Abstract
IgD monoclonal gammopathies are uncommon. They are seen rarely as a monoclonal gammopathy of undetermined significance and are present in 1%-2% of patients with multiple myeloma. In light-chain amyloidosis, IgD monoclonal proteins are found in ap-proximately 1% of patients. When an IgD monoclonal protein is found, amyloidosis is often omitted from the differential diagnosis. In the present study, we reviewed the natural history of IgD-associated amyloidosis among 53 patients seen over 41 years. The distribution of clinical syndromes suggests that these patients have a lower frequency of renal and cardiac involvement. The overall survival of these patients does not appear to be different from that of patients who have light-chain amyloidosis associated with another monoclonal protein.
Introduction
Multiple myeloma associated with IgD monoclonal protein is a well-recognized, albeit rare, entity. Five studies, each including at least 20 patients with IgD-associated multiple myeloma, have been published to date.1-5 This entity appears to have clinical differences compared with non-IgD myeloma, including a younger age at disease presentation and a higher incidence of extramedullary disease. Some have suggested that patients with IgD myeloma have a greater degree of renal insufficiency at presentation. The higher prevalence of renal insufficiency may relate to the high levels of light-chain excretion in the urine. Stem-cell transplantation may be particularly effective for the treatment of this myeloma subgroup.3
At Mayo Clinic, IgD monoclonal proteins represent 0.5% of all serum monoclonal proteins detected (R.A.K., verbal communication, January 2011). In a review of patients in Olmsted County, MN, with monoclonal gammopathy of undetermined significance (MGUS), no occurrences of IgD-associated MGUS were recognized.6 IgD in MGUS is very rare. The first report from Mayo Clinic of an IgD form of MGUS was published in 1994.4 This patient remained stable after 8 years of follow-up.
In a study including 1024 patients with multiple myeloma, 2% had IgD monoclonal protein,7 and in a review of 474 patients with Ig light-chain amyloidosis at Mayo Clinic, 1% had IgD monoclonal protein.8 The occurrence of an IgD protein in amyloidosis has been reported in single cases but never as a patient series. In the present study, we review Mayo Clinic experience with Ig light-chain amyloidosis associated with IgD monoclonal protein.
Methods
This protocol was approved by Mayo Clinic Institutional Review Board in accordance with the Declaration of Helsinki and state regulations of Minnesota. All patients gave written informed consent. At our institution, data pertaining to patients with amyloidosis are captured prospectively in a dysproteinemia database. This database is continuously updated, and deaths are entered from the social security death index. Annual follow-up correspondence is sent to ensure that patients are not lost to follow-up. We retrospectively searched this database for the records of all patients with amyloidosis seen at Mayo Clinic, Rochester, MN, from May 15, 1969 through January 21, 2010. Among all patients identified, we further searched for patients who had evidence of an IgD protein. Patients with amyloidosis who did not have a detectable IgG, IgA, or IgM heavy chain had been further screened with antisera to IgD and IgE heavy chains by immunodiffusion. This was the basis for diagnosing IgD amyloidosis, and no diagnoses were made at autopsy. Clinical and demographic data for all patients in the final IgD amyloidosis group were retrieved from the patient records.
To compare the characteristics of patients with amyloidosis who did and did not have IgD protein, we identified a non-IgD comparator group from among patients who were registered as part of 2 trials of therapy for amyloidosis.9,10 Follow-up in these studies was complete, and the therapy was uniform among patient cohorts. Patients in the study received comprehensive investigation of the amyloidosis. We then compared factors of interest between the patients with amyloidosis who did and did not have an IgD protein. These factors included age, sex, albumin level, and BM plasma cells. Differences between the groups were compared using the χ2 or Fisher exact test for nominal variables and the nonparametric Wilcoxon rank sum test for continuous variables. Survival was evaluated with Kaplan-Meier analysis in a Cox proportional hazards model. Differences in timed end points were tested for statistical significance with 2-tailed log-rank testing. Data analysis was conducted by the senior author (M.A.G.) using JMP Version 8.0 statistical software (SAS Institute). P < .05 was considered statistically significant.
Results
Patient and disease characteristics
Among 3955 patients with Ig light-chain amyloidosis seen during the study period of 41 years, 53 patients (1.3%) had a serum IgD monoclonal protein. Characteristics of these 53 patients are shown in Table 1. Fifty-seven percent of the patients were men, and the median age was 60.5 years. Median time from the histologic diagnosis of amyloidosis to the first evaluation at Mayo Clinic was 1.5 months (interquartile range [IQR], 0-3.25 months). Of the 11 patients who are currently alive, the median follow-up was 57 months. Of the 41 patients who have died, 10 (24%) died within 4.7 months and 30 (73%) died within 5 years of histologic diagnosis.
Patient and clinical characteristics (N = 53)
| Men, n (%) | 30 (57) |
| Median age, y (range) | 60.5 (34.7-80.9) |
| Race, n (%) | |
| White | 52 (98) |
| Black | 1 (2) |
| Symptoms and signs, n (%) | |
| Fatigue | 32 (60) |
| Lower extremity edema | 23 (43) |
| Paresthesias | 17 (32) |
| Weight loss | 17 (32) |
| Dyspnea on exertion | 15 (28) |
| Carpal tunnel syndrome | 14 (26) |
| Enlarged tongue | 11 (21) |
| Hepatomegaly | 9 (17) |
| Neuropathy | 8 (15) |
| Purpura | 7 (13) |
| Bleeding | 3 (6) |
| Pain | 2 (4) |
| Status at last follow-up, n (%) | |
| Dead | 41 (77) |
| Alive | 11 (21) |
| Lost to follow-up | 1 (2) |
| Cause of death, n (%)* | |
| Unknown | 24 (59) |
| Cardiac failure or arrest | 10 (24) |
| Infection | 3 (7) |
| Myeloma | 1 (2) |
| Renal failure | 1 (2) |
| Cachexia | 1 (2) |
| Treatment-related leukemia | 1 (2) |
| Men, n (%) | 30 (57) |
| Median age, y (range) | 60.5 (34.7-80.9) |
| Race, n (%) | |
| White | 52 (98) |
| Black | 1 (2) |
| Symptoms and signs, n (%) | |
| Fatigue | 32 (60) |
| Lower extremity edema | 23 (43) |
| Paresthesias | 17 (32) |
| Weight loss | 17 (32) |
| Dyspnea on exertion | 15 (28) |
| Carpal tunnel syndrome | 14 (26) |
| Enlarged tongue | 11 (21) |
| Hepatomegaly | 9 (17) |
| Neuropathy | 8 (15) |
| Purpura | 7 (13) |
| Bleeding | 3 (6) |
| Pain | 2 (4) |
| Status at last follow-up, n (%) | |
| Dead | 41 (77) |
| Alive | 11 (21) |
| Lost to follow-up | 1 (2) |
| Cause of death, n (%)* | |
| Unknown | 24 (59) |
| Cardiac failure or arrest | 10 (24) |
| Infection | 3 (7) |
| Myeloma | 1 (2) |
| Renal failure | 1 (2) |
| Cachexia | 1 (2) |
| Treatment-related leukemia | 1 (2) |
n = 41.
The initial clinical symptoms and signs are shown in Table 1. Median duration of fatigue (n = 32) before the histologic diagnosis was 7.5 months (IQR, 4-15 months). The median duration of paresthesias (n = 17) was 7.5 months (IQR, 5-24), the duration of dyspnea (n = 15) was 6 months (IQR, 3-14), and the amount of weight loss (n = 17) was 7.5 kg (IQR, 3.8-11.3). Median duration that various signs were present before histologic diagnosis of amyloidosis was 6 months for edema and 12 months for carpal tunnel syndrome. Median liver size for the 9 patients with hepatomegaly was 7 cm below the right costal margin (IQR, 4-10 cm). Palpable splenomegaly was reported in only 2 patients, each at 4 cm below the left costal margin. Only 2 patients reported pain. Clinical syndromes associated with amyloidosis included the presence of cardiac amyloid in 21 patients, renal amyloid in 19, carpal tunnel syndrome in 14, hepatic amyloid in 9, peripheral neuropathy in 8, and malabsorption and orthostatic hypotension in 3 patients each.
Laboratory findings
Laboratory values for the 53 patients are shown in Table 2. Only 10% (5 of 52 patients tested) had a hemoglobin value < 10 g/dL, and only 3 patients had a platelet count > 500 × 109/L. Troponin T level, which has only been measured in routine practice since 2005, was recorded in 14 patients, 8 of whom had a value > 0.03 ng/mL. In our experience, patients in the past 10 years have been diagnosed earlier and therefore have better survival; troponin levels may be higher in patients seen earlier in the study period because of a delayed diagnosis, so our values may not represent what might be seen in current practice. Initial serum creatinine level was > 2 mg/dL in 8 patients. Only 3 patients had a bilirubin level > 2.0 mg/dL, and all 3 had a direct bilirubin level > 1 mg/dL. Only 10% (5 of 50 patients tested) had a serum albumin level < 2 g/dL.
Laboratory values for patients with IgD amyloidosis (N = 53)
| Test . | Value* . | Reference range . |
|---|---|---|
| Serum values | ||
| Hemoglobin, g/dL | 13.3 (11.6-13.8) | 12.0-15.5 |
| WBCs, × 109/L | 7.6 (6.1-9.1) | 3.5-10.5 |
| Platelets, × 109/L | 291 (107-588)† | 150-450 |
| Troponin T, ng/mL (n = 14) | 0.04 (0.01-0.20) | ≤ 0.01 |
| Creatinine, mg/dL | 1.1 (0.6-3.3) | Men: 0.8-1.3 |
| Women: 0.6-1.1 | ||
| Bilirubin, mg/dL | 0.5 (0.4-0.9) | 0.1-1.0 |
| β2-microglobulin, μg/mL (n = 26) | 2.77 (2.15-5.37) | 0.70-1.80 |
| M protein, g/dL (n = 14) | 0.7 (0.3-1.5) | None |
| Albumin, g/dL | 2.97 (2.40-3.54) | 3.5-5.0 |
| IgD, mg/dL (n = 15) | 51 (16-203) | < 10 |
| Urine values (n = 51) | ||
| Total protein, g/24 h | 1.5 (0.3-5.1) | < 0.102 |
| M protein, g/24 h (n = 43) | 0.28 (0.1-3.8)† | None |
| Test . | Value* . | Reference range . |
|---|---|---|
| Serum values | ||
| Hemoglobin, g/dL | 13.3 (11.6-13.8) | 12.0-15.5 |
| WBCs, × 109/L | 7.6 (6.1-9.1) | 3.5-10.5 |
| Platelets, × 109/L | 291 (107-588)† | 150-450 |
| Troponin T, ng/mL (n = 14) | 0.04 (0.01-0.20) | ≤ 0.01 |
| Creatinine, mg/dL | 1.1 (0.6-3.3) | Men: 0.8-1.3 |
| Women: 0.6-1.1 | ||
| Bilirubin, mg/dL | 0.5 (0.4-0.9) | 0.1-1.0 |
| β2-microglobulin, μg/mL (n = 26) | 2.77 (2.15-5.37) | 0.70-1.80 |
| M protein, g/dL (n = 14) | 0.7 (0.3-1.5) | None |
| Albumin, g/dL | 2.97 (2.40-3.54) | 3.5-5.0 |
| IgD, mg/dL (n = 15) | 51 (16-203) | < 10 |
| Urine values (n = 51) | ||
| Total protein, g/24 h | 1.5 (0.3-5.1) | < 0.102 |
| M protein, g/24 h (n = 43) | 0.28 (0.1-3.8)† | None |
Values are median (IQR) unless otherwise indicated.
Median (range).
A serum monoclonal protein peak (the “M spike”) was visible on serum protein electrophoresis in only 14 patients, and only 5 had an M spike > 1 g/dL. On serum immunofixation, the IgD light-chain protein was κ in 11 patients, λ in 35, and uncertain in 6; 1 patient had a biclonal D λ and G κ protein. The free light-chain ratio, measured in 14 patients, was normal in 1, λ predominant in 8, and κ predominant in 5. Quantitative IgD level, measured in only 15 patients, was a median of 51 (IQR, 16-203) mg/dL (normal, < 10 mg/dL).
The median urinary protein loss in 24 hours was 1.5 g (normal, < 0.102 g; Table 2); 30 patients excreted more than 1 g of protein, and 19 patients had more than 3 g of protein in the urine. A urine monoclonal protein was detected in 43 of 51 patients; urinary immunofixation detected a λ light chain in 33 and a κ light chain in 10. An IgD fragment was found in the urine of 14 patients.
Biopsy and Congo red staining of tissues showed amyloid deposits in the BM in 47% of the patients tested and in the fat aspirate in 73% (Table 3). BM biopsy, performed in 44 patients, showed a median of 10% plasma cells (IQR, 3%-30%). Eleven patients (25%) had more than 30% plasma cells in the BM.
Tissue biopsy findings
| Source of biopsy . | Presence of amyloid . | ||
|---|---|---|---|
| Positive . | Negative . | Equivocal . | |
| BM (n = 38), n (%) | 18 (47) | 20 (53) | |
| Fat aspirate, n (%) | 16 (73) | 5 (23) | 1 (5) |
| Rectum, n | 6 | 1 | 1 |
| Kidney, n | 5 | ||
| Liver, n | 4 | ||
| Small bowel, n | 4 | ||
| Heart, n | 3 | ||
| Skin, n | 1 | ||
| Sural nerve, n | 1 | ||
| Source of biopsy . | Presence of amyloid . | ||
|---|---|---|---|
| Positive . | Negative . | Equivocal . | |
| BM (n = 38), n (%) | 18 (47) | 20 (53) | |
| Fat aspirate, n (%) | 16 (73) | 5 (23) | 1 (5) |
| Rectum, n | 6 | 1 | 1 |
| Kidney, n | 5 | ||
| Liver, n | 4 | ||
| Small bowel, n | 4 | ||
| Heart, n | 3 | ||
| Skin, n | 1 | ||
| Sural nerve, n | 1 | ||
Treatment
Data on initial therapy were available for 39 of the 53 patients. An alkylator-corticosteroid combination or multiple alkylators with a corticosteroid was administered in 23. Dexamethasone alone was administered to 2 patients, 1 at Mayo Clinic, described in the next paragraph. Initial therapy was colchicine, VAD (vincristine, doxorubicin, dexamethasone), and bortezomib in 1 patient each. An immunomodulatory agent (thalidomide or lenalidomide) was initially administered to 4 patients. Seven patients received stem cell transplantations, 6 at Mayo Clinic, as discussed 2 paragraphs below.
Detailed treatment information was available for 9 patients. Three patients received chemotherapy; 2 of these patients were treated in a study of vincristine, carmustine, melphalan, cyclophosphamide, and prednisone for amyloidosis.9 A 41-year-old woman with calf claudication, arthropathy, tongue enlargement, and an IgD λ protein at the time of diagnosis achieved a very good partial response with this chemotherapy but died of myelodysplastic syndrome/acute leukemia 40.9 months after the initiation of therapy. A 61-year-old man with an IgD λ monoclonal protein at diagnosis died of cardiac arrest, with known cardiac amyloidosis, 29.8 months after beginning therapy. The other patient, a 66-year-old woman with cardiac and liver amyloidosis associated with an IgD λ protein, was in a dexamethasone therapy study.11 She responded to single-agent dexamethasone for 81 months. At disease progression, she received melphalan and prednisone for 24 months and died of progressive hepatic failure 111 months after the initiation of therapy.
Six patients (3 men and 3 women 47-63 years of age) underwent autologous stem cell transplantation between October 2003 and May 2010. Four had biopsy-proven renal amyloid. Two had cardiac amyloid (septal thicknesses: 15 and 20 mm), 1 biopsy proven. Two had peripheral neuropathy. The IgD protein was λ in 5 and κ in 1. All 6 patients had a hematologic response, 4 of which were complete, and 4 had organ response. One patient had relapse of disease and is now on dialysis, and another had relapse and is alive with salvage chemotherapy. One patient had disease relapse and died of progressive intestinal amyloidosis 22 months after beginning therapy. The other 5 patients are alive at a median of 68 months (range, 7.5-83.5 months) after the initiation of therapy.
Comparison of IgD and non-IgD amyloidosis
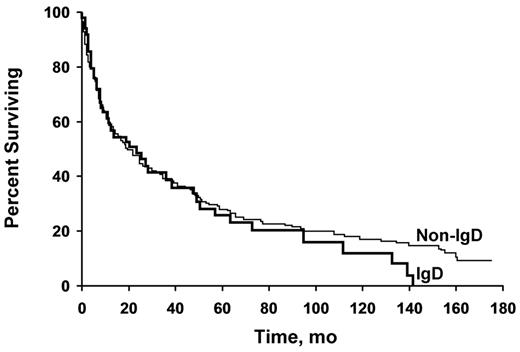
We compared the 53 patients with IgD amyloidosis with a group of 144 patients with non-IgD amyloidosis (Table 4).9,10 Findings that were significantly different in the IgD group compared with the non-IgD group included a lower frequency of renal amyloidosis (P = .005), which corresponded to a lower cholesterol level (P = .047) related to the decreased prevalence of nephrotic-range proteinuria, and a lower prevalence of cardiac amyloidosis (P = .047). There was also a higher serum albumin level (P = .04) related to the lower level of proteinuria. However, the difference in median 24-hour urine protein between the 2 groups did not reach statistical significance. No difference in survival was seen between the groups; the survival curves were virtually superimposable (Figure 1). Variables that might affect survival—liver size, performance status, septal thickness, serum creatinine level, and β2-microglobulin level—were not different between the groups (Table 4). The echocardiographic ejection fraction may predict survival; the result was not significant (P = .08), but only 31 of the 53 patients underwent echocardiography (data not shown). No other clinical features predicted survival (data not shown), although an insufficient number of patients had measurement of B-natriuretic peptide or troponin levels for entry into the statistical model.
Comparison of IgD and non-IgD amyloidosis
| . | IgD (n = 53) . | Non-IgD (n = 144) . | P . |
|---|---|---|---|
| Median age, y (IQR) | 60 (50-67) | 61.5 (53-68) | .34 |
| Men, n (%) | 30 (57) | 94 (65) | .31 |
| Hepatomegaly, n (%) | 9 (17) | 39 (27) | .16 |
| Interventricular septal thickness, mm, median (IQR) | 15 (11-17) | 13 (11-15) | .11 |
| Ejection fraction, %, median (IQR) | 60 (44-67) | 60 (50-68) | .17 |
| Creatinine, mg/dL, median (IQR) | 1.1 (0.9-1.6) | 1.1 (0.9-1.5) | .34 |
| Total cholesterol, mg/dL, median (IQR) | 196 (146-299) | 242 (186-328) | .047 |
| β2-microglobulin, μg/mL, median (IQR) | 2.77 (2.15-5.38) | 2.83 (2.07-3.99) | .39 |
| Albumin, g/dL, median (IQR) | 2.97 (2.43-3.54) | 2.69 (2.07-3.37) | .04 |
| Urine protein, g/24 h, median (IQR) | 1.5 (0.3-5.1) | 2.9 (0.4-7.4) | .12 |
| Urine M spike, g/24 h, median (IQR) | 0.28 (0.02-0.63) | 0.19 (0.05-0.69) | .90 |
| Serum M spike | Immunofixation only (fixation only, 0.6 g/dL) | Immunofixation only (fixation only, 0.9 g/dL) | .31 |
| BM plasma cells, %, median (IQR) | 10 (3-30)* | 8 (4-16)† | .09 |
| Renal amyloid, n (%) | 19 (36) | 84 (58) | .005 |
| Cardiac amyloid, n (%) | 24 (45) | 81 (56) | .047 |
| Neuropathy, n (%) | 5 (9) | 19 (13) | .47 |
| Autonomic failure, n (%) | 3 (6) | 10 (7) | .74 |
| . | IgD (n = 53) . | Non-IgD (n = 144) . | P . |
|---|---|---|---|
| Median age, y (IQR) | 60 (50-67) | 61.5 (53-68) | .34 |
| Men, n (%) | 30 (57) | 94 (65) | .31 |
| Hepatomegaly, n (%) | 9 (17) | 39 (27) | .16 |
| Interventricular septal thickness, mm, median (IQR) | 15 (11-17) | 13 (11-15) | .11 |
| Ejection fraction, %, median (IQR) | 60 (44-67) | 60 (50-68) | .17 |
| Creatinine, mg/dL, median (IQR) | 1.1 (0.9-1.6) | 1.1 (0.9-1.5) | .34 |
| Total cholesterol, mg/dL, median (IQR) | 196 (146-299) | 242 (186-328) | .047 |
| β2-microglobulin, μg/mL, median (IQR) | 2.77 (2.15-5.38) | 2.83 (2.07-3.99) | .39 |
| Albumin, g/dL, median (IQR) | 2.97 (2.43-3.54) | 2.69 (2.07-3.37) | .04 |
| Urine protein, g/24 h, median (IQR) | 1.5 (0.3-5.1) | 2.9 (0.4-7.4) | .12 |
| Urine M spike, g/24 h, median (IQR) | 0.28 (0.02-0.63) | 0.19 (0.05-0.69) | .90 |
| Serum M spike | Immunofixation only (fixation only, 0.6 g/dL) | Immunofixation only (fixation only, 0.9 g/dL) | .31 |
| BM plasma cells, %, median (IQR) | 10 (3-30)* | 8 (4-16)† | .09 |
| Renal amyloid, n (%) | 19 (36) | 84 (58) | .005 |
| Cardiac amyloid, n (%) | 24 (45) | 81 (56) | .047 |
| Neuropathy, n (%) | 5 (9) | 19 (13) | .47 |
| Autonomic failure, n (%) | 3 (6) | 10 (7) | .74 |
n = 44.
n = 137.
Patients with IgD and non-IgD amyloidosis have similar survival. Kaplan-Meier analysis of survival in patients with IgD-associated (n = 53) and non–IgD-associated (n = 144) amyloidosis shows nearly overlapping survival curves. No difference between the groups was seen (P = .51).
Patients with IgD and non-IgD amyloidosis have similar survival. Kaplan-Meier analysis of survival in patients with IgD-associated (n = 53) and non–IgD-associated (n = 144) amyloidosis shows nearly overlapping survival curves. No difference between the groups was seen (P = .51).
Discussion
To our knowledge, this study reports the first case series of IgD amyloidosis. Our findings suggest that these patients have similar survival and appear to have a lower incidence of renal involvement than their non-IgD counterparts. Six (12%) of the patients underwent autologous stem cell transplantation, only 1 of whom has died to date, and the other 5 have a median follow-up of 68 months.
IgD monoclonal proteins are rare and historically have been associated with the presence of multiple myeloma. In 1970, a patient was reported who had IgD multiple myeloma with multiple extramedullary amyloid-containing tumors and amyloid casts in the kidney.12 In 1973, another patient was reported who had IgD myeloma and amyloid arthropathy that led to periarticular infiltration with subcutaneous nodules mimicking rheumatoid arthritis.13 Both of these reports predated the first recognition that amyloidosis was a clear plasma-cell dyscrasia associated with monoclonal Ig.14 In contrast to these isolated case reports, IgD-associated multiple myeloma was well recognized. One study reviewed the cases of 133 patients with IgD-associated multiple myeloma; these IgD-associated cases constituted 0.8% of the M components and 2.1% of the myelomas seen at the institution.1 In these patients, IgD levels were recognized to be low despite the presence of a monoclonal IgD protein, and λ light chains were found in 90%. On the basis of these findings, we might suspect amyloidosis to be more prevalent in IgD myeloma than in non-IgD myeloma because of its λ preponderance, but this does not appear to be the case.1 In 1975, amyloid fibril protein was immunochemically identified in a patient with IgD myeloma, and it demonstrated reactivity with both D and λ, suggesting that the amyloid fibril protein was an intact circulating polypeptide light chain.2 IgD light-chain deposition disease has also been reported in the kidney with an IgD λ protein and no fibrillar deposit.15 In a series of 2038 patients with a monoclonal protein, 9 IgD monoclonal proteins were found (0.44%).16 All of these patients had multiple myeloma and none was recognized as having amyloidosis. There is only 1 report of IgD λ amyloidosis developing in a patient with MGUS 11 years after that diagnosis.17 The patient received a stem cell transplantation and had an excellent response.
IgD myeloma has been shown to have some laboratory similarities with IgD amyloidosis. In a Mayo Clinic series, an M spike was only detectable in 60% of the 53 patients with IgD myeloma, and light-chain proteinuria was present in 96%.18 The light chain was λ in 60%, although these patients appeared to have shorter survival than patients with non-IgD myeloma.18 An estimate of the prevalence of IgD myeloma is 0.2%-0.3% of all patients having a monoclonal protein.19 In one series, myeloma cast nephropathy was present in all patients with IgD myeloma, perhaps reflecting the high prevalence of light-chain proteinuria.20 Of 20 000 patients undergoing autologous stem cell transplantation for multiple myeloma, 379 (1.9%) had IgD.21 These patients appeared to have higher complete remission rates with significantly worse survival than patients with non-IgD myeloma.
In a review of 133 patients with IgD myeloma, amyloidosis was found at autopsy in 44%.1 Therefore, the amyloidosis was either subclinical or was simply not recognized by clinicians at presentation. When we reviewed the cases of patients with asymptomatic light-chain amyloidosis found at the time of diagnostic BM biopsy, amyloid deposits were found in only 2 of 144 specimens (1%), and neither patient had an IgD monoclonal protein.22 Asymptomatic amyloidosis is uncommon and IgD amyloidosis is rare.
The recognition of IgD amyloidosis can be difficult. The IgD monoclonal protein level is very small, often visible only by immunofixation, which may delay the diagnosis. The inability to detect a light chain in 6 of the patients in our study is not surprising. As noted in Table 4, most patients did not have a measurable M spike (detectable by immunofixation only). When low-level monoclonal gammopathies are seen, it is not always easy to discern a monoclonal band. Moreover, IgD monoclonal proteins are so closely linked to the diagnosis of multiple myeloma in the minds of clinicians that the possibility of amyloidosis may be overlooked.
The survival data reported in this paper may not reflect current anticipated outcomes. The therapies administered over the 41 years of the study would not meet the standard of care today. Patients seen at Mayo Clinic after September 1, 2006 have had improved 4-year overall survival at 42%, compared with 21% before that date.23 High-dose chemotherapy followed by stem cell transplantation and recently introduced novel agents have been important in enhancing the outcomes for this patient population. One series reported a complete response rate of 49% using high-dose chemotherapy followed by stem cell transplantation.24 Complete eradication of light-chain production is believed to be necessary to prevent further tissue deposition of amyloid. We recently reported results in 430 patients with amyloidosis undergoing stem cell transplantation.25 The patients with a complete response have not attained median survival; for those with a partial response, median survival was 107 months. Organ responses were seen in 47%.25
Lenalidomide is a newer therapy that has been used with some success. When lenalidomide was combined with dexamethasone in the treatment of primary amyloidosis, the progression-free survival of patients with complete response was 49.8 months.26 The 3-drug combination of lenalidomide, melphalan, and dexamethasone used in patients with primary amyloidosis resulted in a hematologic response rate of 58%, which was complete in 42%.27 The 2-year event-free survival and the overall survival were 54% and 81%, respectively. In another study of 35 patients with amyloidosis given lenalidomide, cyclophosphamide, and dexamethasone, the hematologic response rate was 60% and the median overall survival was 16.1 months.28 The maximum tolerated dose of lenalidomide in combination with melphalan has been found to be 15 mg.27
Bortezomib is another drug that has shown promise for primary amyloidosis. A phase 1 dose-escalation study of bortezomib given either twice weekly (days 1, 4, 8, and 11) every 21 days or once weekly (days 1, 8, 15, and 22) every 35 days reported hematologic responses in 50% of patients.29 The 1-year hematologic progression-free rates were 72.2% and 74.6% and the 1-year survival rates were 93.8% and 84%, respectively, for the twice-weekly and once-weekly regimens. Among 70 patients, there were 20 renal responses (29%) and 9 cardiac responses (13%). The once-weekly regimen was associated with less neurotoxicity and a lower rate of discontinuation and dose reduction due to toxicity than the twice-weekly regimen. Both dosing schedules represent active, well-tolerated regimens in relapsed amyloidosis.29
A limitation of the current study was in the identification of a suitable control group. The 2 cohorts of patients with IgD and non-IgD amyloidosis were well matched for clinical features known to affect outcomes but not for the therapy they received. Over 41 years, the therapy was heterogeneous, with half receiving an alkylator-corticosteroid combination that might not be used today. The control group also lacked patients undergoing transplantation or receiving modern anti–plasma cell drugs. As a consequence of this shortcoming, a firm conclusion about the lack of survival differences between the groups is somewhat tenuous. It was challenging to find a contemporaneous group of patients for which all data were available for analysis, so a compromise was reached in using the selected 144 patients.
In conclusion, IgD amyloidosis is a distinct entity, predominantly of the λ light-chain type, that causes the typical amyloidosis syndromes of cardiomyopathy, nephrotic-range proteinuria, hepatomegaly, and peripheral neuropathy. The median survival in our series was 19.9 months. It appears that cardiac and renal involvement are less common with this heavy-chain type. Amyloidosis must be kept in the differential diagnosis when a patient with an IgD monoclonal protein is recognized.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.A.G. designed the research; M.A.G., F.K.B., and S.R.H. analyzed the data; M.A.G., F.K.B., S.R.H., D.D., A.D., P.R.G., S.K.K., M.Q.L., J.A.L., N.L., S.V.R., S.J.R., S.R.Z., J.R.M., V.R., and R.A.K. edited the manuscript; D.D., A.D., P.R.G., and S.K.K. contributed analytical input; M.Q.L., J.A.L., N.L., S.V.R., and S.J.R. helped with the data interpretation; M.A.G. performed the statistical analysis; and all authors read and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Morie A. Gertz, MD, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: gertz.morie@mayo.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal