Abstract
Tipifarnib (T) exhibits modest activity in elderly adults with newly diagnosed acute myelogenous leukemia (AML). Based on preclinical synergy, a phase 1 trial of T plus etoposide (E) yielded 25% complete remission (CR). We selected 2 comparable dose levels for a randomized phase 2 trial in 84 adults (age range, 70-90 years; median, 76 years) who were not candidates for conventional chemotherapy. Arm A (T 600 mg twice a day × 14 days, E 100 mg days 1-3 and 8-10) and arm B (T 400 mg twice a day × 14 days, E 200 mg days 1-3 and 8-10) yielded similar CR, but arm B had greater toxicity. Total CR was 25%, day 30 death rate 7%. A 2-gene signature of high RASGRP1 and low aprataxin (APTX) expression previously predicted for T response. Assays using blasts from a subset of 40 patients treated with T plus E on this study showed that AMLs with a RASGRP1/APTX ratio of more than 5.2 had a 78% CR rate and negative predictive value 87%. This ratio did not correlate with outcome in 41 patients treated with conventional chemotherapies. The next T-based clinical trials will test the ability of the 2-gene signature to enrich for T responders prospectively. This study is registered at www.clinicaltrials.gov as #NCT00602771.
Introduction
The development of tolerable and effective therapies for adults with acute myelogenous leukemia (AML) remains challenging, especially for the elderly patient. Although part of the poor outcome in elderly AML (particularly age 75 and older) relative to younger adults (under age 55) reflects differences in the host's ability to tolerate intensive therapy, the disease itself is biologically more resistant to the cytotoxic effects of traditional chemotherapy.1-5 These AMLs often evolve from antecedent myelodysplastic syndromes (MDSs) and are genetically complex as a result of toxin exposure and cumulative DNA damage.6-8 Recent microarray gene expression studies demonstrate that AMLs in older adults are more likely to overexpress ras, src, and tumor necrosis factor (TNF) genes with downstream pathway activation and therefore exhibit a decreased sensitivity to chemotherapy agents, such as anthracyclines.8 The net result is a lack of benefit of intensive chemotherapy for elderly adults with AML.
Farnesyltransferase inhibitors (FTIs) are small molecule signal transduction inhibitors that impede critical cell growth and survival signals.9-12 These agents are potent and selective inhibitors of farnesyltransferase (FTase), an intracellular enzyme that catalyzes the transfer of a 15-carbon farnesyl moiety to various polypeptide acceptors, including the chaperone HDJ-2, nuclear lamins, centromeric proteins that interact with microtubules to promote completion of mitosis, and small GTP-binding polypeptides of the Ras, Rho, and Rheb families.13-16 Inhibiting farnesylation of these polypeptides leads to diminished cell proliferation and, in some model systems, tumor cell death.
Tipifarnib (T), an orally bioavailable nonpeptidomimetic methylquinolinone FTI, exhibits modest activity in refractory AML17,18 and in elderly adults with newly diagnosed, poor-risk AML when given as a single agent.19,20 In a phase 2 study of 158 adults with untreated poor-risk AML (93% > 65 years, 75% with secondary AML, 47% with adverse cytogenetics),20 complete remissions (CR) were achieved in 14%, of whom 82% had prior MDS and 40% had adverse cytogenetics. Whereas median overall survival (OS) for all 158 patients was 5.3 months, median OS for those achieving CR was 18.3 months. More recently, a phase 3 study of single agent T versus best supportive care, including hydroxyurea (HU) in 457 patients older than 70 years with newly diagnosed AML who were deemed “not fit” for conventional chemotherapy, was conducted in Europe and Canada.19 Although CRs with disease-free survival (DFS) 8 months and OS 22 months were achieved in 8% of those randomized to T compared with no CRs in the supportive care/HU arm, no statistically significant survival advantage was appreciated in those patients treated with T.
In an attempt to increase CR rate and duration, we combined T with other antileukemic agents in vitro.13 In primary AML samples, T inhibited signaling downstream of the farnesylated small G protein Rheb and synergistically enhanced etoposide (E)-induced antiproliferative effects.13 These findings led to a multicenter phase 1 trial of oral T (300-600 mg twice daily for 14 or 21 days) plus oral E (100-200 mg daily days 1-3 and 8-10 for each cycle) in 84 adults older than 70 years who were not candidates for conventional induction therapy, on the basis of both host and disease biology.13 T + E led to higher response rates than those seen in the single-agent studies, with CR rate 25% across multiple dose levels of both drugs, DFS 9.8 months, and a median OS 22 months for CR patients. Notably, these results occurred in patients with multiple poor-risk features, including multiple comorbidities, adverse cytogenetics, and secondary AML.
To gain further understanding of the optimal doses of T and E that will produce the maximal CR rate with the most acceptable toxicity profile, we have now conducted a randomized phase 2 study to compare 2 T + E schedules. The schedules selected from the phase 1 study generated CRs in 3 of 6 for each arm at the maximal tolerated dose) and no deaths or dose-limiting toxicities in their respective 6-patient cohorts. The rationale was to obtain additional clinical information that would allow us to select one of 2 different schedules of T + E, based on both response and toxicity, so that the better performing arm could be moved forward for further comparative trials.
The ability to select patients who are likely to benefit from a T-containing regimen would provide an important therapeutic option for elderly AML patients who are unlikely to tolerate or benefit from intensive chemotherapy approaches. Raponi et al conducted serial studies of gene expression profiling in the context of AML cell lines and primary AML marrow blasts exposed to T in vitro,21 marrow blasts from patients with relapsed and refractory AML undergoing treatment with T alone,22 and marrow blasts from elderly adults with previously untreated AML with poor-risk features who received T alone as induction therapy.23 Studies in the newly diagnosed cohort uncovered a 2-gene transcript signature consisting of high RASGRP1 (which encodes the Ras-activating guanine nucleotide exchange factor RASGRP1) and low APTX (which encodes the DNA excision repair protein aprataxin), the ratio of which can positively predict clinical response.23 Retrospectively, in the context of this current clinical trial, we investigated and confirmed that the 2-gene signature correlated with clinical response in a cohort of the elderly AML patients treated with T + E.
Methods
Patient eligibility and selection
Between January 2008 and December 2009, 110 elderly adults (age > 70 years) with pathologically confirmed (using World Health Organization criteria)24 newly diagnosed de novo or secondary (MDS, myeloproliferative disorder, treatment-related) AML excluding acute promyelocytic leukemia, were evaluated for eligibility using previously described criteria.25 Patients were ineligible if they had Eastern Cooperative Oncology Group performance status more than 3, peripheral blast count more than 30 000/μL or a projected doubling time of less than 2 days, but cytoreduction with hydroxyurea was permitted until 24 hours before T + E. Prior therapy for MDS (cytokines, thalidomide/lenalidomide, interferon, 5-azacytidine/decitabine) was not exclusionary. All patients provided written informed consent following the Declaration of Helsinki, and the clinical trial was approved by the institutional review boards of each participating institution.
Treatment schema
Patients were randomly assigned to one of 2 dose schedules of T + E. In arm A, T was 600 mg orally twice a day for 14 days and E was 100 mg orally on days 1 to 3 and 8 to 10. In arm B, T was 400 mg orally twice a day for 14 days and E was 200 mg orally on days 1 to 3 and 8 to 10. Each treatment cycle was 28 days, followed by a rest period of up to 35 days to allow count recovery. Subsequent cycles began on days 29 to 64 of the previous cycle. Patients were eligible to receive a second cycle if CR, partial response (PR), hematologic improvement (HI), or stable disease was achieved. Patients achieving CR after cycle 1 or cycle 2 were permitted up to 6 additional cycles of T + E after CR had been attained. Patients achieving PR or HI could receive T + E until disease progression or unacceptable toxicity ensued. All patients received supportive care as previously described.13,20 Growth factors were not permitted. Dose reductions in one or both drugs were permitted in both arms for grade 2 or greater neurotoxicity or nephrotoxicity and/or grade 3 or greater other nonhematologic toxicities, including hepatic dysfunction. For arm A, the dose of T was decreased to 400 mg twice a day and E remained at 100 mg. For arm B, if there was no neurotoxicity, only E was decreased to100 mg on days 1 to 3 and 8 to 10, with T decreased to 300 g twice a day only for the presence of neurotoxicity.
Response and toxicity evaluation
Bone marrow aspiration and biopsy were performed before treatment and at hematologic recovery or when leukemia regrowth was suspected clinically, typically 14 to 21 days after the last dose of tipifarnib. Hematologic recovery and response criteria were as previously described.13,20,25 National Cancer Institute Common Toxicity Criteria Version 3.0, were used to describe and grade all adverse events. T + E was discontinued for progressive disease or grade 4 nonhematologic toxicity. T + E was withheld temporarily for more than or equal to grade 2 neurotoxicity or nephrotoxicity; grade 3 other nonhematologic toxicity (excluding alopecia or controlled nausea and vomiting); or grade 4 granulocytopenia or platelets < 20 000/μL lasting > 3 weeks after completion of each 28-day cycle. T + E could be resumed at a lower tipifarnib dose (400 mg orally twice a day for arm A) after resolution to < grade 1 nonhematologic toxicity within 28 days of first occurrence.
Laboratory correlates
Two-gene signature.
Bone marrow blasts were obtained from 2 groups of adults with AML before beginning treatment: group 1, T + E phase 2 study: 40 T + E patients who were treated on this protocol and had adequate aspirated pretreatment marrow sample (a subset of the total 84 patient group); and group 2, specificity study: 41 adults with newly diagnosed AML who were undergoing induction timed sequential therapy with cytosine arabinoside (Ara-C) and daunorubicin followed by etoposide (AcDVP16)26 or flavopiridol followed by Ara-C and mitoxantrone (FLAM)27 and who had adequate pretreatment marrow samples. RNA isolation from cell pellets was performed using RNeasy Midi Kit (QIAGEN). TaqMan-based quantitative RT-PCR assays were developed in a single-tube triplex quantitative PCR format using a commercially available RNA-to-Ct One Step RT-PCR Kit from Life Technologies and also using GMP-grade reagents from the Veridex Breast Lymph Node (BLN) RT-PCR Kit (GeneSearch BLN Test Kit, IVD) for the 2 T-related markers RASGRP1 and APTX, and HMBS as an internal control. A high correlation between the 2 formats (Pearson R2 range for 3 markers, 0.97-1) with area under the curve = 0.83 (P = .0009) was demonstrated.28 The GMP RT-PCR protocol and sequences of the TaqMan assays are described in supplemental Methods and supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Statistical design and analysis
Analysis of toxicity and efficacy.
The goal of this randomized phase 2 study was to select one of 2 different schedules of T + E, based on both response and toxicity, by determining which arm would yield more favorable efficacy and safety outcomes, and the better performing arm could be moved forward for further comparative trials. We planned a “pick the winner” approach using a Simon 2-stage design to minimize accrual to an arm with an ineffective regimen with a targeted response range of 20% (null) versus 40% (alternative). In the first stage, 21 patients were enrolled in each arm; if 4 or fewer CRs were observed, the arm was closed. If 5 or more CRs are observed, then the arm could remain open for an additional 21 patients (total 42). An arm would be considered promising if there were at least 13 CRs in 42 patients. This design can distinguish between 20% and 40% CR rates with 6% false-positive (type 1) and 10% false-negative (type II) error rates. At the interim look, toxicity would be evaluated to determine whether both regimens were safe to continue enrollment. If, at the end of the study, both arms had continued to the maximum sample size and the CR rates were approximately the same, then the less toxic arm would be selected. If there were differences in both CR and toxicity, then a clinically based algorithm would be used to make the selection. Response differences and toxicity differences would be given approximately the same weight in the selection decision. To determine “dose-selecting toxicity,” we used an overall toxicity score as follows29 : 0 indicates no toxicity; 1, any toxicity (hospitalization, fever/infection, mucositis, hyperbilirubinemia, neurotoxicity); 2, hospitalization more than 10 days, neurotoxicity grade 2, all other toxicities grade 3; 3, neurotoxicity more than grade 3, all other toxicities grade 4; and 4, death.
Time to event outcomes (OS and DFS) were analyzed using Kaplan-Meier methods. CR rates and clinical benefit were estimated using binomial approaches with 95% CIs. Continuous toxicity scores at interim analysis were compared using Wilcoxon rank-sum test. Summary statistics, such as mean, median, and range, are reported for continuous outcomes, and 95% exact CIs are reported for proportions. The estimate, 95% CI, and test of the null hypothesis for the primary endpoint (CR) were performed accounting for the interim look.30
Two-gene analysis.
Exclusion criteria of sample analysis were based on the defined cycle threshold (Ct) cutoff values of 3 markers. If a sample had one of 2 markers RASGRP1 or APTX above the Ct cutoff of 35, it was considered as nonevaluable. If a sample had a control marker HMBS value above the Ct cutoff of 30 but 2 other markers below Ct of 35, this sample was considered evaluable.
During development of the 2-gene predictive quantitative RT-PCR assay in a multiplexed single reaction, the normalization procedure for determining relative expression of RASGRP1/APTX was refined to include an external normalization control within each assay run. Use of an external reference gene for normalization is a common analytical technique for relative gene expression data and is commonly referred to as the 2−ΔΔCt, or δ-delta Ct method.31 The following algorithm was used for the 2-gene ratio calculation r = 2−̂([A − B] − [C − D]), where A is sample RASGRP1 Ct value, B is J Y control RNA RASGRP1 Ct value, C is sample APTX Ct value, and D is JY RNA APTX Ct value.
Logistic regression analysis was used to evaluate the association between the 2-gene ratio and CR. Based on the resulting receiver operator characteristic (ROC) curve from the regression model, an optimal threshold of 5.2 was chosen based on the ROC curve algorithm in MedCalc Version 11.6 software (May 11, 2011) which, in turn, is based on the maximized sum of threshold specificity and sensitivity values. Using the threshold, patients were divided into 2 groups and the groups were compared with respect to CR rate and OS. Kaplan-Meier curves were generated for OS for each group. For CR, positive predictive value, negative predictive value (NPV), sensitivity, and specificity were calculated using the threshold. Cox regression was used to estimate the hazard ratio comparing risk of death between the groups and to generate a P value.
Results
Patient demographics
Of the 110 patients evaluated for eligibility, 84 were enrolled. Reasons for ineligibility included screen failure because of diagnosis being MDS or myeloproliferative neoplasm rather than AML (7), patients declining therapy (9), alternate therapy (3), and Eastern Cooperative Oncology Group performance status more than 3 (8). As detailed in Table 1, median age for the 84 patients enrolled was 77 years (range, 70-90 years), with 61 (73%) being older than 75 years, and median Eastern Cooperative Oncology Group performance status of 1 (range, 0-2), with only 9 (11%) have a performance status of 0. Fifty-two patients (62%) had adverse cytogenetics and 46 (55%) had secondary AML, with the duration of antecedent hematologic disorder ranging from 1 month to more than 10 years and with 15 (33%) having received some type of treatment for their antecedent hematologic disorder. Of the 22 patients 70 to 74 years of age, 17 (77%) had secondary AML (12 patients) and/or adverse cytogenetics (12 patients). Almost all patients (80 of 84, 95%) had at least one nonhematologic comorbidity and 49 (58%) had 3 or more.
Demographic and biologic characteristics of 84 elderly adults with newly diagnosed AML treated with T + E
| . | Arm A (n = 63) . | Arm B (n = 21) . | Total (n = 84) . |
|---|---|---|---|
| Sex, no. (%) | |||
| Male | 39 (63) | 14 (67) | 53 (63) |
| Female | 24 (38) | 7 (33) | 31 (37) |
| Median age, y (range) | 76 (70-90) | 78 (71-90) | 76 (70-90) |
| Biologic disease features, no. (%) | |||
| Secondary AML | 36 (57) | 10 (48) | 46 (55) |
| MDS/AML | 24 | 7 | 31 |
| t-AML | 12 | 3 | 12 |
| Prior therapy* | 10 (28) | 5 (50) | 15 (33) |
| Adverse cytogenetics† | 38 (60) | 14 (67) | 52 (62) |
| Single | 10 | 4 | 14 |
| Complex (> 3 lesions) | 19 | 7 | 26 |
| Other | 9 | 3 | 12 |
| No. with > 1 poor risk feature | 47 (75) | 15 (71) | 62 (74) |
| Host comorbidities, no. (%) | |||
| 1 comorbidity | 61 (97) | 19 (90) | 80 (95) |
| 3 comorbidities | 36 (57) | 13 (62) | 49 (58) |
| . | Arm A (n = 63) . | Arm B (n = 21) . | Total (n = 84) . |
|---|---|---|---|
| Sex, no. (%) | |||
| Male | 39 (63) | 14 (67) | 53 (63) |
| Female | 24 (38) | 7 (33) | 31 (37) |
| Median age, y (range) | 76 (70-90) | 78 (71-90) | 76 (70-90) |
| Biologic disease features, no. (%) | |||
| Secondary AML | 36 (57) | 10 (48) | 46 (55) |
| MDS/AML | 24 | 7 | 31 |
| t-AML | 12 | 3 | 12 |
| Prior therapy* | 10 (28) | 5 (50) | 15 (33) |
| Adverse cytogenetics† | 38 (60) | 14 (67) | 52 (62) |
| Single | 10 | 4 | 14 |
| Complex (> 3 lesions) | 19 | 7 | 26 |
| Other | 9 | 3 | 12 |
| No. with > 1 poor risk feature | 47 (75) | 15 (71) | 62 (74) |
| Host comorbidities, no. (%) | |||
| 1 comorbidity | 61 (97) | 19 (90) | 80 (95) |
| 3 comorbidities | 36 (57) | 13 (62) | 49 (58) |
t-AML indicates treatment-related AML.
Includes previous therapy for MDS and t-MDS with growth factors, demethylating agents, thalidomide, or lenalidomide individually or in combinations.
Adverse cytogenetics: −5/5q, −7/7q, abnormal 3/3q, abnormal 11q23, abnormal 17p, −20q, +13, t(6;9), t(9;22), complex (> 3 abnormalities).
Interim analysis
After the first 42 patients were enrolled in the trial, an interim analysis was performed to evaluate the safety and efficacy of each dose schedule of T + E (n = 21 patients per arm). As shown in Table 2, patients randomized to arm A (T 600 mg twice a day × 14 days, E 100 mg on days 1-3 and 8-10) versus arm B (T 400 mg twice a day × 14 days, E 200 mg on days 1-3 and 8-10) were similar in terms of host and disease biologic features. Both arms surpassed the efficacy early stopping rule, with 7 of 21 (33%) in arm A and 6 of 21 (29%) in arm B achieving CR. However, although the frequency of hospitalization during cycle 1 was similar between the arms, the number of days of hospitalization was different, with median 4 days for arm A vs median 16 days for arm B. Moreover, the occurrence of grade 3 or greater nonhematologic toxicities was greater in arm B than arm A (33% vs 19%). One patient (5%) died of toxicity in arm A, whereas 5 (24%) died of toxicity in arm B (90% CI, 10%-43%). The median total toxicity score (0-4) was 1 for arm A and 2 for arm B (P = .09). Based on the inferior toxicity profile observed in arm B, enrollment to this arm was halted. Given lack of standard treatment options for patients eligible for this trial, accrual to arm A was continued for an additional 42 patients, for a total of 84 patients in the study (63 patients in arm A and 21 patients in arm B). Because of early withdrawal of one patient in arm A after 2 days of T + E, there are 62 patients and 21 patients eligible for efficacy analysis in arms A and B, respectively.
Toxicity and efficacy outcomes of arm A and arm B at interim analysis
| . | Arm A (n = 21) . | Arm B (n = 21) . |
|---|---|---|
| Efficacy | ||
| No. of cycles, median (range) | 2 (1-10) | 1 (1-6) |
| No. (%) of patients receiving second cycle | 14 (67) | 9 (43) |
| Median OS, mo (range) | 12 (0.5-37.4) | 7 (0.6-22.5) |
| OS > 12 mo, no. (%) | 10 (48) | 7 (33) |
| CR, no. (%) | 7 (33) | 6 (29) |
| Median DFS, mo (range) | 11 (6.6-27.1) | 8 (2.2-21.2) |
| OS > 12 mo, no. (%) | 6 (86) | 5 (83) |
| Overall Toxicity Score, no. (%)* | ||
| 0 | 7 (33) | 4 (19) |
| 1 | 6 (29) | 5 (24) |
| 2 | 6 (29) | 5 (24) |
| 3 | 1 (5) | 2 (10) |
| 4 | 1 (5) | 5 (24) |
| No. (%) of Toxicity Score > 3 | 2 (10) | 7 (33) |
| No. (%) > grade 3 nonhematologic toxicities | 4 (19) | 7 (33) |
| No. (%) > grade 3 infection | 5 (24) | 8 (38) |
| Bloodstream | 4 | 1 |
| Lung | 1 | 7 |
| Fatal infection | 1 (5) | 4 (19) |
| . | Arm A (n = 21) . | Arm B (n = 21) . |
|---|---|---|
| Efficacy | ||
| No. of cycles, median (range) | 2 (1-10) | 1 (1-6) |
| No. (%) of patients receiving second cycle | 14 (67) | 9 (43) |
| Median OS, mo (range) | 12 (0.5-37.4) | 7 (0.6-22.5) |
| OS > 12 mo, no. (%) | 10 (48) | 7 (33) |
| CR, no. (%) | 7 (33) | 6 (29) |
| Median DFS, mo (range) | 11 (6.6-27.1) | 8 (2.2-21.2) |
| OS > 12 mo, no. (%) | 6 (86) | 5 (83) |
| Overall Toxicity Score, no. (%)* | ||
| 0 | 7 (33) | 4 (19) |
| 1 | 6 (29) | 5 (24) |
| 2 | 6 (29) | 5 (24) |
| 3 | 1 (5) | 2 (10) |
| 4 | 1 (5) | 5 (24) |
| No. (%) of Toxicity Score > 3 | 2 (10) | 7 (33) |
| No. (%) > grade 3 nonhematologic toxicities | 4 (19) | 7 (33) |
| No. (%) > grade 3 infection | 5 (24) | 8 (38) |
| Bloodstream | 4 | 1 |
| Lung | 1 | 7 |
| Fatal infection | 1 (5) | 4 (19) |
Overall Toxicity Scoring Criteria: 0 indicates no toxicity; 1, any toxicity (hospitalization < 10 days; fever/infection; grade 1 or 2 mucositis, elevated bilirubin, or creatinine; grade 1 neurotoxicity); 2, hospitalization > 10 days; grade 2 neurotoxicity; all other toxicities grade 3; 3, neurotoxicity > grade 3; all other toxicities grade 4; and 4, death.
Toxicities
A total of 273 cycles were given to 84 patients, with the median number of cycles per patient being 2 (range 1-10); 46 patients (56%) received at least 2 cycles, 27 (32%) received at least 3 cycles, and 18 (21%) received 4 or more cycles. Hospitalizations (Table 3) were required in 49 of 84 (58%) during cycle 1 for a median 8 days (range, 2-36 days), but only during 17 (9%) of the remaining 189 cycles, for an overall hospitalization rate of 66 of 273 cycles (24%). The 63 patients treated with arm A received 234 cycles, and the 21 patients treated with arm B received 39 cycles. A total of 27 dose reductions for nonhematologic toxicities were implemented for 24 of 84 patients (29%). For arm B patients, 6 of 21 (29%) required dose reductions of T alone (2 patients) or both T + E (4 patients) during cycle 1. There were a total of 18 of 63 patients (29%) in arm A requiring dose reduction of T alone, 15 patients during cycle 1, and 3 during cycle 2 or 3.
Summary of toxicities encountered during T + E therapy
| . | Cycle 1, no. (%) of 84 patients . | Total, no. (%) of 273 patients . |
|---|---|---|
| Hospitalizations | 49 (58) | 66 (24) |
| Documented infections | 30 (36) | 50 (18) |
| Skin/cellulitis | 6 | 8 |
| Pneumonia | 18 | 23 |
| Sinusitis | 1 | 2 |
| Gastrointestinal | 1 | 2 |
| Genitourinary | 4 | 5 |
| Bacteremia | 7 | 10 |
| Neutropenic fever | 20 (24) | 31 (11) |
| Drug-related toxicities | ||
| Neurotoxicity (grade 1-4) | 19* | 22‡ |
| Oral mucositis (> grade 2) | 6 | 9 |
| Gastrointestinal (> grade 2) | 2 | 4 |
| Hyperbilirubinemia (> grade 2) | 10 | 16 |
| Renal (> grade 2) | 14 | 17 |
| Dehydration (> grade 2) | 13 | 16 |
| Rash (> grade 2) | 3 | 4 |
| Fatigue (> grade 2) | 15 | 26 |
| Cardiac (grade 1-5) | 3† | 6§ |
| Death | 10 (12) | 14 (5) |
| Infection | 6 | 9 |
| Blood | 3 | 3 |
| Lung | 3 | 6 |
| Cardiac | 1 | 2 |
| Multiorgan | 3 | 3 |
| . | Cycle 1, no. (%) of 84 patients . | Total, no. (%) of 273 patients . |
|---|---|---|
| Hospitalizations | 49 (58) | 66 (24) |
| Documented infections | 30 (36) | 50 (18) |
| Skin/cellulitis | 6 | 8 |
| Pneumonia | 18 | 23 |
| Sinusitis | 1 | 2 |
| Gastrointestinal | 1 | 2 |
| Genitourinary | 4 | 5 |
| Bacteremia | 7 | 10 |
| Neutropenic fever | 20 (24) | 31 (11) |
| Drug-related toxicities | ||
| Neurotoxicity (grade 1-4) | 19* | 22‡ |
| Oral mucositis (> grade 2) | 6 | 9 |
| Gastrointestinal (> grade 2) | 2 | 4 |
| Hyperbilirubinemia (> grade 2) | 10 | 16 |
| Renal (> grade 2) | 14 | 17 |
| Dehydration (> grade 2) | 13 | 16 |
| Rash (> grade 2) | 3 | 4 |
| Fatigue (> grade 2) | 15 | 26 |
| Cardiac (grade 1-5) | 3† | 6§ |
| Death | 10 (12) | 14 (5) |
| Infection | 6 | 9 |
| Blood | 3 | 3 |
| Lung | 3 | 6 |
| Cardiac | 1 | 2 |
| Multiorgan | 3 | 3 |
Seven grade 1, 7 grade 2, 3 grade 3, and 2 grade 4.
One grade 2, 1 grade 3, and 1 grade 5.
Seven grade 1, 9 grade 2, 3 grade 3, and 3 grade 4.
One grade 2, 3 grade 3, and 2 grade 5.
Table 3 delineates the spectrum of nonhematologic toxicities encountered during one or more cycles of T + E. Overall, the toxicity profile recapitulates that detected during the phase 1 trial of T + E.13 For the entire group of 84 patients, 6 (7%) deaths occurred before day 30 (1 renal failure, 2 multiorgan failure, and 3 infection). An additional 4 patients died days 38 to 54 after cycle 1 from sudden death (n = 1) or progressive fungal pneumonia (n = 3). Thus, a total of 10 of 84 (12%) died of toxicity during or after cycle 1. Of the 46 patients who went on to receive a second cycle, 4 (9%) died (1 sudden death and 3 pulmonary infection). No patients died of T + E-related toxicities in cycle 3 or thereafter. The < 60-day death rate was 16 of 84 (19%), with 11 deaths being related to toxicity and 5 because of progressive AML.
Clinical outcome
For the 84 patients enrolled and eligible for efficacy analysis, 21 (25%) achieved CR and 9 (11%) achieved PR/HI, for an overall response rate of 36% (95% CI, 26%-47%). Median OS is 6.6 months (95% CI, 5.4-9.7 months; range, 0.5-37.2 months), with a 12-month survival rate of 33% (95% CI, 24%-44%). For the 21 patients achieving CR, median OS is 19.2 months (95% CI, 12.9 months to infinity; range, 3.7-37.4 months); for those achieving PR/HI, median OS is 10.5 months (95% CI, 7.34 months to infinity; range, 2.2-17.4 months). Median DFS for the 21 CR patients was 6 months (95% CI, 4.6-19.9 months; range, 1.7-26.3 months). Characteristics of the 21 patients achieving CR (Table 4) are similar to patients achieving CR in the Lancet phase 2 single-arm trial20 and the phase 1 T + E combination trial13 in terms of age, secondary AML, comorbidities, and cytogenetics.
Clinical and cytogenetic characteristics of 21 patients achieving complete remission in response to T + E
| Age, y/sex . | Prior sAML/treatment . | PS . | Comorbidities . | Cytogenetics . | Arm . | DFS/OS arm . |
|---|---|---|---|---|---|---|
| 85/male | No/no | 1 | Aortic aneurysm, HBP nephrolithiasis | 47 XY, +8 | A | 10.9/13 |
| 77/male | No/no | 2 | Abdominal aneurysm, HBP nephrolithiasis, gastrointestinal bleed | 47 XY, +11 | A | 6.6*/37.4+ |
| 76/male | No/no | 2 | CAD, diabetes, sleep apnea | NG | B | 2.8/3.7 |
| 80/male | No/no | 2 | COPD, lower gastrointestinal bleed, HBP | 46 XY | A | 14.1/26.1 |
| 82/male | Yes/yes | 2 | CAD/stents | 46 XY | B | 10.7/14 |
| 75/female | Yes/no | 2 | CAD/stents, atrial fibrillation peripheral vascular disease | 46 XX, −20q | B | 21/22.8 |
| 79/male | Yes/yes | 1 | Prostate cancer/XRT, HBP, GERD | 45XY,-7,t(3;3) | B | 5.9/16.3 |
| 81/female | No/no | 1 | HBP, ↑ lipids, gout | 46XY | A | 21.3*/33.8+ |
| 81/male | No/no | 2 | Prostate cancer/XRT esophageal stricture | 47 XY,+13 + 9q34 | B | 21.1/22.6 |
| 88/male | Yes/yes | 1 | HBP, pneumonia | 46XY | B | 2.2/14.3 |
| 74/male | No/no | 2 | CAD, DOE, ”B”symptoms | ND | A | 7.0/23.6 |
| 74/male | Yes/yes | 2 | Prostate cancer/XRT, DOE, CAD carotid endarterectomy | 46XY | A | 6.9/12 |
| 76/male | Yes/yes | 1 | CAD/post-CABG, BPH | 45XY,dic(1;11) der 2, t(1;2) | A | 18.8*/27.5+ |
| 77/male | No/no | 2 | CAD, DOE, stomatitis | 46XY | A | 4.7/6.2 |
| 76/male | No/no | 1 | Osteoarthritis | 46XY,t(2;11) | A | 5.3/19.3 |
| 71/male | Yes/yes | 1 | HBP, prostate cancer, Grover disease | 46XY | A | 13.7/23.2+ |
| 79/female | No/no | 2 | None | ND | A | 6.6/13 |
| 72/male | Yes/yes | 2 | Myeloma, pneumonia, HBP GERD, hypothyroidism, leukemia cutis | 46XY | A | 4.6/6.1 |
| 75/male | No/no | 2 | HBP, pharyngeal obstruction | 47XY,+8 | A | 7.8/10.8 |
| 71/female | Yes/no | 1 | Arthralgias, fatigue | 46XX | A | 16+/17.5+ |
| 76/male | Yes/no | 1 | HBP, BPH | 46XX | A | 16+/17+ |
| Age, y/sex . | Prior sAML/treatment . | PS . | Comorbidities . | Cytogenetics . | Arm . | DFS/OS arm . |
|---|---|---|---|---|---|---|
| 85/male | No/no | 1 | Aortic aneurysm, HBP nephrolithiasis | 47 XY, +8 | A | 10.9/13 |
| 77/male | No/no | 2 | Abdominal aneurysm, HBP nephrolithiasis, gastrointestinal bleed | 47 XY, +11 | A | 6.6*/37.4+ |
| 76/male | No/no | 2 | CAD, diabetes, sleep apnea | NG | B | 2.8/3.7 |
| 80/male | No/no | 2 | COPD, lower gastrointestinal bleed, HBP | 46 XY | A | 14.1/26.1 |
| 82/male | Yes/yes | 2 | CAD/stents | 46 XY | B | 10.7/14 |
| 75/female | Yes/no | 2 | CAD/stents, atrial fibrillation peripheral vascular disease | 46 XX, −20q | B | 21/22.8 |
| 79/male | Yes/yes | 1 | Prostate cancer/XRT, HBP, GERD | 45XY,-7,t(3;3) | B | 5.9/16.3 |
| 81/female | No/no | 1 | HBP, ↑ lipids, gout | 46XY | A | 21.3*/33.8+ |
| 81/male | No/no | 2 | Prostate cancer/XRT esophageal stricture | 47 XY,+13 + 9q34 | B | 21.1/22.6 |
| 88/male | Yes/yes | 1 | HBP, pneumonia | 46XY | B | 2.2/14.3 |
| 74/male | No/no | 2 | CAD, DOE, ”B”symptoms | ND | A | 7.0/23.6 |
| 74/male | Yes/yes | 2 | Prostate cancer/XRT, DOE, CAD carotid endarterectomy | 46XY | A | 6.9/12 |
| 76/male | Yes/yes | 1 | CAD/post-CABG, BPH | 45XY,dic(1;11) der 2, t(1;2) | A | 18.8*/27.5+ |
| 77/male | No/no | 2 | CAD, DOE, stomatitis | 46XY | A | 4.7/6.2 |
| 76/male | No/no | 1 | Osteoarthritis | 46XY,t(2;11) | A | 5.3/19.3 |
| 71/male | Yes/yes | 1 | HBP, prostate cancer, Grover disease | 46XY | A | 13.7/23.2+ |
| 79/female | No/no | 2 | None | ND | A | 6.6/13 |
| 72/male | Yes/yes | 2 | Myeloma, pneumonia, HBP GERD, hypothyroidism, leukemia cutis | 46XY | A | 4.6/6.1 |
| 75/male | No/no | 2 | HBP, pharyngeal obstruction | 47XY,+8 | A | 7.8/10.8 |
| 71/female | Yes/no | 1 | Arthralgias, fatigue | 46XX | A | 16+/17.5+ |
| 76/male | Yes/no | 1 | HBP, BPH | 46XX | A | 16+/17+ |
sAML indicates secondary AML (MDS, treatment-related)/treatment for MDS; PS, performance status; HBP, high blood pressure; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; XRT, irradiation; GERD, gastroesophageal reflux disease; DOE, dyspnea on exertion; CABG, coronary artery bypass graft; and BPH, benign prostatic hyperplasia.
Reversion to MDS.
For the 62 patients in arm A, 15 achieved CR (26%; 95% CI, 15%-42%, adjusted for interim look), 8 (13%) achieved PR or HI, and the remaining 39 (61%) had no response, yielding an overall response rate of 37% (95% CI, 24%-49%). The P value for testing the null hypothesis (CR rate 20%) is 0.17. OS for all 63 patients and by responder status is shown in Figures 1 and 2, respectively. Median OS was 23.4 months (95% CI, 12.9 months to infinity) for the 15 CR patients, 10.2 months (95% CI, 7.3 months to infinity) for the 8 achieving PR/HI, and 3.7 months (95% CI, 2.4-7.1 months) for the 39 nonresponders. Among the 15 patients achieving CR in arm A, the median DFS was 6 months (95% CI, 5.1 months to infinity; Figure 3). There was no difference in DFS between arm A CR patients and the total CR population (15 arm A plus 6 arm B).
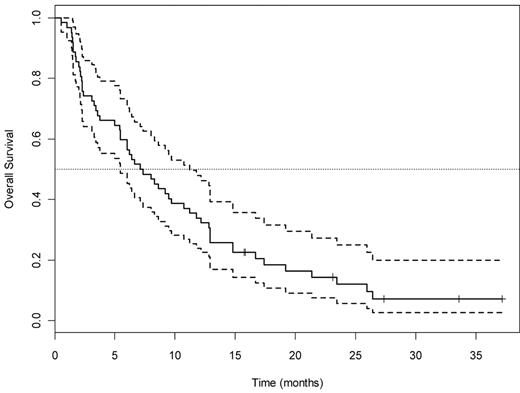
OS for all 63 patients treated with the T + E arm A. Dotted lines indicate 95% CI.
OS for all 63 patients treated with the T + E arm A. Dotted lines indicate 95% CI.
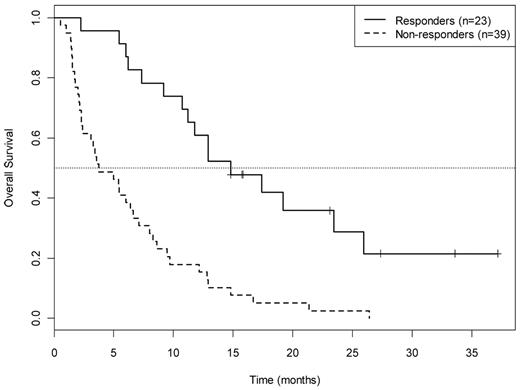
OS by response category for 62 patients treated in arm A. Responders: CR (complete remission) PR/HI (partial remission/hematologic improvement. Nonresponders: SD + PD indicates stable disease plus progressive disease.
OS by response category for 62 patients treated in arm A. Responders: CR (complete remission) PR/HI (partial remission/hematologic improvement. Nonresponders: SD + PD indicates stable disease plus progressive disease.
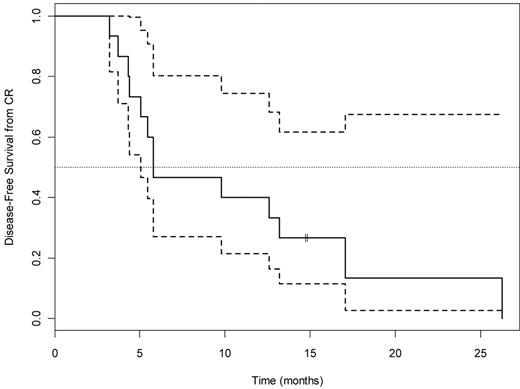
DFS (median, 6; 95% CI, 5.1, Infinity) for the 15 arm A patients who achieved CR. Dotted lines indicate 95% CI.
DFS (median, 6; 95% CI, 5.1, Infinity) for the 15 arm A patients who achieved CR. Dotted lines indicate 95% CI.
RASGRP1/APTX expression ratio
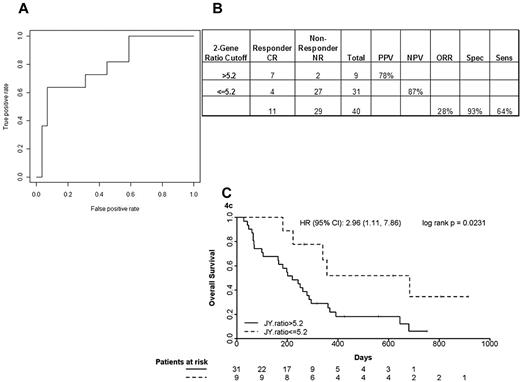
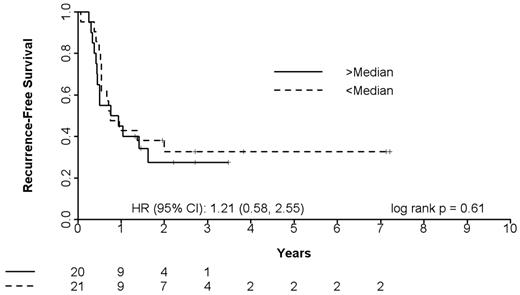
The multiplex single-step quantitative PCR assay was performed on pretreatment bone marrow blasts from 40 newly diagnosed elderly AML patients treated with T + E and 41 newly diagnosed AML patients treated with intensive timed sequential therapy26,27 (Table 5). The 40 T + E patients whose marrow blasts were studied were representative of the entire treatment population: median age, 77 years (range, 70-90 years), 24 (60%) with secondary AML and 26 (65%) with adverse cytogenetics, 11 (28%) achieving CR and 9 (21%) achieving PR or HI. As shown in Figure 4A-B, patients with a RASGRP1/APTX ratio of 5.2 or greater had a CR rate of 78% compared with those with a ratio of less than 5.2, who had a CR rate of 13%. This translates to an NPV of 87%. Kaplan-Meier survival curve analysis of patients stratified using a ratio cutoff of 5.2 indicated a positive trend in favor of utility of the 2-gene ratio assay in predicting response between no response and CR (hazard ratio = 2.96, P = .023) to the combination of T + E in elderly newly diagnosed AML patients (Figure 4C).
Evaluation of RASGRP1/APTX ratio treatment specificity: characteristics of patients receiving (phase 2) and not receiving (specificity) tipifarnib-based induction therapy for newly diagnosed AML
| . | Phase 2 (n = 40) . | Specificity (n = 41) . |
|---|---|---|
| Sex (male/female) | 21/19 | 19/22 |
| Median age, y (range) | 77 (70-90) | 58 (21-71) |
| Therapy | T + E | Ara-C, anthracycline, and third agent (flavopiridol or etoposide) |
| Cytogenetics, no. (%) | ||
| Favorable | 0 | 5 (10) |
| Normal | 12 (30) | 15 (37) |
| Intermediate | 2 (5) | 4 (10) |
| Adverse | 26 (65) | 17 (41) |
| FLT3-ITD, no. (%)* | 0 | 9 (22) |
| Response, no. (%) | ||
| CR | 11 (28) | 25 (61) |
| PR/HI | 9 (22) | 0 |
| Overall | 20 (50) | 25 (61) |
| . | Phase 2 (n = 40) . | Specificity (n = 41) . |
|---|---|---|
| Sex (male/female) | 21/19 | 19/22 |
| Median age, y (range) | 77 (70-90) | 58 (21-71) |
| Therapy | T + E | Ara-C, anthracycline, and third agent (flavopiridol or etoposide) |
| Cytogenetics, no. (%) | ||
| Favorable | 0 | 5 (10) |
| Normal | 12 (30) | 15 (37) |
| Intermediate | 2 (5) | 4 (10) |
| Adverse | 26 (65) | 17 (41) |
| FLT3-ITD, no. (%)* | 0 | 9 (22) |
| Response, no. (%) | ||
| CR | 11 (28) | 25 (61) |
| PR/HI | 9 (22) | 0 |
| Overall | 20 (50) | 25 (61) |
All FLT3-ITD+ patients had normal cytogenetics.
Accuracy of the optimized quantitative PCR assay in a phase 2 study of T + E study in elderly AML. (A) ROC analysis using complete remission (CR) only as response. AUC indicates area under the ROC curve. (B) A 2 × 2 contingency table using the optimal cutoff and 2-gene assay performance characteristics. NR indicates no response; PPV, positive predictive value; NPV, negative predictive value; ORR, overall response rate; Spec, specificity; and Sens, sensitivity. (C) Kaplan-Meier analysis of patients stratified using an optimal ratio cut-off of 5.2. HR indicates hazard ratio.
Accuracy of the optimized quantitative PCR assay in a phase 2 study of T + E study in elderly AML. (A) ROC analysis using complete remission (CR) only as response. AUC indicates area under the ROC curve. (B) A 2 × 2 contingency table using the optimal cutoff and 2-gene assay performance characteristics. NR indicates no response; PPV, positive predictive value; NPV, negative predictive value; ORR, overall response rate; Spec, specificity; and Sens, sensitivity. (C) Kaplan-Meier analysis of patients stratified using an optimal ratio cut-off of 5.2. HR indicates hazard ratio.
To determine whether the 2-gene assay was specific for T responsiveness or alternatively reflected broad sensitivity to mechanistically diverse chemotherapy agents, we examined marrow blasts from 41 adults with newly diagnosed AML undergoing intensive multiagent induction chemotherapy, including Ara-C, anthracyclines, and E. In contrast to the findings in T-treated patients, there was no demonstrable association between the 2-gene ratio and clinical outcome for the total non–T-treated cohort or for either non-T treatment subset (Figure 5). The ROC area under the curve was determined to be 0.5, which demonstrates no significant value in predicting clinical response to non–T chemotherapeutic regimens. Furthermore, when subjects were classified as either high or low ratios based on the median of 2-gene ratio (0.959), 25th quantile (0.561), 75th quantile (1.248), there was no demonstrated benefit in OS. After survival, analyses were performed: (1) all patients, (2) AcDVP16 only, and (3) FLAM only. The results in Figure 5 showed that there were no significant differences in OS (days) between the 2 groups stratified by median ratio. This was also true when using cutpoints at the 25th and 75th quantiles (data not shown).
OS of 41 AML patients treated with intensive induction chemotherapy with Ara-C, anthracycline, and a third agent (flavopiridol or etoposide): stratification by high versus low 2-gene ratio. Median ratio = 0.959. HR indicates hazard ratio.
OS of 41 AML patients treated with intensive induction chemotherapy with Ara-C, anthracycline, and a third agent (flavopiridol or etoposide): stratification by high versus low 2-gene ratio. Median ratio = 0.959. HR indicates hazard ratio.
Discussion
Our randomized phase 2 trial confirms the activity and tolerability of T + E for elderly adults with newly diagnosed AML that was initially detected in the phase 1 trial.13 For this trial, we selected 2 dose schedules that yielded 50% CR rates in the phase 1 study without evidence for dose-limiting toxicities. Expansion and up-front comparison of the 2 T + E schedules demonstrated similar efficacy but greater toxicity in arm B, allowing us to pursue arm A at a relatively early point in the trial, after 42 patients had been randomized. It is somewhat disappointing that the CR rate in the phase 2 expansion was only 26% and the overall response rate was only 37%. Nonetheless, the ability of T-containing regimens to induce durable CRs in older adults with multiple unfavorable prognostic features compares favorably with other nonintensive approaches in elderly adults including low-dose Ara-C,32 clofarabine,33,34 the demethylating agents 5-azacytidine35 and decitabine,36,37 and lenalidomide.38 Moreover, as in other trials of T either alone19,20 or in combination with E,13 the achievement of CR is associated with a significant duration of OS.
Previous observations in AML marrow blasts obtained from elderly adults undergoing antileukemia therapy with single-agent T have defined a ratio of expression of 2 genes, RASGRP1 and APTX, that can predict for clinical response.23 Retrospective analysis of bone marrow aspirates collected in the context of the current T + E trial have validated the 2-gene signature as a reproducible predictor of response to T and suggest that it may be possible to prospectively discriminate those patients with AML who are likely to respond to T versus those who are not. Importantly, our data substantiate the notion that the signature is relatively specific for T because there appeared to be no predictive relationship between the RASGRP1/APTX mRNA ratio and 2 intensive investigational chemotherapy regimens, each of which included Ara-C and an anthracycline with a third agent (flavopiridol or etoposide). Although the positive predictive value of the test in the context of the T + E phase 2 trial was 78%, the NPV of the test was slightly lower (87%) than the NPV reported for T monotherapy studies, which have been in the range of approximately 95%.23 This apparent discrepancy in NPV between single and combination therapies might relate in part to response to E in addition to or instead of T.
The genetic studies lend credence to the initial rationale for development of FTIs, which centered around the requisite posttranslational farnesylation of Ras proteins for activation of Ras-driven signal transduction.9,12,14,15,19 It was originally reasoned that FTIs would have a particular role in malignancies where RAS mutations confer constitutive activation of Ras-mediated signaling. Ironically, studies to date have failed to demonstrate a clear relationship between the presence or absence of Ras mutations and clinical outcome.11,18,20 However, Ras activation can be driven by numerous gene-independent mechanisms.9-16 In particular, previous studies have shown that RASGRP1 serves as a guanine nucleotide exchange factor that activates Ras isoforms, including H-Ras and N-Ras, in model systems.39 Our data support the notion that net Ras activity, driven at least in some instances by high RASGRP1 expression, remains a pivotal factor in the overall mechanism of action and antileukemic activity of T. These results are also consistent with recent experiments showing that down-regulation of RASGRP1 is associated with diminished sensitivity in leukemia cells.39 Moreover, T exhibits substantial single-agent activity in T lineage lymphomas, which (like normal T lymphocytes) express high levels of RASGRP1.40
The contribution of relatively low APTX expression to overall T responsiveness may relate to a decreased ability of T-exposed cells to undergo DNA excision repair. Indeed, in our phase 1 trial of T + E,13 we demonstrated that T induced DNA damage (measured by histone H2AX phosphorylation) and apoptosis (measured by subdiploid DNA) in marrow blasts obtained on day 8 of therapy. DNA damage and apoptosis appeared to be linked in CR patients but not in no response patients, raising the possibility that nonresponsiveness to T could relate in part to APTX-driven repair of T-associated DNA damage. Thus, at least in theory, APTX down-regulation may confer a decreased ability to repair T-associated DNA damage that, in turn, might facilitate AML cell apoptosis.
The ability to reliably predict response to a particular antileukemic drug would be a major step toward the development of clinically applicable drug sensitivity testing for patients diagnosed with AML. Accurate discrimination of T responders from T-nonresponders would allow us to direct T-based strategies to the subset of patients whose cells have a “responsive” 2-gene ratio rather than to the heterogeneous group of elderly patients with newly diagnosed AML, the majority of whom will not respond to T-based therapy. The ability to identify T-responsive patients for T-based therapies may be particularly germane because those patients who do achieve CR or even PR with T-based therapies enjoy significant durations of both DFS and OS.13,18-20 In this regard, our next clinical trials will evaluate the feasibility of conducting 2-gene signature assays in real time to prospectively identify and select elderly AML patients who are likely to derive significant clinical benefit from T-based therapies.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Tim Jatkoe (OCD, Johnson and Johnson) for providing support with statistical analysis of the 2-gene assay outcomes, the Johns Hopkins Sidney Kimmel Cancer Center nursing staff for superb medical care, and the patients and their families, without whose partnership we could never have conducted the trial and from whom we have learned critical information that will help us to improve the treatment of these diseases.
This work was supported in part by the National Cancer Institute (cooperative agreement U01 CA70095, J.E.K.), National Cancer Institute Cancer Center (support grant 2P30 CA06973-46), National Center for Research Resources (grant UL1 RR025005), Leukemia Lymphoma Society Translational Research Program (White Plains, NY; grant 6047-08, S.H.K. and J.E.K.), and philanthropic funds from Dr Robert E. Fischell in memory of his late wife Marian (J.E.K.).
National Institutes of Health
Authorship
Contribution: J.E.K., T.I.V., M.R., and E.G.-M. designed and performed research, analyzed data, and wrote the paper; E.K.R., B.D.S., S.D.G., S.M., S.H.K., and J.J.W. participated in protocol design, helped to analyze data, and performed critical review of the manuscript; J.M.G. served as the multicenter coordinator, performed research, collected data, and assisted with data analysis; V.I. and S.G. performed research, obtained samples, and coordinated sample disbursement for laboratory studies; L.E.M., E.J.F., H.E.C., M.J.L., G.R.R., M.A.M, and C.D.G. performed research and edited the paper; and C.D., J.P., and Y.W. participated in assay development and analysis and edited the paper.
Conflict-of-interest disclosure: T.I.V., C.D., J.P., and Y.W are employees of Ortho Clinical Diagnostics. M.R. is a former employee of Centocor and is currently employed at Clovis Oncology. T.I.V., M.R., C.D., J.P., and Y.W. are patent holders for the 2-gene signature. The remaining authors declare no competing financial interests.
The current affiliation for M.R. is Clovis Oncology, San Francisco, CA.
Correspondence: Judith E. Karp, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, 1650 Orleans St, CRB 1, Rm 2M44, Baltimore, MD 21231-1000; e-mail: jkarp2@jhmi.edu.
References
Author notes
J.E.K. and T.I.V. contributed equally to this study.