In this issue of Blood, Gandhi et al report novel findings on the involvement of von Willebrand factor (VWF) in atherogenesis/atherosclerosis in mice.1
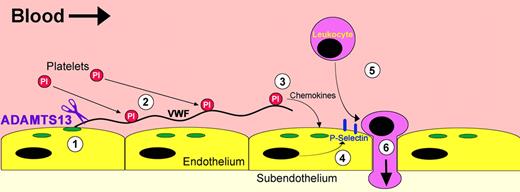
Proinflammatory VWF strings. Hypothetical mechanism through which ADAMTS13 deficiency promotes leukocyte adhesion and extravasation. (1) Endothelial cells at atherosclerosis-prone sites become activated and induce the release of UL-VWF from Weibel-Palade bodies (green). (2) A proportion of the UL-VWF that is secreted remains attached to the endothelium, where it unravels and tethers platelets. (3) Retention of platelets over the endothelial surface promotes the release of a variety of different chemokines that (4) act on the endothelium, thereby inducing the expression of adhesion molecules like P-selectin (blue). (5) P-selectin promotes rolling and adhesion of circulating leukocytes, which in turn can (6) extravasate into the subendothelial layers. In the presence of ADAMTS13 (purple), VWF strings are rapidly proteolysed from the endothelial surface to prevent this proinflammatory sequence.
Proinflammatory VWF strings. Hypothetical mechanism through which ADAMTS13 deficiency promotes leukocyte adhesion and extravasation. (1) Endothelial cells at atherosclerosis-prone sites become activated and induce the release of UL-VWF from Weibel-Palade bodies (green). (2) A proportion of the UL-VWF that is secreted remains attached to the endothelium, where it unravels and tethers platelets. (3) Retention of platelets over the endothelial surface promotes the release of a variety of different chemokines that (4) act on the endothelium, thereby inducing the expression of adhesion molecules like P-selectin (blue). (5) P-selectin promotes rolling and adhesion of circulating leukocytes, which in turn can (6) extravasate into the subendothelial layers. In the presence of ADAMTS13 (purple), VWF strings are rapidly proteolysed from the endothelial surface to prevent this proinflammatory sequence.
Whereas the role of VWF in hemostasis is comparatively well understood,2 the contribution of VWF to atherosclerosis has been debated. VWF is expressed by endothelial cells and megakaryocytes, where it multimerises into hyperreactive ultra-large (UL)–VWF. Both endothelial cells and platelets release UL-VWF on activation. In free circulation, VWF adopts a globular conformation that does not normally interact with platelets. However, the collagen-binding site within its A3 domain is constitutively exposed enabling globular VWF to bind extravascular collagen at sites of vessel damage. Once bound to collagen, VWF multimers unfold because of increased tensile forces exerted by the flowing blood on the VWF molecule. This induces the exposure of VWF A1 domain platelet-binding sites, which enables the specific recruitment of platelets to the damaged vasculature. VWF platelet-tethering function is regulated by the plasma metalloprotease, ADAMTS13.3 This enzyme only cleaves VWF in its unravelled conformation as the exosites and scissile bond that ADAMTS13 recognizes are normally buried within the central A2 domain of globular VWF.4 In this way, ADAMTS13 modulates the hemostatic function of VWF in plasma as well as the growth of the platelet plug at sites of vessel injury.
The study by Gandhi et al, however, explores the physiologic consequences of VWF proteolytic regulation by ADAMTS13 in a different location.1 On secretion, a proportion of UL-VWF released from endothelial cells remains tethered to the cell surface, resulting in the formation of unraveled tethered VWF strings capable of binding platelets and recruiting leukocytes to the endothelium. In the presence of ADAMTS13, however, these VWF strings rapidly disappear, as they are proteolytically released into the circulation.5
Gandhi et al compared the development of the atherosclerotic lesions in ApoE−/− mice with those in Adamts13−/−/ApoE−/− double-deficient mice. The major observations from this study included a large increase in adherent leukocytes to the endothelium of the carotid sinus of Adamts13−/−/ApoE−/− double-deficient mice with an accompanied increase in the rate of plaque development that contained elevated numbers of macrophages.1 That these differences were observed as a result of ADAMTS13 deficiency strongly suggests that these effects are VWF-dependent, as ADAMTS13 exhibits remarkable specificity for VWF alone.
How, then, to explain these findings? The authors propose that ADAMTS13 deficiency extends the lifetime of UL-VWF strings on activated endothelial cell surfaces that are most prone to atherosclerosis. This, in turn, facilitates recruitment of both platelets and leukocytes to these regions. The question still remains, though, as to how VWF strings promote the capture of circulating leukocytes. A previous study by Chauhan et al revealed that ADAMTS13 deficiency in mice enhanced P-selectin–dependent leukocyte adhesion to both activated and nonactivated endothelium.6 Although another study reported the ability of leukocytes to roll on platelets bound to endothelial attached UL-VWF strings in vitro,7 it seems more likely (based on the intravital microscopy presented by Gandhi et al) that the leukocytes are adhering directly to the endothelium, rather than to the platelet-decorated strings themselves. It would certainly be of interest to examine leukocyte adhesion in Adamts13−/−/ApoE−/− double-deficient mice both immediately before and after provision of recombinant ADAMTS13 to explore whether VWF proteolysis by its cleaving protease can reduce endothelial surface bound leukocytes. This would represent one way of formally testing whether leukocytes were binding directly to platelet–UL-VWF strings in vivo.
An alternative hypothesis to explain elevated recruitment of leukocytes may be that the binding of platelets to UL-VWF strings over the surface of the endothelium serves to further activate the endothelium and induce the expression of cell surface adhesion molecules to which circulating leukocytes bind. This may be a more favorable possibility given the elevated numbers of macrophages in the lesions of Adamts13−/−/ApoE−/− double-deficient mice (compared with ApoE−/− littermates), suggesting that the bound leukocytes are interacting with and transmigrating across the endothelium. Thus, in the absence of ADAMTS13, endothelial-bound UL-VWF strings mediate platelet recruitment to the arterial surface, which as a result deliver certain platelet chemokines and growth factors that provide an additional proinflammatory stimulus to the arterial wall (see figure). Testing such a hypothesis will, however, require careful experimental design.
A further question that arises from the studies of Gandhi et al is the dependency of ADAMTS13 concentration on the proinflammatory effects of UL-VWF strings. The authors compared Adamts13+/+ with Adamts13−/− mice (and not Adamts13+/− mice) on the ApoE−/− background. Consequently, it remains unclear whether there is a linear relationship between ADAMTS13 concentration and VWF string-dependent leukocyte adhesion, or whether there is a threshold effect in which even very low ADAMTS13 concentrations can protect against the observed recruitment of leukocytes. In ADAMTS13-deficient mice, platelet-decorated UL-VWF strings can be visualized over the activated endothelium by intravital microscopy, whereas such strings seem to appear only transiently in Adamts13+/− or Adamts13+/+ mice. A recent study by de Maeyer et al reported platelet–UL-VWF string survival time over the activated endothelium of ∼ 5 seconds in Adamts13+/+, whereas this was ∼ 13 seconds in Adamts13−/− mice.8 These results might suggest that even a small increase in lifetime of a platelet–UL-VWF string could potentially translate into a proinflammatory stimulus. Whether changes of platelet–UL-VWF string lifetime occur as a function of ADAMTS13 plasma concentration across the normal range in humans will be key to determining whether the proinflammatory effects of VWF strings are pertinent to the pathogenesis of human vascular diseases.
Although there is increasing evidence that ADAMTS13 plasma concentration is inversely correlated with the risk of both myocardial infarction and stroke,9 these data really measure the risk of an occlusive coronary or cerebral thrombotic event rather than the development of the vascular disease that might precipitate such clinical sequelae. Is there any clinical evidence, therefore, to support the involvement of VWF strings in atherogenesis or vascular disease? In a study by Srámek et al examining atherosclerotic plaque development in normal individuals and in patients with type 3 von Willebrand disease, the authors reported no difference in the plaque score between these groups, suggesting that plaque development occurs independently of VWF strings in humans.10 However, it must be considered that human atherosclerosis is a far more complex and multifactorial disease than the monogenic ApoE−/− mouse model of atherosclerosis, which makes isolating the contribution of VWF strings to the progression of this disease in humans very difficult. Consequently, further studies will be necessary to better define any involvement of endothelial UL-VWF strings in human disease. This work by Gandhi et al provides an excellent setting to understand how modulation of VWF function by ADAMTS13 over the endothelial surface may influence the proinflammatory conditions in different vascular diseases.1 This will also provide further clues as to how ADAMTS13 may impact on the vasculature in a manner distinct from its comparatively well-described role in modulating primary hemostasis.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal