Abstract
Chronic inflammation and decreased frequency of regulatory T cells (Tregs) in visceral adipose tissue contribute to the propagation of insulin resistance to diabetes mellitus. We tested the hypothesis that new-onset posttransplantation diabetes mellitus (PTDM) is associated with measurable changes in Treg subsets after allogeneic hematopoietic stem cell transplantation (HSCT). PTDM before day 100 and Treg phenotype at engraftment were determined in 36 HSCT recipients without preceding history of diabetes mellitus. Among patients with new-onset PTDM (N = 24), the frequency of circulating CLA+ (skin-homing) Tregs was decreased (1.53% vs 3.99%; P = .002) and the percentage of α4β7+ (gut-homing) Tregs was increased (17.9% vs 10.7%; P = .048). In multivariate analysis, patients with PTDM continued to demonstrate elevated ratios of α4β7+ Tregs to CLA+ Tregs (odds ratio, 18.1; P = .020). PTDM is associated with altered immune regulation after HSCT and could represent a target to modulate alloreactivity.
Introduction
Posttransplantation diabetes mellitus (PTDM) occurs in 10% to 30% of patients undergoing allogeneic hematopoietic cell transplantation (HSCT).1-3 Insulin resistance is a prominent characteristic of the metabolic syndrome. This pro-inflammatory disorder is highly prevalent in survivors of HSCT and is associated with increased risk for cardiovascular mortality.4,5 Regulatory T cells (Tregs) are an immunosuppressive subset of CD4+ T cells that have been implicated in the prevention of both autoimmune disorders, such as type I diabetes mellitus and the inhibition of alloimmune responses, including GVHD.6-8 Recent data suggest that immune dysregulation and impaired accumulation of Tregs in visceral adipose tissue are associated with the development of insulin resistance in animal models.9,10 Therefore, we tested the hypothesis that patients with PTDM have altered frequencies of Tregs early after HSCT.
Methods
Patients 18 years of age or older undergoing myeloablative or reduced intensity conditioning followed by related or unrelated donor transplant were eligible for 2 independent Vanderbilt University Institutional Review Board–approved trials examining either PTDM or tissue-specific Tregs. Thirty-six HSCT recipients with hematologic malignancies but without preexisting diabetes were enrolled in both studies concurrently and were followed for 100 days after HSCT for PTDM, defined as fasting glucose more than or equal to 126 mg/dL or random glucose more than or equal to 200 mg/dL. Fasting glucose levels were assessed weekly from day 0 to day 100. All patients received GVHD prophylaxis with calcineurin inhibitor and either methotrexate or mycophenolate mofetil. The diagnosis and grading of acute GVHD followed standard criteria.11
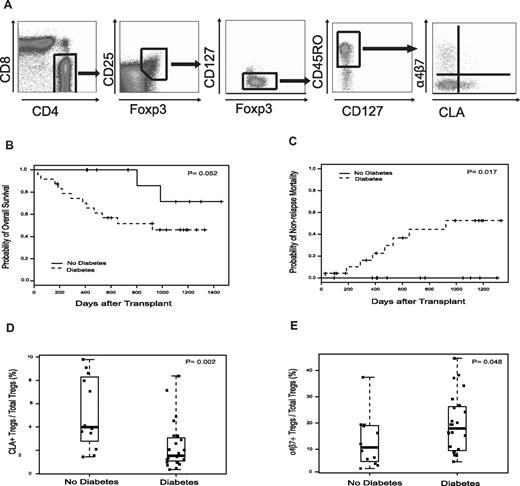
Peripheral blood mononuclear cells were isolated at engraftment (absolute neutrophil count ≥ 0.5 × 109/L for 3 days). Tregs were analyzed by flow cytometry (see Figure 1A).12 The frequency of CD45RO+CD25+Foxp3+CD127lo Tregs was expressed as the percentage of positive cells in the total CD4+ gate. CLA+ Tregs (skin-homing) and α4β7+ Tregs (gut-homing) were enumerated as subpopulations within the total Treg population. CD4+ memory T cells (CD4+CD45RO+), CD4+CLA+ T cells, and CD4+α4β7+ T cells also were quantified.
Groups were compared using χ2 for categorical variables and Mann-Whitney U test for continuous outcomes. The primary aim was to test for an association between PTDM and Treg subsets using Mann-Whitney U test and logistic regression for univariate and multivariate analysis, respectively. Kaplan-Meier method was used to estimate overall survival and disease-free survival, and groups were compared using the log-rank test. Cumulative incidence was used to estimate nonrelapse mortality, defined as any death occurring in the absence of malignancy relapse or progression. P values were 2-tailed and considered significant at P < .05.
Results and discussion
Among 36 HSCT recipients with prospectively collected metabolic and Treg data, 24 (66.7%) patients developed new-onset PTDM during the first 100 days of transplantation (median, 24.5 days after HSCT; range, 7-100 days). Table 1 summarizes cohort characteristics. Grade 2 to 4 acute GVHD occurred in 28 (77.8%) patients (median, 22 days after HSCT; range, 7-93 days). Acute GVHD was treated with corticosteroids in 28 (77.8%) persons: grade 1 (N = 2) and grade 2 to 4 (N = 26). PTDM was diagnosed before grade 2 to 4 acute GVHD or the start of systemic steroids in 12 (50%) and 14 (58%) patients, respectively. Among surviving patients (N = 22), median follow-up was 2.9 years (range, 0.5-4.0 years). PTDM developing before day 100 days was associated with inferior overall survival and increased nonrelapse mortality (Figure 1B-C). Causes of nonrelapse mortality included: infection (N = 4), gastrointestinal GVHD (N = 1), diffuse alveolar hemorrhage (N = 1), thrombotic thrombocytopenic purpura (N = 1), and myocardial infarction (N = 1). PTDM did not impact disease-free survival (data not shown).
Characteristics of total cohort, 36 patients undergoing HSCT
| Clinical characteristics . | No. of patients with characteristic, stratified by PTDM status (percentage) . | P . | |
|---|---|---|---|
| No diabetes . | Diabetes . | ||
| Total no. of patients | 12 | 24 | |
| Age, y | |||
| Median | 45 | 43.5 | .545 |
| Range | 24-62 | 21-65 | |
| Sex | |||
| Male | 7 (58) | 10 (42) | .345 |
| Female | 5 (42) | 14 (58) | |
| Pretransplantation BMI, kg/m2 | |||
| Median | 27.6 | 26.2 | .159 |
| Range | 24.4-49.5 | 22.5-49.5 | |
| Pretransplantation HTN | |||
| Absent | 6 (50) | 17 (71) | .220 |
| Present | 6 (50) | 7 (29) | |
| Family history of DM | |||
| Absent | 3 (25) | 10 (42) | .326 |
| Present | 9 (75) | 14 (58) | |
| Pre-HCT laboratory data | |||
| Fasting glucose, mg/dL | |||
| Median | 95 | 97 | .545 |
| Range | 88-119 | 79-121 | |
| Fasting insulin, μU/mL | |||
| Median | 9.95 | 18.2 | .664 |
| Range | 6.7-109 | 1.60-194 | |
| Fasting C-peptide, ng/mL | |||
| Median | 2.95 | 5.95 | .307 |
| Range | 1.80-129 | 1.70-63.4 | |
| Transplantation characteristics | |||
| Diagnosis | |||
| AML + ALL | 7 (58) | 10 (42) | |
| CML | 3 (25) | 0 | |
| MDS + MPD | 1 (8) | 2 (8) | |
| NHL + HL | 1 (8) | 8 (33) | |
| Other malignancies | 0 | 4 (17) | |
| CIBMTR disease risk | |||
| Low/intermediate | 10 (83) | 17 (71) | .414 |
| High | 2 (17) | 7 (29) | |
| Conditioning regimen | |||
| Myeloablative | 8 (67) | 19 (79) | .414 |
| Reduced intensity | 4 (33) | 5 (21) | |
| Donor | |||
| Related | 9 (75) | 12 (50) | .151 |
| Unrelated | 3 (25) | 12 (50) | |
| Stem cell source | |||
| Peripheral blood | 10 (83) | 14 (58) | .134 |
| Other | 2 (17) | 10 (42) | |
| HLA | |||
| Matched | 11 (92) | 21 (88) | 1.00 |
| Mismatched | 1 (8) | 3 (12) | |
| Acute GVHD prophylaxis | |||
| CSA + methotrexate | 7 (58) | 17 (71) | .453 |
| CSA/FK506 + MMF | 5 (42) | 7 (29) | |
| Acute GVHD* | |||
| Grade 0 or 1 | 4 (33) | 4 (17) | .257 |
| Grade 2-4 | 8 (67) | 20 (83) | |
| GVHD organ involvement | |||
| Skin | 2 (17) | 14 (58) | .018 |
| Gastrointestinal | 7 (58) | 17 (71) | .453 |
| Liver | 0 | 0 | |
| Systemic steroids | |||
| Absent | 4 (33) | 4 (17) | .257 |
| Present | 8 (67) | 20 (83) | |
| Day 100 malignancy status | |||
| CR or PR | 11 (92) | 19 (79) | .640 |
| Relapse or progression | 1 (8) | 5 (21) | |
| Day 100 survival | |||
| Alive | 12 (100) | 22 (92) | .797 |
| Dead | 0 | 2 (8)† | |
| Clinical characteristics . | No. of patients with characteristic, stratified by PTDM status (percentage) . | P . | |
|---|---|---|---|
| No diabetes . | Diabetes . | ||
| Total no. of patients | 12 | 24 | |
| Age, y | |||
| Median | 45 | 43.5 | .545 |
| Range | 24-62 | 21-65 | |
| Sex | |||
| Male | 7 (58) | 10 (42) | .345 |
| Female | 5 (42) | 14 (58) | |
| Pretransplantation BMI, kg/m2 | |||
| Median | 27.6 | 26.2 | .159 |
| Range | 24.4-49.5 | 22.5-49.5 | |
| Pretransplantation HTN | |||
| Absent | 6 (50) | 17 (71) | .220 |
| Present | 6 (50) | 7 (29) | |
| Family history of DM | |||
| Absent | 3 (25) | 10 (42) | .326 |
| Present | 9 (75) | 14 (58) | |
| Pre-HCT laboratory data | |||
| Fasting glucose, mg/dL | |||
| Median | 95 | 97 | .545 |
| Range | 88-119 | 79-121 | |
| Fasting insulin, μU/mL | |||
| Median | 9.95 | 18.2 | .664 |
| Range | 6.7-109 | 1.60-194 | |
| Fasting C-peptide, ng/mL | |||
| Median | 2.95 | 5.95 | .307 |
| Range | 1.80-129 | 1.70-63.4 | |
| Transplantation characteristics | |||
| Diagnosis | |||
| AML + ALL | 7 (58) | 10 (42) | |
| CML | 3 (25) | 0 | |
| MDS + MPD | 1 (8) | 2 (8) | |
| NHL + HL | 1 (8) | 8 (33) | |
| Other malignancies | 0 | 4 (17) | |
| CIBMTR disease risk | |||
| Low/intermediate | 10 (83) | 17 (71) | .414 |
| High | 2 (17) | 7 (29) | |
| Conditioning regimen | |||
| Myeloablative | 8 (67) | 19 (79) | .414 |
| Reduced intensity | 4 (33) | 5 (21) | |
| Donor | |||
| Related | 9 (75) | 12 (50) | .151 |
| Unrelated | 3 (25) | 12 (50) | |
| Stem cell source | |||
| Peripheral blood | 10 (83) | 14 (58) | .134 |
| Other | 2 (17) | 10 (42) | |
| HLA | |||
| Matched | 11 (92) | 21 (88) | 1.00 |
| Mismatched | 1 (8) | 3 (12) | |
| Acute GVHD prophylaxis | |||
| CSA + methotrexate | 7 (58) | 17 (71) | .453 |
| CSA/FK506 + MMF | 5 (42) | 7 (29) | |
| Acute GVHD* | |||
| Grade 0 or 1 | 4 (33) | 4 (17) | .257 |
| Grade 2-4 | 8 (67) | 20 (83) | |
| GVHD organ involvement | |||
| Skin | 2 (17) | 14 (58) | .018 |
| Gastrointestinal | 7 (58) | 17 (71) | .453 |
| Liver | 0 | 0 | |
| Systemic steroids | |||
| Absent | 4 (33) | 4 (17) | .257 |
| Present | 8 (67) | 20 (83) | |
| Day 100 malignancy status | |||
| CR or PR | 11 (92) | 19 (79) | .640 |
| Relapse or progression | 1 (8) | 5 (21) | |
| Day 100 survival | |||
| Alive | 12 (100) | 22 (92) | .797 |
| Dead | 0 | 2 (8)† | |
BMI indicates body mass index; HTN, hypertension; DM, diabetes mellitus; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; CML, chronic myeloid leukemia; MPD, myeloproliferative disorder; NHL, non-Hodgkin lymphoma; HL, Hodgkin lymphoma; CIBMTR, Committee of International Bone Marrow Transplant Registry; CSA, cyclosporine; FK506, tacrolimus; MMF, mycophenolate mofetil; CR, complete response; and PR, partial response.
Grade of acute GVHD (0 or 1 vs 2-4) indicates maximal grade of acute GVHD during first 100 days of transplantation.
Causes of death included: relapse of malignancy (n = 1) and infection (n = 1).
Engraftment samples were collected at a median of 19 days (range, 12-27 days) after HSCT and before the diagnosis of diabetes in 15 (62.5%) patients. Because of severe lymphopenia, only Treg percentages were examined. The percentage of circulating CLA+ Tregs at engraftment was significantly lower in patients with PTDM compared with patients without the disorder (1.53% vs 3.99%; P = .002). Conversely, PTDM was associated with increased frequencies of α4β7+ Tregs (17.9% vs 10.7%; P = .048; Figure 1D-E). After excluding patients whose diabetes diagnosis preceded Treg analysis (N = 9), PTDM continued to be associated with decreased CLA+ Tregs (P = .002) and increased α4β7+ Tregs (P = .071), indicating that immune changes occur before clinical manifestations of diabetes. The skewing of the Treg phenotype toward increased α4β7 expression and decreased CLA expression in all patients with PTDM was further demonstrated by the ratio of α4β7+ Tregs to CLA+ Tregs, which was significantly higher in patients with PTDM (8.93 vs 1.85; P = .004). Furthermore, the incidence of day 100 PTDM was 75.0% in persons with ratios of α4β7+ Tregs to CLA+ Tregs greater than or equal to the cohort median of 6.32, compared with 44.4% in those with a ratio less than the median (P = .005). Analyses were repeated using only patients with grade 2 to 4 acute GVHD treated with corticosteroids (N = 26). Results were similar with PTDM associated with decreased CLA+ Tregs (1.51% vs 4.12%; P = .013), increased α4β7+ Tregs (19.4% vs 6.86%; P = .048), and an elevated ratio of α4β7+ Tregs to CLA+ Tregs (10.9 vs 1.78; P = .007). PTDM was not associated with the percentage of CD4+ memory T cells, CD4+CLA+ T cells, CD4+α4β7+ T cells, or total Tregs (not accounting for homing; data not shown).
Treg tissue-homing subsets, PTDM, and transplant outcomes. (A) Multiparameter analysis of Treg tissue-homing subsets. Treg tissue-homing subset identification using 10-color multiparametric flow cytometry. Gates were initially set on CD3+ while excluding cells expressing CD14 or marking with the amine viability dye (data not shown). Viable CD4+ T cells were then selected by gating on CD4+CD8− cells. To ensure adequate cell numbers for accurate gating, quadrants were created using 2-parameter comparisons assessing the expression of Foxp3, CD25, CD127, CD45R0, α4β7, and CLA by the total CD4+ cell population (data not shown). These gates were then sequentially applied to the CD4+ cell population to determine Treg subset frequency. (B-C) Overall survival and nonrelapse mortality of patients undergoing HSCT stratified by the presence of PTDM. (D-E) PTDM was associated with changes in Treg tissue-homing phenotype. The percentage of CLA+ Tregs and α4β7+ Tregs was determined by multiparametric flow cytometry at the time of neutrophil engraftment. Box plots define the values for median, range, and 25th and 75th percentiles. Two-tailed P values were calculated using the Mann-Whitney U test to assess for differences in the median percentage of CLA+ Tregs and α4β7+ among patients with or without PTDM.
Treg tissue-homing subsets, PTDM, and transplant outcomes. (A) Multiparameter analysis of Treg tissue-homing subsets. Treg tissue-homing subset identification using 10-color multiparametric flow cytometry. Gates were initially set on CD3+ while excluding cells expressing CD14 or marking with the amine viability dye (data not shown). Viable CD4+ T cells were then selected by gating on CD4+CD8− cells. To ensure adequate cell numbers for accurate gating, quadrants were created using 2-parameter comparisons assessing the expression of Foxp3, CD25, CD127, CD45R0, α4β7, and CLA by the total CD4+ cell population (data not shown). These gates were then sequentially applied to the CD4+ cell population to determine Treg subset frequency. (B-C) Overall survival and nonrelapse mortality of patients undergoing HSCT stratified by the presence of PTDM. (D-E) PTDM was associated with changes in Treg tissue-homing phenotype. The percentage of CLA+ Tregs and α4β7+ Tregs was determined by multiparametric flow cytometry at the time of neutrophil engraftment. Box plots define the values for median, range, and 25th and 75th percentiles. Two-tailed P values were calculated using the Mann-Whitney U test to assess for differences in the median percentage of CLA+ Tregs and α4β7+ among patients with or without PTDM.
In multivariate analysis adjusting for pretransplant fasting C-peptide, corticosteroids, and unrelated donor, patients with PTDM continued to show an altered distribution of Treg subsets (Table 2).13
Multivariate analyses of risk factors for PTDM
| Factor . | Odds ratio* . | 95% CI . | P . |
|---|---|---|---|
| α4β7+ Tregs to CLA+ Tregs > median (6.32) | 18.1 | 1.57-209.5 | .020 |
| Treatment with systemic steroids | 1.09 | 0.12-9.70 | .939 |
| Pretransplantation fasting C-peptide | 1.22 | 0.92-1.60 | .170 |
| Transplantation with unrelated donor | 2.78 | 0.29-26.5 | .374 |
| Factor . | Odds ratio* . | 95% CI . | P . |
|---|---|---|---|
| α4β7+ Tregs to CLA+ Tregs > median (6.32) | 18.1 | 1.57-209.5 | .020 |
| Treatment with systemic steroids | 1.09 | 0.12-9.70 | .939 |
| Pretransplantation fasting C-peptide | 1.22 | 0.92-1.60 | .170 |
| Transplantation with unrelated donor | 2.78 | 0.29-26.5 | .374 |
CLA indicates cutaneous lymphocyte antigen.
Odds ratio greater than 1 indicates increased odds of developing PTDM.
These data confirm that early PTDM is a common complication after HSCT and is associated with inferior transplant outcomes. We found a higher than previously reported incidence of PTDM (66.7%), probably because of prospective data collection, strict diagnostic criteria, and early time-frame after HSCT. We also found that the distribution of Tregs with specific tissue-homing properties was markedly altered in patients who developed PTDM with increased α4β7+ Tregs and decreased CLA+ Tregs. Remarkably, these changes in Treg phenotype remained significant after both stratified and multivariate analysis, despite the relatively small study cohort. These data suggest a strong association between PTDM and Tregs, which could have implications for other important HSCT outcomes.
Animal models have shown a relationship between chronic low-grade inflammation, decreased frequency of Tregs in visceral adipose tissue, and the progression of insulin resistance to diabetes mellitus.9,10 This observation is potentially mediated by leptin, an adipocyte-secreted hormone associated with obesity and diabetes, which can decrease the proliferation of Tregs and promote an inflammatory Th1 phenotype.14-16 In experimental models, adipocyte inflammation and insulin resistance can be reversed by infusion of Tregs.17 Thus, adipocyte physiology is closely linked to cellular immune responses.
Among HSCT recipients, we suspect that early PTDM is caused by progressive insulin resistance related to increased metabolic demands from medications, alloimmune responses, and chronic host adipose tissue inflammation. Adipocyte inflammation and macrophage recruitment to these tissues also could serve as a priming mechanism for GVHD and alloreactivity, thus perpetuating the progression of insulin resistance. Unexpectedly, we found that patients with PTDM had increased frequencies of circulating α4β7+ Tregs (gut-homing). We hypothesize that, among patients with PTDM, the preferential expansion of donor α4β7+ Tregs is a compensatory response meant to suppress the underlying visceral adipose tissue inflammation in diabetes-prone HSCT recipients. This type of adaptive Treg proliferation has been demonstrated in patients with obesity and insulin resistance.17
We have previously shown that increased frequencies of CLA+ or α4β7+ Tregs were associated with decreased skin or gut acute GVHD events, respectively.12 As predicted by the Treg phenotype, PTDM was associated with skin but not gut GVHD (Table 1). Paradoxically, attempted suppression of metabolic inflammation may deplete the pool of α4β7+ Tregs needed for gut GVHD prevention in patients with PTDM. Further studies will be required to understand the complex interplay between Treg subsets, glycemic control, adipocyte physiology, and GVHD.
To our knowledge, this is the first report to show an association between Treg tissue-homing phenotype and the development of diabetes mellitus after HSCT. These data suggest that PTDM and insulin resistance may affect cellular immune responses after HSCT, including Treg subset frequencies and immune regulation. PTDM and the related metabolic syndrome could represent an important new therapeutic target to modulate alloreactivity and clinical outcomes after HSCT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (grant K12CA090625, B.G.E.; grant K08HD061607, J.-H.W.; and grant K08DK090146, D.J.M.), the American Cancer Society (Institutional Research Grant 58-009-48), and the Sartain-Lanier Family Foundation.
National Institutes of Health
Authorship
Contribution: B.G.E., J.E.C., and M.J. designed research, analyzed and interpreted data, and wrote the manuscript; M.L.G., B.N.S., S.M.Y., M.T.R., and S.M.J. analyzed and interpreted data and revised the manuscript; A.A.K., J.-H.W., and D.J.M. contributed to research design and edited the manuscript; and P.L. performed statistical analysis and prepared the figures.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Madan Jagasia, Vanderbilt University Medical Center, 1301 Medical Center Dr, Nashville, TN 37232; e-mail: madan.jagasia@vanderbilt.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal