Signal transduction, which broadly refers to communicating information from the plasma membrane to the nucleus, plays an essential role in regulating the size and composition of hematopoietic populations. Deregulated signal transduction leading to abnormal numbers of immature and differentiated cells is a hallmark of MPN. Somatic mutations in genes encoding signaling molecules are common in the neoplastic cells of patients with MPN, and expressing many of these proteins in primary mouse cells recapitulates the corresponding human diseases.3 These data and the impressive clinical activity of targeted kinase inhibitors in some MPNs definitively established cause-and-effect by linking expression of mutant proteins identified in MPN to the underlying hematologic phenotypes. The mutations found in MPNs frequently alter relatively “proximal” components of signaling networks. From a clinical perspective, there are many examples of strong genotype/phenotype correlations such as the associations of NRAS and CBL mutations with chronic and juvenile myelomonoytic leukemias, of JAK2V617F with polycythemia vera (PV), essential thrombocytosis, and myelofibrosis, and of the BCR-ABL fusion with chronic myeloid leukemia (CML) and high-risk B lineage acute lymphoblastic leukemia (ALL).
On ligand binding, JAK2 trans-phosphorylates tyrosine residues on hematopoietic growth factor receptors that lack intrinsic receptor tyrosine kinase activity such as the erythropoietin (EPO) and granulocyte-macrophage colony stimulating factor receptors (see figure). These modifications create docking sites for regulatory molecules and ultimately lead to the activation of multiple effectors including SHP-2, Src family kinases, Ras, and phosphoinositide-3-kinase (PI3K). JAK2 also directly phosphorylates STAT5, which translocates to the nucleus and induces the transcription of multiple target genes, many of which serve antiapoptotic functions.4 Transduction and transplantation of a constitutively activated Stat5 allele results in multilineage leukemia in mice; however, STAT5 mutations have not been reported in human cancer. Yan and coworkers made use of a conditional Jak2V617F “knockin” mutation that accurately models many aspects of PV when activated by the Mx1-Cre transgene,5 in the setting of an elegant allele generated in the Henninghausen laboratory in which loxP sites were inserted 110 kb apart to permit simultaneous deletion of Stat5a and Stat5b loci on Cre recombinase expression.6 The authors intercrossed these strains to generate Mx1-Cre, Jak2V617F mice that were also homozygous for the targeted Stat5a/5b mutation and then simultaneously induced JAK2V617F protein expression and deleted Stat5a/5b. Genetic ablation of Stat5a/5b completely abrogated all signs of PV-like MPNs, including myelofibrosis. This impressive hematologic response was associated with normalization of progenitor populations and loss of EPO-independent erythroid colony growth, which is a hallmark of PV. Restoring STAT5 expression in hematopoietic cells from compound mutant mice rescued the ability of Jak2V617F to induce MPNs, and in vitro experiments showed that Stat5a/5b-deficent bone marrow remained susceptible to transformation by oncogenic KrasG12D. Yan and coworkers also found that ablating Stat5a/5b reduced p70S6 kinase phosphorylation as well as Bcl-XL, cyclin D2, and Pim-1 expression in Mx1-Cre, Jak2V617F mice, suggesting that one or more of these proteins might contribute to the transformed phenotype. Walz et al used a retroviral transduction system and the same strain of Stat5a/5b conditional mutant mice to demonstrate that loss of STAT5 expression greatly attenuated, but did not eliminate, disease in an established model of Jak2V617F-induced MPN.2 The phenotypic differences identified in these studies likely reflect differential effects of endogenous levels of JAK2V617F expression compared with higher levels in the retroviral model. Thus, STAT5 is an essential effector of JAK2V617F-induced MPN in vivo.
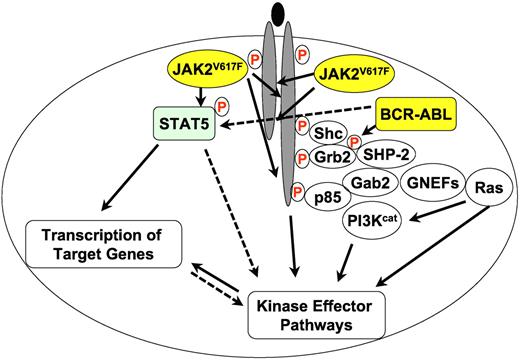
A greatly simplified overview of JAK2V617F and BCR-ABL (yellow circles) signaling in MPN. Filled lines indicate known biochemical interactions and dotted lines indicate possible interactions. Protein phosphorylation is indicated by a circled red “P.” Other proteins are shown as open circles. PI3Kcat designates the catalytic subunits of PI3K proteins and GNEFs are guanine nucleotide exchange factors for Ras.
A greatly simplified overview of JAK2V617F and BCR-ABL (yellow circles) signaling in MPN. Filled lines indicate known biochemical interactions and dotted lines indicate possible interactions. Protein phosphorylation is indicated by a circled red “P.” Other proteins are shown as open circles. PI3Kcat designates the catalytic subunits of PI3K proteins and GNEFs are guanine nucleotide exchange factors for Ras.
Hoelbl and colleagues have shown that retrovirally expressed v-Abl cannot initiate and maintain MPN in the absence of STAT5 function.7,8 In studies that employed two complementary approaches to somatically inactivate Stat5a/b, Walz et al reached a similar conclusion with respect to the inability of BCR-ABL to induce MPN in cells lacking STAT5 expression.2 They also observed a clear effect of Stat5a/b gene dosage on disease phenotype with aggressive MPN emerging rapidly in the context of normal levels of STAT5, and a mixed pattern of MPN and B lineage ALL seen in recipients that received cells lacking one Stat5a/b allele. By contrast, efficient homozygous inactivation of Stat5a/b abolished the ability of BCR-ABL to cause MPN. These authors also found that STAT5 is not absolutely required for BCR-ABL to induce abnormal growth of B lineage precursors in vitro or to cause B-ALL in vivo. Interestingly, it is known that mutating a Grb2 binding site in BCR-ABL (Y177) or inactivating the Gab2 adaptor abrogates BCR-ABL–induced MPN in mice, but many animals ultimately develop B lineage leukemogenesis.9 Consistent with these observations in mice transplanted with BCR-ABL–expressing cells, pharmacologic inhibition of MEK profoundly reduces MPN in Kras mutant mice, but some animals ultimately die of T-cell leukemia despite continuous treatment.10 Together, these data suggest that inhibiting aberrant signaling networks has a profound effect on MPN, but does not fully suppress oncogene-induced lymphoid leukemia.
Mouse models that accurately recapitulate human malignant neoplasms can direct novel therapeutic approaches. While a number of JAK2 inhibitors have been evaluated in patients with myelofibrosis, the clinical benefits observed to date have been modest relative to what has been observed in MPNs treated with BCR-ABL and PDGFR inhibition.11,12 Ineffective inhibition of JAK2 kinase activity is one possible explanation for these lackluster results. While this problem might be overcome by developing more potent and selective inhibitors, sustained inhibition of normal JAK2 activity could also be associated with unacceptable toxicity. Alternatively, it is possible that STAT5 may become reactivated in the malignant clone despite effective ongoing JAK2 inhibition. The ability to directly antagonize STAT5 would be appealing under either scenario. The evolving data implicating STAT5 activity as essential for the survival of JAK2V617F- and BCR-ABL–driven MPN has led to considerable interest in clinically combining inhibitors of JAK2 and BCR-ABL in CML. However, how STAT5 is phosphorylated in CML cells is a central unresolved question, and data suggest that JAK2-independent phosphorylation of STAT5 may predominate in the context of BCR-ABL expression. For example, SRC (which may or may not be downstream of JAK2) can phosphorylate STAT5 in CML cells.13 More recently, Hantschel et al reported that BCR-ABL can efficiently phosphorylate STAT5 in vitro, and showed that pharmacologic JAK2 inhibition does not contribute substantially to STAT5 dephosphorylation in CML cell lines.14
Taken together, the existing data suggest that therapeutics that directly target STAT5 hold great therapeutic promise. As a dimeric transcription factor, STAT5 poses substantial challenges for drug development. Interestingly, however, Nelson et al recently identified the approved neuroleptic agent pimozide in a high-throughput screen as an inhibitor of STAT5 phosphorylation and an inducer of apoptosis in CML cell lines.15 The underlying mechanism of action is unknown. Targeting the SH2 domain of STAT5 with small molecules is an alternative approach that is currently being pursued. In addition to directly targeting STAT5 itself, therapeutic efficacy might also result from inhibiting mediator(s) of activated STAT5. In this regard, the observation of Yan et al that the elevated levels of phosphorylated p70S6 kinase in cells expressing JAK2V617F are dependent on STAT5 expression1 is provocative as it raises the possibility that inhibitors of JAK2 and the TORC complex might act synergistically. Although the absence of somatic mutations has allowed STAT5 to masquerade as an innocent bystander, mounting evidence that it is a coconspirator in the pathogenesis of MPNs raises exciting new therapeutic possibilities.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal