, IGVL1-44 LCs circulated at significant lower concentrations than non-IGVL1-44 LCs from cardiac patients (IGVL1-44 LCs, mean 132.73 mg/L; IQR 86-185; non-IGVL1-44 LCs, mean 690.13 mg/L, IQR 171-463; P = .009).
IGVL1-44 sequences were not dissimilar in terms of antigenic selection rates (75% vs 61%, respectively, P = .42) and calculated isoelectric point (median 4.95 vs 4.35 isoelectric point, respectively, P = .67) to heart-sequences derived from other genes. Similarly, with the present database of sequences, no significant difference between IGVL1-44–positive and –negative heart patients was recorded in terms of: (1) NT-proBNP concentrations (IGVL1-44, mean 6334 ng/L, IQR 3142-7658; other germline genes, mean 10 273 ng/L, IQR 3060-13 142, P = .49); (2) interventricular septum thickness (IGVL1-44, mean 16.15 mm, IQR 13-19; other germline genes, mean 16.55, IQR 15-18, P = .58); or (3) left ventricular ejection fraction (IGVL1-44, mean 42.63%, IQR 39.5-46; others, 51.53%, IQR 37-65, P = .21).
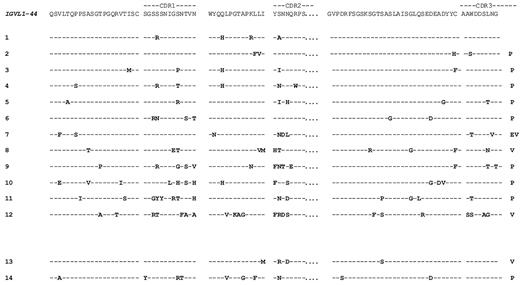
Figure 6 reports the amino acid–derived sequences of the V regions of IGVL1-44 LC compared with the germline sequence, both from heart-positive (nos. 1-12) and heart-negative (nos. 13 and 14) patients. High sequence variation is observed, with amino acid changes focused in the CDR. At inspection, the present database did not allow identification of an obvious pattern, or amino acid sequence motif, shared by VL with major heart involvement (Figure 6 nos. 1-12), or that could help to differentiate them from the heart negative ones (Figure 6 nos. 13 and 14).
Deduced amino acid sequences of the IGVL1-44-derived amyloid VL regions. Amino acid changes from the germline donor, IGVL1-44, are highlighted. Sequences 1-12 were from the dominant heart population, and sequences 13 and 14 were from control patients.
Deduced amino acid sequences of the IGVL1-44-derived amyloid VL regions. Amino acid changes from the germline donor, IGVL1-44, are highlighted. Sequences 1-12 were from the dominant heart population, and sequences 13 and 14 were from control patients.
Discussion
In this study we determined the repertoire of monoclonal LC from a well-defined, adequately numerous series of AL patients with major and predominant heart involvement at diagnosis. We reasoned that if LC genetics were a major determinant of the phenomenon of organ targeting, the repertoire of LC from cardiac patients would differ from that observed in noncardiac dominant patients, and if so, germline genes with intrinsic propensity to target the heart would probably exist. The present results provided evidence that strongly support this hypothesis.
Fundamental features for repertoire analysis are patient population and sequencing strategy. The Pavia center coordinates a National Amyloid Program, and patients were referred from throughout the country for both diagnosis and the various forms of treatment, including conventional and high-dose chemotherapy, and they should constitute, “bona-fide,” a population free of referral bias. A BM study was performed in all cases at presentation, as part of the routine workup, and the sequencing strategy has already proven its suitability for repertoire analysis.15 Using primers located on the constant region (see the section “Sequencing of monoclonal Ig LC-variable regions and identification of Vλ and Jλ germline segments”), no part of VL was covered by the primers: for this reason, PCR amplification efficiency is maximal, independent on the VL sequence, and the amplified fragment comprises the full-length VL with the original sequence.23 Identification of monoclonal VL was obtained in all cases, and sequences were all potentially functional, being free of stop codons or other alterations.
A crucial point for studying the relationship between LC genetics and organ targeting was the categorization of patients according to the major organ involved at presentation. For the definition of heart involvement, standardized criteria were adopted21 and the 2 populations were found to be balanced for several parameters, including age, sex, and type of monoclonal component. The clinical blinded definition of heart involvement as dominant was correct because the 2 populations differed according to parameters typical of major heart involvement, such as serum NT-proBNP and survival.19 The observation that heart patients had a number of plasma cells in their BM that was almost twice as much that of control patients was somewhat expected. Previous works identified the relevance of the clonal plasma cell burden for prognosis and heart involvement.14,26
Use of the available germline gene repertoire was restricted, with just 5 gene segments used to encode approximately 70%-80% of the amyloid λ LC. Restriction is an established feature of AL amyloidosis.14-16 However, in agreement with the notion that LC genetics is major determinant of organ targeting in AL amyloidosis, the use of these 5 gene segments markedly differed in the 2 series. Dominant heart patients presented a 4-fold increased use of IGVL1-44 germline and, according to our data, 2 of 10 cardiac AL patients express LC on the basis of the rearrangement of this germline (Figure 3). Furthermore, increased expression of IGVL1-44 gene in dominant cardiac patients (21%) was not only relative to the control amyloid population (5%), but it holds true compared with the IGVL1-44 rearrangement frequency observed under normal conditions, such as in polyclonal marrow plasma cell populations (4% rearrangement frequency).15
On the contrary, IGVL6-57 gene, known to be associated with kidney involvement,14-16 was underrepresented in this series, stressing the power of our observation. The amyloid-associated gene IGVL3-1 was less frequently observed in cardiac LC too, albeit not in a statistically significant fashion. Indeed, IGVL3-1 was found to be more frequently associated with soft-tissue involvement in other series.16
The relationship between IGVL1-44 expression and cardiac amyloid was maintained when additional variables were introduced into the analysis, most importantly plasma cell numbers. The ODD risk and its 95% CI were clearly favoring major cardiac involvement. Therefore, IGVL1-44 expression needs to be considered a new powerful predictor of major amyloid heart disease.
No apparent clinical or simple biochemical characteristics typical of IGVL1-44 cardiac patients were identified. However, the number of identified patients with IGVL1-44 LC was limited (n = 12), and analyses will be reliably performed when more extended sequencing database will be available. Lack of homology in somatic mutations is a recognized feature in AL amyloidosis, and it was therefore expected.1,2,27 Mutations are clearly relevant for destabilization of the LC molecule, a critical aspect related to amyloidogenicity.1,2,27 In this view, it will be of interest to compare amyloid-forming IGVL1-44 LC with LC from POEMS syndrome,28 a rare nonamyloid plasma cell disorder that is characterized by significant overuse of IGVL1-44 sequences,29 to identify possible disease-related structural features. Unlike IGVL3-1 and IGVL6-57, IGVL1-44 is then clearly not exclusively associated with amyloidosis.
An interesting observation was that dominant heart patients with LC expressing IGVL1-44 gene demonstrated significantly lower concentrations of free LC compared with dominant heart patients expressing other germline genes, despite similar marrow plasma cell infiltration. Because high serum concentrations are not a feature of IGVL1-44 LC, this finding supports the notion that biochemical properties are central to the mechanism of IGVL1-44 targeting to the heart. Lower concentrations with similar plasma cell numbers may reflect lower secretory capacity, increased catabolism, but also more rapid deposition in target organs.
Determination of antigenic selection rates24,25 and isoelectric points failed to discriminate IGVL1-44–positive and –negative cardiac LC. The absence of enhanced imprint of antigenic selection argues against an antigen-antibody interaction within the heart. We deem that targeting of IGVL1-44 LC might be mediated by other forms of interactions, not necessarily via CDR, given the paramount importance of the germline gene donor. It is conceivable that IGVL1-44 gene segment owns germline-encoded structural features that could account for the preferential targeting to the heart. Interaction with extracellular chaperones, as recently outlined for clusterin,30 might be a possibility.
Along this line of thought, in addition to confer amyloidogenicity, somatic mutations in certain positions might also play a relevant role in contrasting the inherent susceptibility of IGVL1-44 sequences to cause preferential heart deposition (because IGVL1-44 sequences without heart involvement are clearly identified). When the database of heart-positive and heart-negative amyloid IGVL1-44 sequences will be sufficiently extended, we foresee it will be possible to recognize via sequence analysis, recombinant mutant and experimental model approaches, key residues or sequence motif responsible for LC/tissue target interaction, hence providing original clues to the understanding of amyloidogenesis. In addition, identification of the underlying mechanisms might help to develop molecules interacting with critical areas, disclosing innovative therapeutic strategies for the one fifth of heart patients expressing the IGVL1-44 germline gene.
In summary, in this study we presented the characterization of the germline gene repertoire of LC causing major heart amyloidosis. We demonstrated that the repertoire is restricted to the use of a few genes, with a remarkable frequency distribution pattern characterized by significant increase in the rearrangement of the IGVL1-44 gene. Expression of this gene increased 5 times the odds of major heart disease, whereas IGVL6-57 expression (a gene known to be associated with dominant kidney deposition) was protective. These findings provide substantial evidence to support the notion that germline gene use is a key determinant in the pathogenesis of a fundamental, obscure, and clinically relevant phenomenon, the diverse pattern of amyloid organ involvement at presentation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Andrea Foli and Paola Russo for their invaluable support in clinical assistance and the participating Centers to the National Amyloid Program.
This work was funded by Fondazione IRCCS Policlinico San Matteo, Università di Pavia. The funders have no role in study design, collection and analysis of data, decision of publish, or manuscript writing.
Authorship
Contribution: V.P. designed the study, conducted research, collected and analyzed data, and wrote the manuscript; G.P. collected and analyzed clinical features and contributed significantly to data interpretation and manuscript writing; S.C., V.N., and P.R. performed molecular biology studies; L.O. was involved in patient management; R.I. was involved in patient management and bone marrow studies; S.P. performed cardiologic studies and echocardiography; C.K. performed statistical analysis and significantly contributed to data interpretation and manuscript writing; and G.M. was involved in study design, data interpretation, and major critical revisions of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vittorio Perfetti, MD, Medical Oncology and Amyloid Center Fondazione IRCCS Policlinico S Matteo, Ple Golgi 19, 27100 Pavia, Italy; e-mail: v.perfetti@smatteo.pv.it.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal