Abstract
The endothelial protein C receptor (EPCR) limits thrombus formation by enhancing activation of the protein C anticoagulant pathway, and therefore may play a role in the etiology of thrombotic disorders. The rs867186 single-nucleotide polymorphism in the PROCR gene (g.6936A > G, c.4600A > G), resulting in a serine-to-glycine substitution at codon 219, has been associated with reduced activation of the protein C pathway, although its association with thrombosis risk remains unclear. The present study is a highly comprehensive systematic review and meta-analysis, including unpublished genome-wide association study results, conducted to evaluate the evidence for an association between rs867186 and 2 common thrombotic outcomes, venous thromboembolism (VTE) and myocardial infarction (MI), which are hypothesized to share some etiologic pathways. MEDLINE, EMBASE, and HuGE Navigator were searched through July 2011 to identify relevant epidemiologic studies, and data were summarized using random-effects meta-analysis. Twelve candidate genes and 13 genome-wide association studies were analyzed (11 VTE and 14 MI, including 37 415 cases and 84 406 noncases). Under the additive genetic model, the odds of VTE increased by a factor of 1.22 (95% confidence interval, 1.11-1.33, P < .001) for every additional copy of the G allele. No evidence for association with MI was observed.
Introduction
Protein C (PC) is a major component of the coagulation/fibrinolysis cascade. Circulating in plasma as an inactive zymogen, PC is activated at the endothelial surface by the membrane-bound thrombin-thrombomodulin complex.1 When activated PC (APC) is bound to its cofactor, protein S, it inactivates the procoagulant factors FVa and FVIIIa, limiting the coagulation cascade and fibrin formation.1,2 PC activation is enhanced approximately 20-fold when PC binds to the endothelial PC receptor (EPCR),3 a type I transmembrane protein. EPCR is primarily localized on the endothelial cells of large blood vessels (ie, the arteries and veins) and is very sparse or absent in the microvascular endothelium of most tissues.4 EPCR-bound APC triggers protease-activated receptor-1 (PAR-1) cleavage, resulting in anti-inflammatory and cytoprotective (eg, antiapoptotic) effects.2,5 In addition to its APC-mediated effects, EPCR also works to limit thrombus formation by binding procoagulant FVII/FVIIa, facilitating the clearance of FVIIa and limiting downstream activation of the tissue factor (extrinsic) coagulation pathway.6,7 These findings strongly favor an important role for EPCR in thrombosis and inflammation.1
A soluble form of EPCR (sEPCR) also circulates in the plasma. sEPCR binds PC/APC with the same affinity as membrane-bound EPCR, but does not enhance PC activation by the thrombin-thrombomodulin complex.8 Furthermore, sEPCR-bound APC is incapable of inactivating FVa8 and may also impede PAR-1 cleavage.2 By limiting APC generation and function, elevated levels of sEPCR may exert procoagulant and proinflammatory effects; in 2 case-control studies,9,10 elevated levels of sEPCR were associated with increased risk of VTE. Likewise, a small family study found a higher occurrence of VTE in those with above-normal values of sEPCR compared with those with normal levels.11
The PROCR gene is located on chromosome 20q11.2, spans 6 kilobases, and possesses 4 exons.12 The mature protein comprises 221 amino acids, including an extracellular domain, a 25-amino acid transmembrane domain, and a 3–amino acid intracytoplasmic sequence. Animal experiments have demonstrated the importance of PROCR in normal embryonic development; in PROCR knock-out mice, fibrin deposition in trophoblast giant cells results in thrombosis at the maternal-embryonic interface.13 Death occurs by embryonic day 10.5.
Gene variants and frequency
Mutations in the PROCR gene that influence protein expression, function, and/or the concentration of sEPCR may be functionally relevant. Rare point mutations in the gene14 and its promoter region15 have been described, but effects on thrombosis and gene expression remain unknown.16
The rs867186 diallelic single nucleotide polymorphism in the PROCR gene (g.6936A>G, c.4600A>G), resulting in a serine-to-glycine substitution at codon 219 in the membrane-spanning domain of EPCR, explains between 56% and 87% of the variations in sEPCR levels.10,17-19 The G allele tags the A3 haplotype (4 PROCR haplotypes have been identified in whites) and is associated with increased shedding of EPCR from the endothelial membrane, both by rendering the receptor more sensitive to cleavage20 and by leading to a truncated mRNA through alternative splicing.21 The overall frequency of the G allele is 0.074 among individuals included to date in the 1000 Genomes Project22 ; however, there are large variations across the population (eg, 0.53 among Papuan New Guineans and 0.0 among South-American Amerindians from the Human Genome Diversity Cell Line Panel).23 In a genome-wide association study (GWAS) of more than 23 000 cohort participants of European ancestry, the G-allele frequency was 0.101.24
Disease
Venous thromboembolism (VTE) results from an obstruction of blood in the venous system25 by a RBC-rich thrombus composed of platelets and fibrin at sites with low blood flow and shear rate and where the vein wall is normal.26 In contrast, arterial thrombosis (ischemic stroke and coronary artery disease) results from platelet-rich thrombi induced by the rupture of an atherosclerotic plaque at arterial sites where shear rates are high. Although arterial and venous thromboses have traditionally been viewed as distinct, recent studies suggest that the 2 conditions share common etiologic pathways. Evidence in support of this is 3-fold (for review, see Prandoni27 or Lowe26 ): (1) several observational studies have demonstrated increased risk of subsequent arterial vascular disease among patients with VTE; (2) drugs affecting coagulation and hemostasis have some effect in preventing and treating various atherosclerotic disorders; and (3) VTE and myocardial infarction (MI) share common risk factors (albeit of different magnitudes), including genetic determinants such as the Factor V Leiden (F5L) and prothrombin G20210A gene mutations28,29 and variants in the ABO gene,30,31 as well as age, obesity, hypertension, diabetes mellitus, smoking, and hypercholesterolemia.32 All of these risk factors increase susceptibility to thrombus formation by modifying the coagulation and fibrinolytic systems.33
The PROCR rs867186 variant has emerged as a candidate risk factor for both arterial and venous thrombotic disease because of the involvement of EPCR in APC- and FVII/FVIIa-mediated clotting and inflammation. However, evidence for an association between the PROCR rs867186 variant and arterial and venous thrombosis has been conflicting. We therefore undertook a systematic review and meta-analysis of observational studies to evaluate the evidence for an association between the PROCR rs867186 variant and 2 common thrombotic outcomes, VTE and MI, which are hypothesized to share etiologic pathways. Although ischemic stroke is also a common thrombotic outcome, we chose not to include it in our review because the outcome was highly variable (ie, ischemic and hemorrhagic stroke cases were often indistinguishable) and because the number of studies of stroke was low.
Methods
Search strategy
MEDLINE, EMBASE, and the HuGE Navigator Genopedia database were searched through July 2011 using a combination of keywords, including “thromboembolism,” “thrombosis,” “pulmonary embolism,” “myocardial infarction,” “EPCR,” and “PROCR” (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Titles and abstracts of identified articles were screened by 2 reviewers, and those that discussed any PROCR variant in the context of VTE or MI proceeded to a full-text screening. The full text of articles was screened by 2 reviewers and case-control, cohort, or cross-sectional studies examining an association between the PROCR rs867186 polymorphism and VTE or MI were selected for inclusion, with disagreements resolved by consensus. Reference lists of included studies and review articles were searched for additional articles. Genetic linkage studies, review articles, animal studies, and conference proceedings were excluded.
GWAS of VTE or MI in which the PROCR rs867186 variant, or variants in close linkage disequilibrium with rs867186, might potentially have been included on the genotyping platform were located using keyword searches of the HuGE Navigator GWAS Integrator database. Unpublished GWAS were identified by keyword searches of the National Center for Biotechnology Information database of genotypes and phenotypes (dbGaP). Authors of relevant GWAS were contacted to request counts of events and nonevents by PROCR rs867186 genotype (ultimately, all identified GWAS had genotyped this single-nucleotide polymorphism).
Information on study design, location, demographics, ascertainment of subjects, case definition, and genotype and allele frequencies was extracted from included studies by 2 independent reviewers using a standardized data abstraction form. When this information was not available from the article, it was sought from other publications reporting on the same study population, if available. Crude odds ratios (ORs) and their 95% confidence intervals (95% CIs) were calculated from genotype frequencies presented in the published article; when separate genotype counts were not presented, they were requested from the study authors.
Meta-analysis
Inverse variance-weighted random effects meta-analysis was used to estimate the summary effect and 95% CI for VTE, MI, and VTE and MI combined, using Stata Version 11 software. The G allele was considered the at-risk allele, and the per-allele effect estimate was calculated as the OR per unit score (0, 1, or 2 copies of the G allele) using logistic regression. The per-allele OR is the risk of disease per one-allele increase, and the P value of the OR tests the hypothesis of zero slope for a line that best fits the 3 genotypic risk estimates.34 The per-allele model is powerful for detecting additive genetic effects34 and the additive model is in accordance with the observed variation in sEPCR values according to the number of copies of the G allele.18,19,35 In secondary analyses, 4 additional genotype contrasts were tested for each outcome: AG versus AA, GG versus AA, GG + AG versus AA (dominant model), and GG versus AG + AA (recessive model). The intensity and significance of between-study heterogeneity were assessed with, respectively, the I2 statistic with its 95% uncertainty interval, and the Cochran Q statistic. I2 values of 25%, 50%, and 75% indicate low, medium, and high between-study heterogeneity, respectively, and P > .05 for the Cochran Q statistic suggests no statistically significant heterogeneity.36 Publication bias was assessed graphically using funnel plots and the Egger test quantified the asymmetry of the plot. The latter tests the null hypothesis that small studies give the same results as large studies37 ; P < .05 was deemed statistically significant.
Four subgroup analyses were subsequently carried out to explore possible explanations for heterogeneity. In the first, GWAS were excluded from the meta-analysis; in the second, candidate gene studies were excluded from the meta-analysis; in the third, analyses were restricted to studies of white populations; and in the fourth, studies with deviation from Hardy-Weinberg equilibrium (HWE), a potential indicator of poor genotyping quality or ascertainment issues, were removed. HWE was investigated in controls of case-control studies and in the entire samples of cohort studies using the standard χ2 goodness of fit test as well as the relative excess heterozygosity (REH).38 The χ2 goodness-of-fit statistic tests the null hypothesis that the data are consistent with HWE. In contrast, the REH approach quantifies Hardy-Weinberg disequilibrium by measuring the ratio of the actual proportion of heterozygotes to the proportion of heterozygotes expected in a population, which conforms to HWE.38 A significant deviation of the ratio from unity, as measured by the 2-sided 95% CI, is evidence of Hardy-Weinberg disequilibrium. Among studies of MI, a fifth subgroup analysis was carried out in which analysis was restricted to studies that had used a history of MI as the case definition.
The Venice criteria for assessing the strength of cumulative evidence in genetic association studies were used.39 Briefly, this semiquantitative index classifies the credibility of cumulative epidemiologic evidence into 3 categories, “weak,” “moderate,” and “strong,” taking into consideration criteria such as amount of evidence (eg, sample size, power, and false-discovery rate), replication (eg, attention to phenotype definition, models, and I2), and protection from bias (eg, population stratification and measurement errors). This review was prepared following the guidance of the HuGE Review Handbook Version 1.0.40
Results
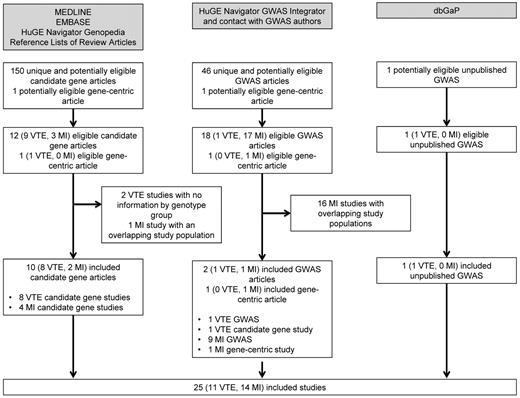
The search of MEDLINE, EMBASE, and HuGE Navigator Genopedia returned 150 unique candidate gene articles, of which 12 (9 VTE and 3 MI) were eligible for inclusion (Figure 1). The study populations overlapped in 3 studies of VTE,9,17,41 so de-duplicated counts were obtained from one of the authors and included. The population in one study of MI19 overlapped with that in the gene-centric meta-analysis42 included in the systematic review (see next paragraph), so this article was excluded from further analysis. A second candidate gene article,43 as well as a gene-centric study of VTE identified from the reference list of a review paper,44 was excluded because genotype counts were not available separately for each of the 3 genotype groups.
Articles and studies identified from a systematic search of the literature on the PROCR rs867186 polymorphism and venous and arterial thrombosis.
Articles and studies identified from a systematic search of the literature on the PROCR rs867186 polymorphism and venous and arterial thrombosis.
The search of HuGE Navigator GWAS Integrator returned 46 GWAS, of which 18 (1 VTE and 17 MI) were eligible for inclusion (Figure 1). Contact with GWAS authors resulted in the identification of an additional gene-centric (> 2000 genes) study not yet published when our search was executed.42 This study, as well as the most recent GWAS of MI,31 were meta-analyses that included 21 studies. These 2 studies encompassed almost all of the previously published GWAS results; therefore, no other GWAS of MI beyond these 2 meta-analyses were included in this systematic review. Study-specific genotype counts were obtained from the GWAS meta-analysis31 for use in the present meta-analysis. In the gene-centric meta-analysis,42 because the per-allele OR was presented but genotype counts were not, the per-allele OR was used in the present meta-analysis. One additional unpublished GWAS of VTE was identified from dbGaP (J.A.H. and M.d.A., unpublished data, March 21, 2011; referred to hereafter as “Heit 2011”) for a total of 2 included GWAS of VTE. The published GWAS of VTE30 also included a candidate gene study that had not been identified in previous searches.
Overall, the systematic review included 25 studies (11 VTE and 14 MI) for a total of 37 415 cases (4821 with VTE and 32 594 with MI) and 84 406 noncases (6070 VTE noncases and 78 336 MI noncases).
Characteristics of studies included in the meta-analysis are presented in supplemental Table 2. All 11 VTE studies used a case-control design, of which 29,41 were family-based (controls were first-degree relatives of cases), one45 was nested within a cohort study, and 2 (Tregouet et al30 and Heit 2011), were GWAS designs. Six studies9,17,30,35,41 included white subjects only, 3 studies (Yamagishi et al,45 Pecheniuk et al,46 and Heit 2011) included white and nonwhite subjects, one study47 included Chinese subjects, and one study10 did not report the race/ethnicity of subjects. Cases and controls were matched by age and gender in 6 studies (Uitte de Willige et al,10 Saposnik et al,35 Yamagishi et al,45 Pecheniuk et al,46 Chen et al,47 and Heit 2011). In all but 2 non-matched studies, age and gender distributions were similar in cases and controls.30 Two studies were of first-event VTE patients,10,30 all cases of VTE were objectively diagnosed, and the case definition included deep vein thrombosis and pulmonary embolism, except in 2 studies10,47 that were limited to deep vein thrombosis only. Cases were recruited from thrombosis clinics in 8 studies, from a venous thrombosis registry in one study,46 and from a community-based cohort in another study.45 Controls were selected from hospital personnel,17 general medical examination clinics (Tregouet et al,30 Saposnik et al,35 and Heit 2011), partners and acquaintances of cases,10 first-degree relatives of cases,9,41 the community,30,46 the underlying cohort,45 or from a clinical trial of antioxidant supplementation.30 In one study,47 the source of cases and controls was not stated.
The 14 MI studies included 1 cohort study, 3 case-control studies, 9 GWAS case-control studies, and 1 case-control gene-centric meta-analysis (supplemental Table 2). Twelve studies included white subjects only; the remaining 2 studies presented results for white subjects and South-Asian subjects separately.18,42 This stratification by race/ethnicity was maintained in the present meta-analysis for a total of 16 MI studies analyzed. Cases and controls were matched by age and gender in 3 studies,18,31,48 whereas the age and/or gender distribution of cases and controls was either not reported or differed considerably in all but 142 of the other studies. One study was of first-event MI patients18 and the case definition was a history of MI in 6 studies, whereas a composite case definition including coronary intervention procedures, angina, > 50% stenosis of coronary vessels, and MI was used in the other 11 studies. Cases and noncases were population, hospital, or clinic based.
Genotype and minor allele frequency distributions were similar between studies (Table 1), with the exception of the studies of South Asians. Whereas the frequency of the G allele among controls or in the underlying cohort was 13% or less in white populations, the frequency was 19%-21% among South Asian controls. There was evidence of deviation from HWE in 3 studies (2 of VTE17,45 and one of MI48 ), with both methods of HWE assessment yielding similar conclusions.
Distribution of PROCR rs867186 genotypes among cases and non-cases in studies of VTE and MI
| Included study* . | AA genotype . | AG genotype . | GG genotype . | MAF† . | Deviation from Hardy-Weinberg equilibrium . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases . | Non-cases . | Cases . | Non-cases . | Cases . | Non-cases . | ||||||||||
| n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | χ2P value . | REH (95% CI)‡ . | ||
| VTE | |||||||||||||||
| Medina 200417 | 291 | 82 | 327 | 82 | 62 | 17 | 74 | 18 | 2 | 1 | 0 | 0 | 0.09 | .04 | |
| Saposnik 200435 | 249 | 74 | 278 | 82 | 85 | 25 | 58 | 17 | 4 | 1 | 2 | 1 | 0.09 | .58 | 1.23 (0.59-2.58) |
| Uitte de Willige 200410 | 345 | 73 | 361 | 77 | 116 | 25 | 100 | 21 | 10 | 2 | 10 | 2 | 0.13 | .33 | 0.83 (0.58-1.21) |
| Medina 200541 | 77 | 81 | 145 | 80 | 17 | 18 | 35 | 19 | 1 | 1 | 1 | 1 | 0.10 | .47 | 1.45 (0.52-4.10) |
| Navarro 20089 | 58 | 69 | 128 | 86 | 24 | 29 | 21 | 14 | 2 | 2 | 0 | 0 | 0.07 | .35 | |
| Pecheniuk 200846 ) | 82 | 72 | 87 | 76 | 27 | 24 | 24 | 21 | 5 | 4 | 3 | 3 | 0.13 | .40 | 0.74 (0.37-1.50) |
| Trégouët 2009–GWAS30 | 309 | 75 | 1003 | 82 | 92 | 22 | 216 | 18 | 9 | 2 | 8 | 1 | 0.09 | .32 | 1.21 (0.83-1.75) |
| Trégouët 2009–MARTHA30 | 885 | 79 | 654 | 82 | 222 | 20 | 141 | 18 | 16 | 1 | 5 | 1 | 0.09 | .38 | 1.23 (0.77-1.97) |
| Yamagishi 200945 | 417 | 84 | 844 | 83 | 72 | 15 | 158 | 16 | 7 | 1 | 14 | 1 | 0.09 | .04 | 0.73 (0.53-0.99) |
| Chen 201147 | 49 | 75 | 63 | 89 | 15 | 23 | 7 | 10 | 1 | 2 | 1 | 1 | 0.06 | .15 | 0.44 (0.13-1.52) |
| Heit 2011 | 978 | 77 | 1029 | 79 | 264 | 21 | 257 | 20 | 28 | 2 | 16 | 1 | 0.11 | .99 | 1.00 (0.76-1.32) |
| MI | |||||||||||||||
| Ireland 2005–EDSC-EW18 | 33 | 65 | 231 | 85 | 17 | 33 | 37 | 14 | 1 | 2 | 4 | 1 | 0.08 | .09 | 0.61 (0.34-1.10) |
| Ireland 2005–EDSC-IA18 | 63 | 55 | 222 | 64 | 48 | 42 | 108 | 31 | 3 | 3 | 19 | 5 | 0.21 | .23 | 0.83 (0.62-1.12) |
| Ireland 2005–HIFMECH18 | 440 | 84 | 455 | 81 | 83 | 16 | 101 | 18 | 3 | 1 | 7 | 1 | 0.10 | .60 | 0.89 (0.59-1.36) |
| Ireland 2005–NPHSII18 | 166 | 85 | 1883 | 82 | 26 | 13 | 387 | 17 | 4 | 2 | 14 | 1 | 0.09 | .57 | 1.08 (0.84-1.38) |
| Medina 200848 | 606 | 88 | 554 | 79 | 80 | 12 | 142 | 20 | 3 | 0 | 1 | 0 | 0.10 | .01 | 3.02 (1.12-8.16) |
| Schunkert 2011–ADVANCE31 | 221 | 79 | 262 | 84 | 55 | 20 | 49 | 16 | 2 | 1 | 1 | 0 | 0.08 | .41 | 1.51 (0.55-4.20) |
| Schunkert 2011 CADomics31 | 1642 | 79 | 2324 | 79 | 409 | 20 | 589 | 20 | 25 | 1 | 37 | 1 | 0.11 | .96 | 1.00 (0.84-1.20) |
| Schunkert 2011 deCODE CAD31 | 5509 | 83 | 22 711 | 82 | 1078 | 16 | 4640 | 17 | 51 | 1 | 244 | 1 | 0.09 | .68 | 0.99 (0.92-1.06) |
| Schunkert 2011 GerMIFS I31 | 670 | 77 | 1225 | 77 | 184 | 21 | 344 | 22 | 11 | 1 | 23 | 1 | 0.12 | .84 | 1.02 (0.81-1.29) |
| Schunkert 2011 GerMIFS II31 | 981 | 80 | 1007 | 78 | 227 | 19 | 265 | 21 | 14 | 1 | 15 | 1 | 0.11 | .60 | 1.08 (0.81-1.43) |
| Schunkert 2011 GerMIFS III31 | 922 | 80 | 1359 | 78 | 222 | 19 | 360 | 21 | 13 | 1 | 29 | 2 | 0.12 | .36 | 0.91 (0.73-1.12) |
| Schunkert 2011 MedStar31 | 369 | 83 | 721 | 82 | 76 | 17 | 148 | 17 | 2 | 0 | 6 | 1 | 0.09 | .59 | 1.13 (0.73-1.73) |
| Schunkert 2011 MIGen31 | 1035 | 81 | 1151 | 82 | 224 | 18 | 240 | 17 | 12 | 1 | 14 | 1 | 0.10 | .71 | 0.95 (0.71-1.27) |
| Schunkert 2011 OHGS131 | 1207 | 82 | 1141 | 81 | 257 | 18 | 263 | 19 | 4 | 0 | 11 | 1 | 0.10 | .33 | 1.17 (0.85-1.62) |
| IBC 50K CAD Consortium (European)42 § | 0.10 | ||||||||||||||
| IBC 50K CAD Consortium (South Asian)42 § | 0.19 | ||||||||||||||
| Included study* . | AA genotype . | AG genotype . | GG genotype . | MAF† . | Deviation from Hardy-Weinberg equilibrium . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases . | Non-cases . | Cases . | Non-cases . | Cases . | Non-cases . | ||||||||||
| n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | χ2P value . | REH (95% CI)‡ . | ||
| VTE | |||||||||||||||
| Medina 200417 | 291 | 82 | 327 | 82 | 62 | 17 | 74 | 18 | 2 | 1 | 0 | 0 | 0.09 | .04 | |
| Saposnik 200435 | 249 | 74 | 278 | 82 | 85 | 25 | 58 | 17 | 4 | 1 | 2 | 1 | 0.09 | .58 | 1.23 (0.59-2.58) |
| Uitte de Willige 200410 | 345 | 73 | 361 | 77 | 116 | 25 | 100 | 21 | 10 | 2 | 10 | 2 | 0.13 | .33 | 0.83 (0.58-1.21) |
| Medina 200541 | 77 | 81 | 145 | 80 | 17 | 18 | 35 | 19 | 1 | 1 | 1 | 1 | 0.10 | .47 | 1.45 (0.52-4.10) |
| Navarro 20089 | 58 | 69 | 128 | 86 | 24 | 29 | 21 | 14 | 2 | 2 | 0 | 0 | 0.07 | .35 | |
| Pecheniuk 200846 ) | 82 | 72 | 87 | 76 | 27 | 24 | 24 | 21 | 5 | 4 | 3 | 3 | 0.13 | .40 | 0.74 (0.37-1.50) |
| Trégouët 2009–GWAS30 | 309 | 75 | 1003 | 82 | 92 | 22 | 216 | 18 | 9 | 2 | 8 | 1 | 0.09 | .32 | 1.21 (0.83-1.75) |
| Trégouët 2009–MARTHA30 | 885 | 79 | 654 | 82 | 222 | 20 | 141 | 18 | 16 | 1 | 5 | 1 | 0.09 | .38 | 1.23 (0.77-1.97) |
| Yamagishi 200945 | 417 | 84 | 844 | 83 | 72 | 15 | 158 | 16 | 7 | 1 | 14 | 1 | 0.09 | .04 | 0.73 (0.53-0.99) |
| Chen 201147 | 49 | 75 | 63 | 89 | 15 | 23 | 7 | 10 | 1 | 2 | 1 | 1 | 0.06 | .15 | 0.44 (0.13-1.52) |
| Heit 2011 | 978 | 77 | 1029 | 79 | 264 | 21 | 257 | 20 | 28 | 2 | 16 | 1 | 0.11 | .99 | 1.00 (0.76-1.32) |
| MI | |||||||||||||||
| Ireland 2005–EDSC-EW18 | 33 | 65 | 231 | 85 | 17 | 33 | 37 | 14 | 1 | 2 | 4 | 1 | 0.08 | .09 | 0.61 (0.34-1.10) |
| Ireland 2005–EDSC-IA18 | 63 | 55 | 222 | 64 | 48 | 42 | 108 | 31 | 3 | 3 | 19 | 5 | 0.21 | .23 | 0.83 (0.62-1.12) |
| Ireland 2005–HIFMECH18 | 440 | 84 | 455 | 81 | 83 | 16 | 101 | 18 | 3 | 1 | 7 | 1 | 0.10 | .60 | 0.89 (0.59-1.36) |
| Ireland 2005–NPHSII18 | 166 | 85 | 1883 | 82 | 26 | 13 | 387 | 17 | 4 | 2 | 14 | 1 | 0.09 | .57 | 1.08 (0.84-1.38) |
| Medina 200848 | 606 | 88 | 554 | 79 | 80 | 12 | 142 | 20 | 3 | 0 | 1 | 0 | 0.10 | .01 | 3.02 (1.12-8.16) |
| Schunkert 2011–ADVANCE31 | 221 | 79 | 262 | 84 | 55 | 20 | 49 | 16 | 2 | 1 | 1 | 0 | 0.08 | .41 | 1.51 (0.55-4.20) |
| Schunkert 2011 CADomics31 | 1642 | 79 | 2324 | 79 | 409 | 20 | 589 | 20 | 25 | 1 | 37 | 1 | 0.11 | .96 | 1.00 (0.84-1.20) |
| Schunkert 2011 deCODE CAD31 | 5509 | 83 | 22 711 | 82 | 1078 | 16 | 4640 | 17 | 51 | 1 | 244 | 1 | 0.09 | .68 | 0.99 (0.92-1.06) |
| Schunkert 2011 GerMIFS I31 | 670 | 77 | 1225 | 77 | 184 | 21 | 344 | 22 | 11 | 1 | 23 | 1 | 0.12 | .84 | 1.02 (0.81-1.29) |
| Schunkert 2011 GerMIFS II31 | 981 | 80 | 1007 | 78 | 227 | 19 | 265 | 21 | 14 | 1 | 15 | 1 | 0.11 | .60 | 1.08 (0.81-1.43) |
| Schunkert 2011 GerMIFS III31 | 922 | 80 | 1359 | 78 | 222 | 19 | 360 | 21 | 13 | 1 | 29 | 2 | 0.12 | .36 | 0.91 (0.73-1.12) |
| Schunkert 2011 MedStar31 | 369 | 83 | 721 | 82 | 76 | 17 | 148 | 17 | 2 | 0 | 6 | 1 | 0.09 | .59 | 1.13 (0.73-1.73) |
| Schunkert 2011 MIGen31 | 1035 | 81 | 1151 | 82 | 224 | 18 | 240 | 17 | 12 | 1 | 14 | 1 | 0.10 | .71 | 0.95 (0.71-1.27) |
| Schunkert 2011 OHGS131 | 1207 | 82 | 1141 | 81 | 257 | 18 | 263 | 19 | 4 | 0 | 11 | 1 | 0.10 | .33 | 1.17 (0.85-1.62) |
| IBC 50K CAD Consortium (European)42 § | 0.10 | ||||||||||||||
| IBC 50K CAD Consortium (South Asian)42 § | 0.19 | ||||||||||||||
Studies are identified by the lead author's last name, year of publication, study name (if a publication included more than 1 study), and reference number.
The minor allele (G) frequency (MAF) was calculated in controls or in the underlying cohort.
The REH cannot be calculated in studies with 0 cases or controls with the GG genotype.
Genotype frequencies were not reported in the published article; however, the study authors state that the single-nucleotide polymorphism did not violate HWE in the controls (P > .0001).
VTE
The meta-analysis supported an association between the PROCR rs867186 variant and VTE (Figure 2 and Table 2), with the odds of VTE increasing by a factor of 1.22 (95% CI, 1.11-1.33) for every additional copy of the G allele (P < .001). A statistically significant association was also observed for the dominant model, the recessive model, and for the GG-versus-AA contrast, whereas the AG-versus-AA contrast approached statistical significance. Between-study heterogeneity was low under the per-allele model (20%, 95% uncertainty interval: 0%-52%), but was moderate for the AG-versus-AA contrast and for the dominant model. Although between-study heterogeneity was low under the recessive model and for the GG-versus-AA contrast, this was likely the result of poor precision due to the small number of GG subjects and should not be interpreted as a lack of true between-study heterogeneity.36 Under the per-allele model, the funnel plot (supplemental Figure 1) and the Egger test (P = .23) revealed no evidence of publication bias. Subgroup analyses yielded results similar to those obtained from analysis of all subjects (Table 3).
Results from a random-effects meta-analysis of the association between the PROCR rs867186 polymorphism and VTE (per-allele model). Individual studies are identified by the lead author's last name, year of publication, study name (if a publication included more than 1 study), and reference number. The sample sizes are represented by the size of the squares. Bars indicate 95% CI.
Results from a random-effects meta-analysis of the association between the PROCR rs867186 polymorphism and VTE (per-allele model). Individual studies are identified by the lead author's last name, year of publication, study name (if a publication included more than 1 study), and reference number. The sample sizes are represented by the size of the squares. Bars indicate 95% CI.
Associations between PROCR rs867186 genotypes and VTE or MI in a random-effects meta-analysis
| Genotype contrast . | Included studies, n . | Total cases, n . | Total non-cases, n . | OR . | 95% CI . | I2 . | 95% Uncertainty interval . | Cochran Q P value . |
|---|---|---|---|---|---|---|---|---|
| VTE | ||||||||
| Per-allele model | 11 | 4821 | 6070 | 1.22 | 1.11-1.33 | 20 | 0-52 | .197 |
| AG versus AA | 11 | 4736 | 6010 | 1.21 | 1.05-1.40 | 43 | 0-72 | .063 |
| GG versus AA | 11 | 3825 | 4979 | 1.81 | 1.29-2.56 | 0 | 0-46 | .694 |
| AG + GG versus AA (dominant) | 11 | 4821 | 6070 | 1.25 | 1.08-1.44 | 47 | 0-74 | .041 |
| GG versus AG + AA (recessive) | 11 | 4821 | 6070 | 1.76 | 1.24-2.48 | 0 | 0-42 | .739 |
| MI | ||||||||
| Per-allele model | 16 | 32 594 | 78 336 | 0.94 | 0.88-1.00 | 65 | 40-79 | < .001 |
| AG versus AA | 14 | 16 850 | 42 919 | 0.96 | 0.86-1.06 | 67 | 43-81 | < .001 |
| GG versus AA | 14 | 14 012 | 35 671 | 0.87 | 0.72-1.06 | 0 | 0-53 | .502 |
| AG + GG versus AA (dominant) | 14 | 16 998 | 43 344 | 0.95 | 0.86-1.05 | 65 | 37-80 | < .001 |
| GG versus AG + AA (recessive) | 14 | 16 998 | 43 344 | 0.88 | 0.72-1.06 | 0 | 0-55 | .462 |
| Genotype contrast . | Included studies, n . | Total cases, n . | Total non-cases, n . | OR . | 95% CI . | I2 . | 95% Uncertainty interval . | Cochran Q P value . |
|---|---|---|---|---|---|---|---|---|
| VTE | ||||||||
| Per-allele model | 11 | 4821 | 6070 | 1.22 | 1.11-1.33 | 20 | 0-52 | .197 |
| AG versus AA | 11 | 4736 | 6010 | 1.21 | 1.05-1.40 | 43 | 0-72 | .063 |
| GG versus AA | 11 | 3825 | 4979 | 1.81 | 1.29-2.56 | 0 | 0-46 | .694 |
| AG + GG versus AA (dominant) | 11 | 4821 | 6070 | 1.25 | 1.08-1.44 | 47 | 0-74 | .041 |
| GG versus AG + AA (recessive) | 11 | 4821 | 6070 | 1.76 | 1.24-2.48 | 0 | 0-42 | .739 |
| MI | ||||||||
| Per-allele model | 16 | 32 594 | 78 336 | 0.94 | 0.88-1.00 | 65 | 40-79 | < .001 |
| AG versus AA | 14 | 16 850 | 42 919 | 0.96 | 0.86-1.06 | 67 | 43-81 | < .001 |
| GG versus AA | 14 | 14 012 | 35 671 | 0.87 | 0.72-1.06 | 0 | 0-53 | .502 |
| AG + GG versus AA (dominant) | 14 | 16 998 | 43 344 | 0.95 | 0.86-1.05 | 65 | 37-80 | < .001 |
| GG versus AG + AA (recessive) | 14 | 16 998 | 43 344 | 0.88 | 0.72-1.06 | 0 | 0-55 | .462 |
Associations within subgroups between PROCR rs867186 genotypes and VTE or MI under the per-allele model in a random-effects meta-analysis
| Subgroup . | Included studies, n . | Total cases, n . | Total non-cases, n . | OR . | 95% CI . | I2 . | 95% Uncertainty interval . | Cochran Q P value . |
|---|---|---|---|---|---|---|---|---|
| VTE | ||||||||
| Candidate gene studies | 9 | 2428 | 3968 | 1.19 | 1.07-1.34 | 15 | 0-51 | .271 |
| GWAS | 2 | 1680 | 2529 | 1.28 | 1.02-1.61 | 29 | 0-74 | .240 |
| Studies of white subjects only | 6 | 2405 | 3096 | 1.35 | 1.14-1.59 | 12 | 0-53 | .321 |
| Studies in HWE | 9 | 3970 | 4653 | 1.28 | 1.16-1.41 | 10 | 0-46 | .333 |
| MI | ||||||||
| Candidate gene studies | 5 | 1576 | 4165 | 1.03 | 0.68-1.56 | 85 | 66-93 | < .001 |
| GWAS | 11 | 31 018 | 74 171 | 0.93 | 0.89-0.98 | 36 | 0-68 | .113 |
| Studies of white subjects only | 14 | 28 086 | 73 728 | 0.93 | 0.86-1.00 | 66 | 40-81 | < .001 |
| Studies in HWE | 15 | 31 905 | 77 639 | 0.95 | 0.90-1.01 | 54 | 16-74 | .007 |
| Studies of subjects with a history of MI only | 6 | 5730 | 7292 | 0.87 | 0.75-1.00 | 64 | 14-85 | .016 |
| Subgroup . | Included studies, n . | Total cases, n . | Total non-cases, n . | OR . | 95% CI . | I2 . | 95% Uncertainty interval . | Cochran Q P value . |
|---|---|---|---|---|---|---|---|---|
| VTE | ||||||||
| Candidate gene studies | 9 | 2428 | 3968 | 1.19 | 1.07-1.34 | 15 | 0-51 | .271 |
| GWAS | 2 | 1680 | 2529 | 1.28 | 1.02-1.61 | 29 | 0-74 | .240 |
| Studies of white subjects only | 6 | 2405 | 3096 | 1.35 | 1.14-1.59 | 12 | 0-53 | .321 |
| Studies in HWE | 9 | 3970 | 4653 | 1.28 | 1.16-1.41 | 10 | 0-46 | .333 |
| MI | ||||||||
| Candidate gene studies | 5 | 1576 | 4165 | 1.03 | 0.68-1.56 | 85 | 66-93 | < .001 |
| GWAS | 11 | 31 018 | 74 171 | 0.93 | 0.89-0.98 | 36 | 0-68 | .113 |
| Studies of white subjects only | 14 | 28 086 | 73 728 | 0.93 | 0.86-1.00 | 66 | 40-81 | < .001 |
| Studies in HWE | 15 | 31 905 | 77 639 | 0.95 | 0.90-1.01 | 54 | 16-74 | .007 |
| Studies of subjects with a history of MI only | 6 | 5730 | 7292 | 0.87 | 0.75-1.00 | 64 | 14-85 | .016 |
Of the individual studies included in the meta-analysis, the largest association between the PROCR rs867186 variant and VTE was observed in a study9 of prothrombin G20210A mutation carriers (per-allele OR = 2.63; 95% CI, 1.41-4.87). Although no other included study was conducted exclusively among these mutation carriers, 7 other studies reported the prevalence of G20210A mutation carriers; the prevalence was less than 25% and no obvious gradient in the estimated ORs according to G20210A mutation prevalence was observed (supplemental Figure 2 and supplemental Table 3). One included study was conducted exclusively among F5L mutation carriers.41 Results from this study were null (per-allele OR = 0.98; 95% CI, 0.54-1.77). A large positive association (per-allele OR = 1.49; 95% CI, 1.17-1.90) was observed in the study that included only idiopathic VTE subjects (ie, VTE in the absence of acquired risk factors: surgery, hospitalization, pregnancy, puerperium, oral contraception, cancer, and autoimmune disease; and in the absence of strong known genetic risk factors: antithrombin, protein S, or PC deficiencies, and homozygosity for F5L or prothrombin G20210A).30 In the 7 other studies in which the etiology of VTE was reported, the proportion of idiopathic VTE subjects ranged from 25%-65% and there was no obvious trend in the estimated ORs according to the frequency of idiopathic subjects.
MI
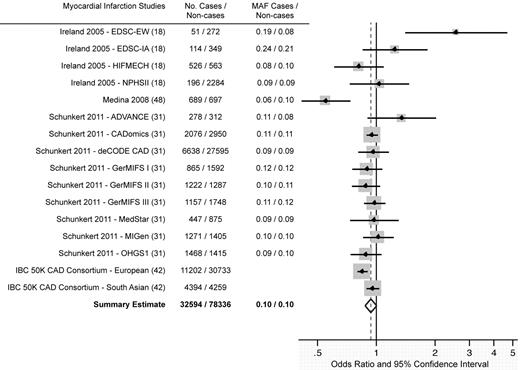
The meta-analysis did not support an association between the PROCR rs867186 variant and MI (Figure 3 and Table 2). None of the investigated genotype contrasts reached statistical significance. Heterogeneity between MI studies under all genetic models was moderate to high (again, the apparent low heterogeneity under the recessive model and for the GG-versus-AA contrast is likely the result of wide CIs in analyses of GG subjects). No publication bias under the per-allele model was observed (supplemental Figure 1), with an Egger test P = .25. When the analysis was restricted to GWAS, under the per-allele model, the I2 was reduced to 36% (95% uncertainty interval, 0%-68%) and the OR for MI was 0.93 (95% CI, 0.89-0.98) for every additional copy of the G allele (P = .005; Table 3). In all other subgroup analyses, heterogeneity remained moderate to high and the effect estimate was similar to that obtained from analysis of all subjects.
Results from a random-effects meta-analysis of the association between the PROCR rs867186 polymorphism and MI (per-allele model). Individual studies are identified by the lead author's last name, year of publication, study name (if a publication included more than 1 study), and reference number. The sample sizes are represented by the size of the squares. Bars indicate 95% CI. Allele frequencies were not reported in the IBC 50K CAD Consortium article.
Results from a random-effects meta-analysis of the association between the PROCR rs867186 polymorphism and MI (per-allele model). Individual studies are identified by the lead author's last name, year of publication, study name (if a publication included more than 1 study), and reference number. The sample sizes are represented by the size of the squares. Bars indicate 95% CI. Allele frequencies were not reported in the IBC 50K CAD Consortium article.
Of the 16 included studies of MI, a statistically significant association between increasing copies of the PROCR rs867186 G allele and MI was found in 3; in 1 of these, the association was positive, and in the other 2, there was an inverse association. The statistically significant positive association was observed in the study18 of white diabetics (per-allele OR = 2.57; 95% CI, 1.41-4.69). Among South-Asian diabetics, the association was also positive, although not statistically significant (per-allele OR = 1.25; 95% CI, 0.85-1.83). The largest inverse association was found in a Spanish study48 of subjects with a history of MI (per-allele OR = 0.55; 95% CI, 0.41-0.74). However, HWE was violated in this study and the results may not be comparable to other included studies. The third statistically significant result was observed in the gene-centric meta-analysis of European subjects42 comprising 10 studies (per-allele OR = 0.85; 95% CI, 0.80-0.90).
Although initially intended, results of VTE and MI studies were not pooled together in a combined analysis because of differences in the direction of the association with the PROCR rs867186 G allele in VTE and MI studies.
Discussion
This meta-analysis of 4821 VTE cases and 6070 controls found a significant association between the PROCR rs867186 variant and VTE. Under an additive genetic model, the odds of VTE increased by 22% for every additional copy of the G allele. In contrast, the meta-analysis found no association between the PROCR rs867186 variant and MI. With nearly 17 000 MI cases and more than 43 000 noncases, and based on our current knowledge of trait and variant characteristics (ie, disease prevalence of approximately 4%,49 population G allele frequency of approximately 10%,24 and an additive disease model), the meta-analysis had > 99% power to detect a per-allele effect as small as 1.1 at P = .05 (supplemental Table 4),50 if such an association existed. However, substantial between-study heterogeneity and other population-specific characteristics may have reduced this theoretical power. Given the present results, the hypothesis of some common pathways underlying venous and arterial thrombosis may not apply to the PROCR gene and the variant may be a genetic risk factor for VTE only.
VTE
The association with VTE is in accordance with the proposed functional implications of the PROCR rs867186 variant, namely, the G allele, causes EPCR shedding from the endothelial membrane, reduced PC activation, and higher FVII/FVIIa levels, eventually leading to thrombosis. Of the 8 studies included in the meta-analysis that examined levels of sEPCR relative to the PROCR rs867186 variant, all observed a significant increase associated with the G allele.9,10,17,18,35,45,47,48 Four studies reported levels of PC or APC by genotype. Of these, two19,45 observed a statistically significant increase in PC associated with the G allele, one9 observed a statistically significant decrease in APC among G allele carriers, and one17 found no association between genotype and APC levels. In a GWAS of plasma levels of PC including more than 8000 participants, the strongest association was observed at the PROCR locus, and the rs867186 polymorphism explained an estimated 10.4% of the variance in PC.51 In 2 studies included in the systematic review that measured prothrombin (fragment 1 and 2) levels relative to genotype, a significant increase was seen for those with the G allele.17,18 The G allele was also associated with elevated levels of FVII and FVIIa in a study of approximately 2000 healthy middle-aged men.6
VTE is multicausal, in that multiple genetic and environmental factors contribute to its etiology.52 Two genetic risk factors for VTE with relatively high population prevalence are well established: the F5L and the prothrombin G20210A mutations. The largest association between the PROCR rs867186 variant and VTE identified in our review was observed in a study of prothrombin G20210A mutation carriers.9 However, further investigation of the modifying role of this mutation was hampered by the low prevalence—or incomplete reporting of the prevalence—of prothrombin G20210A mutation carriers in the other included studies. No association between the PROCR rs867186 variant and VTE was observed in 2 studies of F5L mutation carriers (only 1 of which was included in the review).41,43 In a study (not included in the review) of 2 families with inherited thrombophilia, a stronger association with VTE was observed in those with both the PROCR G allele and a dysfunctional PC gene variant relative to those with a dysfunctional PC gene variant alone.11 Although limited by the small number of studies, these findings suggest that the PROCR rs867186 polymorphism may act in concert with some known genetic risk factors, but not others, to increase VTE risk.
All but 1 study included in the meta-analysis30 used a pooled definition of idiopathic and non-idiopathic VTE cases; only 1 study restricted the case definition to idiopathic VTE patients. In pooling idiopathic and non-idiopathic VTE cases, heterogeneity in the definition of the outcome may have weakened or even masked the effect of the PROCR rs867186 variant on VTE. Providing some evidence for this is the fact that the study restricted to idiopathic VTE patients reported a large positive association with the G allele of PROCR rs867186.
MI
Between-study heterogeneity in MI studies was moderate to high, as indicated by both the I2 and Cochran Q statistics, and is likely attributable to differences in study design, participant selection, and participant characteristics. The meta-analysis pooled results of cohort, case-control, and GWAS designs; in 10 studies, the case definition included MI grouped with other coronary heart diseases, and the severity of MI (fatal versus nonfatal) varied or was unclear across studies. Two studies were restricted to men, some studies included younger subjects (< 60 years of age), whereas the age and gender of subjects was not reported in the study of diabetics. The I2 was less among GWAS, but even among the 9 GWAS in which information on subject characteristics was available, the frequency of cases with a history of MI ranged from 48%-100%, the age and gender distribution of subjects varied considerably, and 2 studies were restricted to cases with a family history of coronary artery disease.
Although no overall association with MI was found, when analysis was restricted to GWAS under the per-allele model, a statistically significant inverse association with the PROCR rs867186 G allele emerged. This finding may be because of reduced heterogeneity in GWAS as a result of harmonization of the case definition, quality control measures, and analysis methods that occurs in consortiums, of which all of the GWAS were part. Our GWAS findings are consistent with a recently published (ie, after our search was updated for the last time) gene-centric case-control study of Italian early-onset MI cases53 ; however, they are nonetheless difficult to explain. The biologic mechanism underlying an inverse association between rs867186 and MI is unclear. It may be that the PROCR rs867186 G allele is in linkage disequilibrium with the true causal variant.
Strengths and limitations
Strengths of the systematic review include the thorough search for articles, the investigation of both venous and arterial thrombotic disease, the large number of included subjects, and the use of a genetic model that is based on biologic evidence. The limitations of the meta-analysis include the possible influences of selection bias and genotype and phenotype misclassification on the results of individual studies and on the results of the meta-analysis, which are difficult to predict. Bias could also have arisen when calculating the crude ORs from genotype frequencies, because this could not be taken into consideration in the analysis when cases and controls were matched. Nonetheless, unmatched effect estimates were almost identical to those presented in the published article, either because matched and unmatched analyses produced similar results or because the investigators had not performed a matched analysis. When estimates adjusted for potential confounders were presented in the published article, these too approximated the crude estimates calculated from genotype frequencies. A major source of confounding in genetic association studies, population stratification, was assessed in the meta-analysis by restricting analysis to white subjects only. Although confounding was not detected, the “white” designation may comprise individuals of various races/ethnicities, and hidden population stratification cannot be completely ruled out.
Whereas selective reporting must be considered in all meta-analyses, because of ready sample availability and low genotyping cost, it is especially a concern in studies of gene-disease associations, in which authors may test many polymorphisms but only report the most interesting or statistically significant findings. Even the means by which publication bias is measured, funnel plots and statistical tests of funnel plot asymmetry, have limited power.40 Although no statistically significant publication bias was detected, it is nonetheless possible that studies with null findings were not published and are missing from the systematic review. In an attempt to overcome this limitation, we included in our search both published and unpublished GWAS data, and authors of all eligible studies were contacted to request genotype counts.
Conclusions
Applying the Venice criteria,39 the strength of cumulative evidence for an association between the PROCR rs867186 variant and VTE is “moderate.” The meta-analysis had > 80% power to detect a per-allele relative risk of 1.15 (supplemental Table 4), there existed some between-study heterogeneity, and the effect of potential biases could not be completely assessed. This corresponds to a score of “B” for amount of evidence, replication, and protection from bias. Using the same criteria, the strength of evidence for an association with MI is “weak.” Despite a considerable sample size indicating large-scale evidence (an “A” rating), there existed substantial between-study inconsistency in MI phenotype definition, study populations, and study findings, and a null overall effect was found (a “C” rating for replication).
In light of the ever-increasing number of gene-disease association studies, especially in the postGWAS era, HuGE reviews are a useful tool to summarize this data. Overall, there is moderate evidence for an association between the PROCR rs867186 polymorphism and VTE, whereas there is weak evidence for an association with MI. This suggests that a common mechanism underlying venous and arterial thrombosis is not mediated by the PROCR rs867186 polymorphism.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Inke König and Dr Jeanette Erdmann for providing genotype counts for the PROCR rs867186 variant from the studies included in the CARDIoGRAM consortium; Dr Martin Farrall for correspondence regarding the CARDIoGRAMplusC4D data; and Dr Francisco España for clarifying the number of non-overlapping cases to be used from each of his 3 studies included in the meta-analysis.
This project was partially funded by the Canadian Institutes of Health Research (grant MOP 86466) and by the Heart and Stroke Foundation of Canada (grant T6484). J.D. holds a Vanier Canada Graduate Scholarship and F.G. holds a Canada Research Chair. The Mayo VTE GWAS was funded by the National Institutes of Health (grant HG 04735).
National Institutes of Health
Authorship
Contribution: F.G., P.E.M., and D.-A.T. conceived the research; J.D., F.G., and C.Y.J designed the study; J.D. led the implementation, analysis, and interpretation of the study; A.S.A. screened articles for eligibility and extracted the data; M.d.A. and J.A.H. contributed to data acquisition; J.D. drafted the manuscript; and all authors critically reviewed the manuscript for intellectual content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: France Gagnon, Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, 155 College Street, Suite 662, Toronto, ON, Canada M5T 3M7; e-mail: france.gagnon@utoronto.ca.