Abstract
Surprisingly little is known about the interaction of human blood mononuclear cells with viruses. Here, we show that monocytes are the predominant cell type infected when peripheral blood mononuclear cells are exposed to viruses ex vivo. Remarkably, infection with vesicular stomatitis virus, vaccinia virus, and a variety of influenza A viruses (including circulating swine-origin virus) induces monocytes to differentiate within 18 hours into CD16−CD83+ mature dendritic cells with enhanced capacity to activate T cells. Differentiation into dendritic cells does not require cell division and occurs despite the synthesis of viral proteins, which demonstrates that monocytes counteract the capacity of these highly lytic viruses to hijack host cell biosynthetic capacity. Indeed, differentiation requires infectious virus and viral protein synthesis. These findings demonstrate that monocytes are uniquely susceptible to viral infection among blood mononuclear cells, with the likely purpose of generating cells with enhanced capacity to activate innate and acquired antiviral immunity.
Introduction
PBMCs in healthy individuals consist of roughly 70% T cells, 10% B cells, 10% monocytes, and 1% each of natural killer (NK) cells and dendritic cells (DCs). Three distinct blood monocyte subsets can be identified on the basis of CD14 and CD16 expression: ∼ 90% CD14++CD16−, 10% CD14+CD16++, and a minor population of CD14++CD16+.1
DCs are potent APCs with a nearly unique capacity to prime naive T lymphocytes.1 Because of their paucity in blood, DCs are typically generated by culturing monocytes ex vivo with cytokines.2,3 Little is known about the interaction of PBMCs with viruses that principally replicate in nonimmune cells. Here, we investigate the effect of infecting PBMCs with 3 representative nonhemotropic viruses that are highly lytic to their normal host cells.
Methods
PBMC infection
Fresh buffy coat preparations were obtained from healthy anoymous donors at the National Institutes of Health (NIH) blood bank who provided informed consent. We also obtained whole blood from healthy donors with informed consent in accordance with the Declaration of Helsinki approved by University of California at Los Angeles Institutional Review Board. PBMCs or purified monocytes were incubated with virus for 1 hour at room temperature in balanced salt solution with 0.1% BSA and then resuspended at 1 × 106 cells per well in complete RPMI 1640. Six or 18 hours after infection, cells were stained with mAbs including PerCP-eFluor 710–conjugated anti-CD3, -CD14, -CD19, and -CD56 or a combination of these “lineage marker” mAbs (all eBioscience); eFluor 450–conjugated anti–HLA-DR, AlexaFluor700 anti-CD11c, and PE-Cy7–conjugated anti-CD123 (all eBioscience); AlexaFluor488 anti-hemagglutinin (Bennink-Yewdell Lab, National Institute of Allergy and Infectious Diseases); PE-Cy7–conjugated anti-TNF, eFluor605NC anti-CD16, and PE anti-CD83 (all eBioscience); PE anti-CD1c (Miltenyi Biotec); PE anti-CD141 (BD Biosciences); FITC anti-CLEC4C (Miltenyi Biotec); and APC anti-CLEC9A (R&D Systems).
Real-time PCR
After 6 hours' infection, total RNA purified from influenza A virus (IAV)–infected monocytes primed with random hexamers was PCR analyzed with Profiler PCR arrays for DC APC and IFN/IFN receptor gene expression (SABiosciences PAHS-406 and PAHS-064), with expression normalized to 5 internal housekeeping genes. A custom array was obtained from SABiosciences for the detection of the human genes CCR7, CD1C, ITGAX (CD11c), CD14, FCGR3A (CD16), CD83, THBD (CD141), CLEC4C (CD303), CLEC9A, CSF2 (GM-CSF), CXCR4, and LAMP3 (DC-LAMP).
Results and discussion
We chose 3 representative viruses: IAV, vesicular stomatitis (VSV), and vaccinia virus (VV). For VV and VSV, we measured infection by expression of enhanced green fluorescent protein inserted into the viral genome. For IAV, infection was monitored by cell surface expression of hemagglutinin. After infecting PBMCs for 6 or 18 hours, cells were characterized by standard flow cytometry phenotypic markers (Figure 1A).
Virus infection of human monocytes induces differentiation into DCs. (A) Human PBMCs were infected for 6 hours with the indicated virus at a multiplicity of infection (MOI) of 10, and surface expression of IAV hemagglutinin or virus-encoded enhanced green fluorescent protein on CD3+ T cells, CD14+ monocytes, CD19+ B cells, CD56+ NK cells, lineage-negative (CD3/14/19/56)−HLA-DR+CD11c+CD123− cDCs, or lineage-negative HLA-DR+CD11c−CD123+ pDCs was determined by flow cytometry. The results represent 1 of 2 separate experiments with similar results. (B) Flow cytometric analysis of T/monocyte/B/NK cells 6 or 18 hours after IAV infection at indicated MOI. % Max indicates percent of maximum. (C) Effect of IAV infection at indicated MOI 6 or 18 hours after infection on lineage-negative HLA-DR+ DC population (red box; left panel) or on cDC (circled CD123lowCD11c+ population)/pDC (circled CD123+CD11c− population) populations (middle panel), and the cell number of monocytes, cDCs, and pDCs in 5 × 105 PBMCs 18 hours after IAV infection (right panel). (D) CD14+ monocyte-depleted PBMCs were infected for 18 hours with IAV and then stained with lineage markers and HLA-DR. The results in panels B through D are representative examples of 5 independent experiments. (E) Purified CD14+ monocytes were infected for 6 and 18 hours with IAV and then stained with anti-hemagglutinin antibody. The results are representative examples of 3 independent experiments.
Virus infection of human monocytes induces differentiation into DCs. (A) Human PBMCs were infected for 6 hours with the indicated virus at a multiplicity of infection (MOI) of 10, and surface expression of IAV hemagglutinin or virus-encoded enhanced green fluorescent protein on CD3+ T cells, CD14+ monocytes, CD19+ B cells, CD56+ NK cells, lineage-negative (CD3/14/19/56)−HLA-DR+CD11c+CD123− cDCs, or lineage-negative HLA-DR+CD11c−CD123+ pDCs was determined by flow cytometry. The results represent 1 of 2 separate experiments with similar results. (B) Flow cytometric analysis of T/monocyte/B/NK cells 6 or 18 hours after IAV infection at indicated MOI. % Max indicates percent of maximum. (C) Effect of IAV infection at indicated MOI 6 or 18 hours after infection on lineage-negative HLA-DR+ DC population (red box; left panel) or on cDC (circled CD123lowCD11c+ population)/pDC (circled CD123+CD11c− population) populations (middle panel), and the cell number of monocytes, cDCs, and pDCs in 5 × 105 PBMCs 18 hours after IAV infection (right panel). (D) CD14+ monocyte-depleted PBMCs were infected for 18 hours with IAV and then stained with lineage markers and HLA-DR. The results in panels B through D are representative examples of 5 independent experiments. (E) Purified CD14+ monocytes were infected for 6 and 18 hours with IAV and then stained with anti-hemagglutinin antibody. The results are representative examples of 3 independent experiments.
For each of the 3 viruses tested, T cells (CD3+), B cells (CD19+), and NK cells (CD56+) expressed negligible or undetectable levels of viral proteins over a range of multiplicities of infection. By contrast, monocytes (CD14+) robustly expressed viral proteins after infection with IAV, VSV, and VV. Similarly, IAV, VSV, and VV infected conventional DCs (cDCs; “lineage negative”; CD3/14/19/56−, HLA-DR+CD11c+CD123−). Intriguingly, plasmacytoid DCs (pDCs; lineage-negative, HLA-DR+CD11c−CD123+) were highly susceptible only to IAV, which might be regulated by TNF-producing non-pDCs in infected PBMCs that may in turn inhibit the capacity of pDCs to secret type I IFNs and thereby enhance the susceptibility of pDCs to IAV.
Six and 18 hours after infection, numbers of T, B, and NK cells were unaltered (Figure 1B). IAV infection slightly reduced monocyte numbers 6 hours after infection and markedly reduced monocytes by 18 hours after infection, particularly at higher multiplicities of infection. In parallel with monocyte reduction, infection increased the numbers of lineage-negative HLA-DR+ DCs (Figure 1C). Additional analysis revealed that all of the increase occurred for cDCs, because pDC numbers were constant (Figure 1C).
There were few propidium iodide–positive/hemagglutinin-positive/CD14+ cells at 6 hours after infection, consistent with differentiation rather than cell death in the disappearance of infected monocytes (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Notably, CD14+ monocyte depletion prevented the IAV-induced increase in DCs (Figure 1D). Conversely, infection of purified monocytes for 18 hours with IAV decreased monocytes (Figure 1E), concomitant with the appearance of cDCs (Figure 2A). Monocyte-DC conversion did not require cell division, because it was unaffected by γ-irradiation of monocytes before IAV infection (Figure 2A).
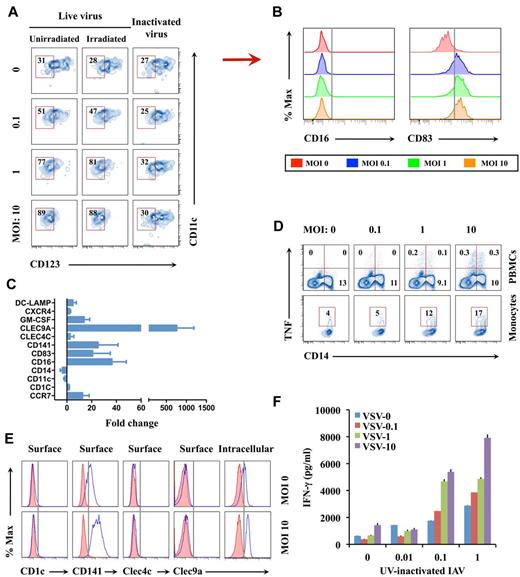
Characterizing virus-induced DCs. (A) Monocytes irradiated with 2000 rad of γ-radiation or nonirradiated cells were infected with live or UV-inactivated IAV. At 18 hours after infection, CD11c+CD123− cDCs instead of CD123+ monocytes or pDCs among the CD14−HLA-DR+ DC population were assessed by flow cytometry. (B) Surface CD16 or CD83 expression on the cDCs in panel A from infected and uninfected samples at 18 hours after IAV infection. The results represent 1 of 3 separate experiments. (C) Quantitative real-time PCR analysis of monocyte/DC-related markers in monocytes after 6 hours after IAV infection at a MOI of 10 vs uninfected sample. The results represent the average of 5 subject samples. (D) Intracellular TNF production by IAV-infected PBMCs or monocytes at 6 hours after infection. (E) Levels of surface CD1c, CD141, CLEC4C, and CLEC9A, as well as intracellular CLEC9A, by IAV-infected monocytes at 18 hours after infection. Shaded and open histograms, respectively, represent isotype and specific antibody staining. (F) Monocytes infected for 6 hours with different doses of VSV were cocultured for 7 days with purified CD4+ T cells from the same donor at a monocyte/T-cell ratio of 1:5 in the presence of varied concentrations of UV-inactivated IAV antigen. IFN-γ levels in supernatants of the cocultures were determined by ELISA. Data in panels D and F are representative of 3 separate experiments. % Max indicates percent of maximum.
Characterizing virus-induced DCs. (A) Monocytes irradiated with 2000 rad of γ-radiation or nonirradiated cells were infected with live or UV-inactivated IAV. At 18 hours after infection, CD11c+CD123− cDCs instead of CD123+ monocytes or pDCs among the CD14−HLA-DR+ DC population were assessed by flow cytometry. (B) Surface CD16 or CD83 expression on the cDCs in panel A from infected and uninfected samples at 18 hours after IAV infection. The results represent 1 of 3 separate experiments. (C) Quantitative real-time PCR analysis of monocyte/DC-related markers in monocytes after 6 hours after IAV infection at a MOI of 10 vs uninfected sample. The results represent the average of 5 subject samples. (D) Intracellular TNF production by IAV-infected PBMCs or monocytes at 6 hours after infection. (E) Levels of surface CD1c, CD141, CLEC4C, and CLEC9A, as well as intracellular CLEC9A, by IAV-infected monocytes at 18 hours after infection. Shaded and open histograms, respectively, represent isotype and specific antibody staining. (F) Monocytes infected for 6 hours with different doses of VSV were cocultured for 7 days with purified CD4+ T cells from the same donor at a monocyte/T-cell ratio of 1:5 in the presence of varied concentrations of UV-inactivated IAV antigen. IFN-γ levels in supernatants of the cocultures were determined by ELISA. Data in panels D and F are representative of 3 separate experiments. % Max indicates percent of maximum.
Additional experiments revealed that a variety of IAVs induced rapid monocyte-to-cDC differentiation, including H3N2 viruses (NT60, HK, Udorn), a more contemporary human H1N1 (Texas/36/91), and importantly, the recent swine-origin IAV A/California/07/09/. We observed the same phenomenon 18 hours after infection with VSV and VV.
The positive correlation between viral gene expression in monocytes and DC differentiation suggests that the virus inoculum is unable to trigger differentiation. Indeed, UV irradiation destroys the capacity of IAV to induce monocyte differentiation (Figure 2A), which indicates that differentiation is not based on the interaction of exogenous viral material with innate immune receptors but requires viral gene expression.
Eighteen hours after infection, IAV, VV, or VSV greatly enhanced cell surface expression of DC maturation marker CD832 (Figure 2B). By contrast, nonclassic monocyte marker CD161 was unchanged. The use of commercial PCR arrays to assess expression of IFN pathway and antigen-presentation genes revealed the expected up-regulation of many IFN-pathway–associated genes and several chemokines and chemokine receptors, adhesion molecules, IL-12α (but not IL-12β), and TNF-α (supplemental Figure 1B). Custom PCR arrays (Figure 2C) revealed the loss of CD11c and CD14 mRNA, consistent with the rapid disappearance of CD14 via flow cytometry. The mRNAs encoding the following cDC marker proteins were strongly up-regulated: GM-CSF, CD141, CLEC9A, CCR7, CD83, and DC-LAMP. By contrast, mRNA encoding CLEC4C, pDC marker protein CD303, CXCR4, and CD1c demonstrated modest or no significant changes.
Flow cytometry and cytometric bead assay confirmed that virus infection induced TNF-α and GM-CSF (Figure 2D; supplemental Figure 1C). We then found that surface CD141 and intracellular CLEC9A, but not surface CD1c or CLEC4C, were strongly up-regulated (Figure 2E), which suggests that the phenotype of virus-induced DCs resembles a blood CD141+ DC subset rather than a blood CD1c+ DC or CLEC4C+ pDC subset.1 To determine to what extent virus-induced DCs also resembled classic GM-CSF plus IL-4 monocyte–derived DCs, we performed a side-by-side comparison of DCs generated with IAV infection versus classic cytokine addition. Again, high levels of surface CD83 and CD141 and intracellular CLEC9A were observed in virus-induced DCs (supplemental Figure 1D). By contrast, high levels of surface CD209 were only found in GM-CSF plus IL-4 monocyte–derived cells, which clearly indicates the differential nature of DCs generated with infection versus cytokine addition. Microarray analysis further revealed that virus-infected cells up-regulated CD141+ DC subset–related IRF8 and BATF3 and down-regulated monocyte lineage-related TLR4 (supplemental Figure 1E). Microarray data were deposited in NCBI Gene Expression Omnibus with accession #GSE35473.
We examined the ability of purified infected versus uninfected monocytes to trigger autologous purified CD4+ T cells (containing anti-IAV T cells) to secrete IFN-γ on addition of UV-inactivated IAV after 7 days of coculture. VSV-infected monocytes alone did not trigger IFN-γ secretion, which demonstrates the antigen specificity of T-cell activation. Without monocyte addition, purified CD4+ T cells failed to produce IFN-γ, which functionally confirms the purity of CD4+ T cells. VSV infection of monocytes resulted in a dose-dependent increase (up to 3-fold) in IFN-γ production (Figure 2E) in parallel with its increase in monocyte-to-DC differentiation.
Taken altogether, the present data demonstrate that virus infection induces the differentiation of monocytes into DCs with a rapidity unprecedented in the literature.
It has been reported that GM-CSF, TNF, IL-15, and IFN-α, all of which demonstrated increases in mRNA in virus-infected cells in the present study, induce monocyte differentiation into DCs.3-5 In addition, virus infection down-regulates mRNA expression of macrophage colony-stimulating factor receptor, which promotes macrophage differentiation from monocytes,4 and CD14 mRNA, which agrees with the CD14-low phenotype of virus-induced DCs.
The selective infectivity of monocytes with IAV among PBMCs is remarkable. Many investigators have reported influenza viremia, the potential for blood-borne influenza transmission, and the need for blood screening for influenza.6,7 It is likely that IAV is present in the blood during human infection and promotes DC differentiation from blood monocytes. In line with this possibility, it would be interesting to examine the potential increase in blood DCs during human infection, with the caveat that DCs may rapidly exit the blood and thereby avoid detection. The resistance of lymphocytes to viral infections demonstrates that blood cell types can severely limit viral infection. As nonhuman viruses, VSV and VV are not expected to evolve specific tropism for human monocytes. This suggests that monocytes evolved to be infected by a variety of viruses, possibly to enhance antigen presentation by enabling endogenous antigen presentation.
The dramatic increase in surface CD141 and intracellular CLEC9A expression on virus-induced DCs is intriguing. CD141 and CLEC9A are related markers for recently identified human blood CD141+ DCs, the equivalent of mouse CD8+ DCs, which are adept at cross-presentation and have been shown to potentiate cytotoxic T-lymphocyte responses.8-13 Contrary to its surface expression on blood CD141+ DCs, CLEC9A is only detected within virus-induced DCs, which suggests a phenotype difference between blood CD141+ and virus-induced DCs. Hence, further studies are required to determine whether or not the changes observed are specific to virus-induced DCs, as well as the relationship of virus-induced DCs with blood CD141+ DCs. Nevertheless, virus-induced DCs could have significant clinical implications in the development of antiviral strategies for therapy and vaccines.
We presume that virus-induced DCs principally function as APCs and immune regulators after emigrating into immune organs. The up-regulation of CXCR4 and particularly CCR7 mRNA is likely involved in DC egress, because these receptors are strongly implicated in emigration of mature blood DCs to lymph nodes.14,15 It appears likely that viral infection of tissue monocytes will similarly result in DC differentiation that could potentially drain via lymph to nodes where they participate in T-cell activation.
In summary, the present data reveal a novel, rapid, virus-induced monocyte-to-DC differentiation pathway that can potentially be exploited for rational vaccine design and antiviral therapeutics.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Department of Transfusion Medicine at the NIH Clinical Center for providing PBMCs; Glennys Reynoso and Eugene Shenderov for assistance with irradiation experiments; Clarmyra Hayes for assistance with cytometric bead array assay; and Drs Timothy Myers, Paul Gardina, and Qin Su for performing microarray experiments and analyzing microarray data.
This work was generously supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (J.R.B. and J.W.Y.) and the National Institutes of Health (R.L.M.).
National Institutes of Health
Authorship
Contribution: W.H., R.L.M., J.R.B., and J.W.Y. designed and interpreted the experiments; W.H., J.S.G., X.L., C.B.B., and D.R. performed the experiments; and W.H., R.L.M., and J.W.Y. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Jonathan W. Yewdell, Bldg 33, Rm 2E13C, 33 North Dr, NIH, Bethesda, MD 20892; e-mail: jyewdell@nih.gov.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal