Abstract
Burkitt lymphoma (BL) predominates in pediatric patients, whereas diffuse large B-cell lymphoma (DLBCL) is uncommon. In contrast to adults, BL and DLBCL are treated similarly in children and both entities have superior outcomes in children compared with adults. Gene expression profiling (GEP) and miRNA expression profiling clearly differentiated pediatric DLBCL from BL, forming distinct clusters regardless of patient age. However, pathway analysis of GEP data identified minor differences between corresponding pediatric and adult tumors. Predominance (6:1) of the germinal center B-cell subtype to activated B-cell subtype was found among pediatric DLBCL. Two cases were molecularly classified as primary mediastinal B-cell lymphoma. We observed frequent abnormalities in 8q24 in pediatric DLBCL, including MYC rearrangement in 31% (5 of 16) and gain or amplification in 50% (6 of 12) nonrearranged cases. MYC rearrangement was present in 96% (23 of 24) BL cases. Array-based CGH analysis identified abnormalities that are shared between adult and pediatric DLBCL (+12q15, +19q13, −6q), and abnormalities unique to the pediatric cases (−4p14, −19q13.32, +16p11.2), suggesting distinct pathogenetic mechanisms relative to age. Elucidation of the underlying target genes may provide insight into factors that modulate outcome and could provide potential novel therapeutic targets with less toxicity for pediatric patients with B-cell non-Hodgkin lymphoma.
Introduction
Lymphoma is the third most frequent type of cancer in children, accounting for approximately 15% of childhood malignancy. The incidence of lymphoma varies from only 3% in children younger than 5 years to 24% in 15 to 19 year olds.1-3 In children, non-Hodgkin lymphoma (NHL) consists predominantly of mature aggressive B-cell lymphomas, with Burkitt lymphoma (BL) being most common in 5 to 14 year olds and diffuse large B-cell lymphoma (DLBCL) predominating in 15 to 19 year olds.1,3 Pediatric BL and DLBCL are treated uniformly with short but high-intensity multiagent chemotherapy regimens designed for BL. Both entities have superior outcomes relative to adults, with overall survival (OS) rates greater than 90%.1,4-8 Despite these advances, intensive chemotherapy is associated with significant morbidity, and more targeted, pathway-specific therapeutic approaches are desirable.8,9 Although adult BL is also treated with a high-intensity regimen, adult DLBCL is treated with R-CHOP or CHOP-like regimens.10,11 The prognosis of adult DLBCL remains significantly worse than DLBCL in children, but it is unclear whether this is because of the ability of children to better tolerate intensive treatment or whether distinct pathogenetic mechanisms modulate disease outcome.
BL and DLBCL are recognized by the World Health Organization (WHO) as separate entities having distinct genetic alterations, tumor morphology, and immunophenotype. However, there is significant overlap in the defining features of BL and DLBCL in some cases, resulting in a group of unclassifiable lymphomas with features intermediate between BL and DLBCL.12 Compared with adults, pediatric DLBCL shares more features with BL, including high proliferation, increased MYC expression, decreased BCL2 expression, higher incidence of MYC translocation, and germinal center (GC) phenotype (75%).13,14 Delineation of homogeneous groups of BL and DLBCL to help identify tumor-specific characteristics therefore remains challenging. Gene expression profiling (GEP) has been used to more precisely classify BL and DLBCL molecularly.15,16 Using GEP-defined groups of molecular BL (mBL), 2 previous studies found no differences in gene expression or DNA copy number alterations (CNAs) between pediatric and adult mBL, despite clinical differences between these 2 groups.17-19 Comparisons of GEP and CNA between adult and pediatric DLBCL has not been reported, however.
Genome-wide miRNA profiling has also been used to molecularly define different types of lymphoma. Using 6 BL cases, 1 study identified miRNAs differentially expressed in BL relative to chronic lymphocytic leukemia, mantle cell lymphoma, and follicular lymphoma.20 A 9-miRNA signature was also found to differentiate the activated B-cell (ABC) and GC B-cell (GCB) subtypes of DLBCL.21 Through coordination of array CGH and miRNA expression data, Li et al identified 63 miRNA that are deregulated in DLBCL by recurrent copy number (CN) changes.22 These studies underscore the contribution of miRNA deregulation in lymphoma pathogenesis and the potential utility of miRNA profiling in classifying tumors. However, miRNA profiles that distinguish BL and DLBCL are still unavailable, and profiling of pediatric lymphomas has not been reported.
DLBCL is a heterogeneous group of entities both clinically and biologically, and includes the GCB and ABC subtypes, which can be defined molecularly by GEP.23-25 After multiagent chemotherapy, with or without rituximab, patients with the GCB subtype have a significantly better OS compared with those with the ABC subtype.23,26 Primary mediastinal large B-cell lymphoma (PMBL) shares morphologic features with DLBCL but is now recognized as a distinct entity that shares some features of classical Hodgkin lymphoma.27 In contrast with other DLBCL subtypes, therapeutic outcomes are worse for children with PMBL compared with adult patients.28,29 By immunohistochemistry, pediatric DLBCL was shown to consist predominantly of the GCB subtype,13,14 which may account for the favorable prognosis in this age group. However, a GEP-based molecular classification of pediatric DLBCL is currently lacking.
In this study, we sought to characterize pediatric BL and DLBCL molecularly using GEP and miRNA analysis, and to determine whether differences in these signatures exist between pediatric and adult tumors. Using homogeneous, molecularly defined cohorts, we also examined whether differences in genetic alterations or molecular pathways between adult and pediatric tumors may explain the clinical differences and provide insight into distinct pathogenetic mechanisms.
Methods
Patient characteristics
Pediatric specimens were collected from the Cooperative Human Tissue Network pediatric NHL repository through the Children's Oncology Group, and adult specimens were collected from the Nebraska Lymphoma Study Group Registry and Tissue Bank. Pediatric patients were defined using a cut-off of 20 years of age or younger and adult patients were defined as older than 20 years. Frozen tissues were obtained from 57 pediatric BL (ages 2-20 years; median, 8 years), 13 pediatric DLBCL (ages 9-18 years; median, 15 years), 26 adult BL (ages 21-85 years; median, 66 years), and 98 adult DLBCL (ages 22-87 years; median, 60 years). Clinical data were available on all adult and 36 pediatric patients. Pathology review of the pediatric cases was done by T.C.G. and W.C.C. using available materials, which included institutional pathology reports and hematoxylin and eosin slides, and adult cases were reviewed by a panel of Leukemia/Lymphoma Molecular Profiling Project pathologists. GEP was done on all pediatric (n = 70) and adult (n = 124) cases. This study was reviewed and approved by the institutional review board at University of Nebraska Medical Center.
GEP
Frozen sections were cut from each of the cryopreserved blocks and examined for adequacy of the materials before other studies. Genomic DNA and total RNA were isolated by All prep DNA/RNA Mini Kit (QIAGEN). GEP was done by Human Genome U133 Plus Version 2.0 array (Affymetrix) and analyzed by BRB-ArrayTools Version 3.7 software (http://linus.nci.nih.gov/BRB-ArrayTools.html),30 as previously described.23 Molecular classification of cases was by the Bayesian compound covariate predictor method25 using a published gene expression signature distinguishing BL from DLBCL,15 as described previously.31 A second published gene signature that distinguishes mBL from other mature aggressive B-cell lymphomas16 was used to confirm the BL and DLBCL molecular classifications. Cases classified as molecular DLBCL were then subclassified into ABC, GCB, and PMBL subtypes of DLBCL.23,27 Gene set enrichment analysis was used to compare pediatric (n = 45) with adult (n = 17) mBL, and pediatric (n = 13) with adult (n = 51) GCB mDLBCL using the Curated Gene Set in the Broad Institute's Molecular Signature Database.
SNP array analysis of DNA CNAs and copy neutral LOH
Genomic DNA (250 ng) was prepared from all 21 pediatric mDLBCL specimens according to the GeneChip Mapping 500K Assay protocol for hybridization on 250K NspI Human Mapping Arrays (Affymetrix). Single nucleotide polymorphism (SNP) genotypes, CN data, and regions of copy neutral loss of heterozygosity (LOH) were generated using Genotyping Console Version 2.1 software from Affymetrix, as previously described.31 CNAs were aligned and the minimal common region (MCR) for each recurrent abnormality was determined. CNA occurring in more than 10% of cases were selected for further analysis. MCRs that were devoid of genes or that showed complete overlap with an annotated CNV of similar CN state were excluded. Gene expression data for all RefSeq genes residing in an MCR were compared with gene CN using the class comparison tool in BRB ArrayTools. The criterion used to define differential gene expression between CNA+ and CNA− groups was P < .05 under the random variance model univariate test.
Analysis for MYC gene rearrangement
Interphase FISH analysis for chromosome 8q24 (MYC) translocations was performed using cryostat tissue sections, as previously described with minor modification.32 Briefly, a MYC break-apart probe (Abbott-Vysis) was used for hybridization. Nuclei were counterstained with 4,6-diamidino-2-phenylindole in Antifade solution, and the slides were visualized using an Olympus BX51 fluorescence microscope. Images were captured and archived using CytoVision Version 4.5.2 software (Applied Imaging). A total of 50 to 100 nuclei per case were scored for the presence of the MYC translocation. The normal cutoff for this FISH assay in tissue sections has been established to be 15% by prior studies.
miRNA isolation and profiling
Total RNA for miRNA profiling was extracted from four 20μM sections (based on 1 cm2 surface area) of cryopreserved tissues using the mirVana miRNA isolation Kit according to the manufacturer's instructions (Ambion). Reverse transcription was done with 300 ng of total RNA using the Megaplex RT Primers and enzyme kit as suggested by the manufacturer (ABI). To enhance assay sensitivity, a preamplification step of 12 cycles was introduced using Megaplex PreAmp Primers. The preamplified cDNA was loaded onto the 384-well format TaqMan microRNA assay plates (TaqMan human miRNA array Version 2.0, ABI). Quantitative real-time PCR was performed on a 7900HT Fast Real-Time PCR System (ABI). Threshold cycle (CT) was defined as the fractional cycle number at which the fluorescence exceeds the fixed threshold of 0.1 with automatic baseline using the RQ Manager Version 1.2 software (ABI). The raw data were uploaded into BRB-ArrayTools (Version 3.7.0) for analysis. Using the expression of all miRNA, classification of BL and DLBCL cases was done using the compound covariate Bayesian predictor, in which specimen labels were assigned by the GEP-based molecular classifier. Classification precisions were evaluated using leave-one-out cross-validation. Differential miRNA expression between groups was determined by random-variance T test and significance-analysis-of-microarrays (SAM).
Clinical correlations
Fisher exact test was used to analyze categorical data, and Wilcoxon rank-sum test was used to analyze continuous data between groups. Fisher exact test was used to examine the association between age and GEP predictor in DLBCL. Posthoc tests were adjusted for multiple comparisons using the Bonferroni method. The Kaplan-Meier method was used to estimate overall survival distributions, and the log-rank test was used to compare survival distributions between groups. SAS Version 9.3 software was used for data analysis (SAS Institute).
Results
Patient characteristics and molecular classification
Table 1 summarizes the pathologic and molecular classifications and characteristics of the 70 pediatric patients. The 57 pediatric BL patients, as determined by pathology, were predominantly male (> 80%) and had a median age at diagnosis of 8 years. The 13 pediatric DLBCL patients by pathology were also mostly male and had a median age at diagnosis of 15 years, which was significantly different from the BL median age (P < 5 × 10−5). For patients with a concordant molecular and pathologic diagnosis, median age at diagnosis was 8 years for mBL and 15 years for mDLBCL. However, mBL cases with a discrepant DLBCL pathologic diagnosis had a median age at diagnosis of 13 years, significantly different from other mBL (P = .02). Similarly, mDLBCLs with discrepant BL pathology had a median age at diagnosis of 6 years, significantly different from other mDLBCLs (P = .0001). Supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) illustrates the significant differences in OS between pediatric and adult cases defined molecularly by GEP. Pediatric DLBCL and BL had OS of 100% and 90%, respectively. As expected, adult DLBCL, whether treated with CHOP or R-CHOP, and adult BL treated aggressively had a significantly decreased OS relative to the pediatric cases.6 Five cases of DLBCL from the Nebraska Lymphoma Study Group Registry and Tissue Bank were younger than 20 years but were treated with a CHOP-like regimen similar to adult DLBCL. Interestingly, these cases had a better OS relative to adult DLBCL patients who were treated similarly.
Classification of pediatric patients
| Pathologic diagnosis . | BL (n = 57) . | DLBCL (n = 13) . |
|---|---|---|
| Median age at diagnosis, y (range) | 8 (2-20) | 15 (9-18) |
| Sex | ||
| Female | 11 | 3 |
| Male | 46 | 10 |
| Molecular diagnosis* | ||
| mBL | 45 | 2† |
| mDLBCL | 10† | 11 |
| NC | 2 | 0 |
| Pathologic diagnosis . | BL (n = 57) . | DLBCL (n = 13) . |
|---|---|---|
| Median age at diagnosis, y (range) | 8 (2-20) | 15 (9-18) |
| Sex | ||
| Female | 11 | 3 |
| Male | 46 | 10 |
| Molecular diagnosis* | ||
| mBL | 45 | 2† |
| mDLBCL | 10† | 11 |
| NC | 2 | 0 |
NC indicates nonclassifiable cases with a diagnostic probability of < 90% by Bayesian classification.
Molecular diagnosis is the Bayesian classification of cases using the Burkitt Lymphoma Gene Signature derived from Dave et al.15
Case with a molecular classification that was discrepant with the pathologic diagnosis.
Using the Bayesian prediction method, we applied a GEP-based molecular classifier to the pediatric cases that robustly distinguishes BL and DLBCL in adult lymphomas.15 The predictor identified 47 mBLs and 21 mDLBCLs with probability more than 99% (Table 1; Figure 1A). Two cases had probabilities intermediate between BL and DLBCL and were not classifiable molecularly. Approximately 80% of all pediatric cases had a molecular classification consistent with the pathologic diagnosis; however, 10 morphologically defined BLs were reclassified as mDLBCL and 2 morphologically defined DLBCLs were reclassified as mBL. To confirm the case reclassifications, we used a second, independently derived gene signature that distinguishes mBL from other mature aggressive B-NHL.16 Cases classified as mBL or mDLBCL were concordant between the 2 gene signatures; however, a higher number of cases were unclassifiable (ie, probability < 90%) by Hummel classifier,16 with the majority of these cases showing probability of more than 70% versus more than 90% by our classifier for the same entity.15
Gene expression profiling of BL and DLBCL. (A) Molecular classification of pediatric lymphomas using the Bayesian compound covariate predictor method and a published gene signature that distinguishes BL from DLBCL.15 GEPs of adult lymphomas used to derive this signature were used as a training (GEO accession no. GSE4732) dataset for the Bayesian predictor. Pediatric lymphomas with a probability ≥ 90% were classified accordingly. (B) Hierarchical clustering of adult (n = 21) and pediatric (n = 49) BL and adult (n = 102) and pediatric (n = 21) DLBCL cases using the gene expression signature used in panel A demonstrated robust distinction of BL and DLBCL regardless of patient age. Pediatric and adult specimens intermingled and did not form distinct subclusters. (C) Hierarchical clustering of BL and DLBCL using 35 significantly differentially expressed miRNAs between BL and DLBCL. Cases clustered by entity regardless of patient age. Two adult DLBCL cases clustered with BL and one pediatric BL case clustered with DLBCL. A separate cluster of DLBCL (far right) demonstrated high expression of both the BL and DLBCL signatures.
Gene expression profiling of BL and DLBCL. (A) Molecular classification of pediatric lymphomas using the Bayesian compound covariate predictor method and a published gene signature that distinguishes BL from DLBCL.15 GEPs of adult lymphomas used to derive this signature were used as a training (GEO accession no. GSE4732) dataset for the Bayesian predictor. Pediatric lymphomas with a probability ≥ 90% were classified accordingly. (B) Hierarchical clustering of adult (n = 21) and pediatric (n = 49) BL and adult (n = 102) and pediatric (n = 21) DLBCL cases using the gene expression signature used in panel A demonstrated robust distinction of BL and DLBCL regardless of patient age. Pediatric and adult specimens intermingled and did not form distinct subclusters. (C) Hierarchical clustering of BL and DLBCL using 35 significantly differentially expressed miRNAs between BL and DLBCL. Cases clustered by entity regardless of patient age. Two adult DLBCL cases clustered with BL and one pediatric BL case clustered with DLBCL. A separate cluster of DLBCL (far right) demonstrated high expression of both the BL and DLBCL signatures.
Subtype classification of the 21 pediatric mDLBCL specimens was done using 2 gene signatures: a predictor that distinguishes GCB and ABC subtypes,23 and a PMBL gene signature.27 These classifiers identified 13 GCB, 2 ABC, 2 PMBL, and 4 nonclassifiable cases (Table 2). Both PMBL cases were female adolescents with a mediastinal mass and amplification of the chromosome 2p REL locus, as detected by array comparative genomic hybridization. A higher proportion of the GCB subtype relative to the ABC subtype was found, predominantly in the discrepant cases (ie, those classified as BL by pathology and mDLBCL by GEP). The supervised hierarchical clustering of all pediatric and adult cases showed 2 distinct clusters of BL and DLBCL patients regardless of patient age (Figure 1B). The pediatric and adult cases intermixed and did not form subclusters, suggesting strong similarity of the respective pediatric and adult tumors at the gene expression level.
Molecular subtype classification of pediatric DLBCL
| Pathologic diagnosis* . | Molecular diagnosis† . | |||
|---|---|---|---|---|
| GCB . | ABC . | PMBL . | NC . | |
| DLBCL | 5 | 1 | 2 | 3 |
| BL | 8 | 1 | 0 | 1 |
| Total | 13 | 2 | 2 | 4 |
| Pathologic diagnosis* . | Molecular diagnosis† . | |||
|---|---|---|---|---|
| GCB . | ABC . | PMBL . | NC . | |
| DLBCL | 5 | 1 | 2 | 3 |
| BL | 8 | 1 | 0 | 1 |
| Total | 13 | 2 | 2 | 4 |
NC indicates nonclassifiable cases with a diagnostic probability of < 90% by Bayesian classification.
Pathologic diagnosis is the specimen diagnosis based on pathology review provided by the Cooperative Human Tissue Network.
Differential gene expression and pathway enrichment in adult and pediatric lymphomas
Using molecular classification, a sufficient number of pediatric GCB mDLBCL (n = 13) and mBL (n = 45) cases were available for comparison with adult GCB mDLBCL (n = 51) and adult mBL (n = 17) cases. SAM analysis identified only 132 differentially expressed genes between adult and pediatric GCB mDLBCL (supplemental Table 1; supplemental Figure 2) and 63 differentially expressed genes between adult and pediatric mBL (supplemental Table 2; supplemental Figure 3). Gene set enrichment analysis identified pathways and gene signatures that differed significantly between pediatric and adult tumors (Table 3). Comparison of adult and pediatric GCB mDLBCL revealed enrichment for B-cell surface molecules and GC markers in adult GCB cases. Genes within these pathways that were up-regulated in adult GCB relative to pediatric GCB included CD19, CD20, CD40, CD52, CD72, CD79a, CD79b, CXCR5, and BLNK. Ingenuity analysis found further enrichment for pathways involved in inflammation and altered T- and B-cell signaling in adult relative to pediatric GCB mDLBCL. Enrichment for apoptosis pathways, BCR signaling, and GC markers, G1 to S cell cycle transition, BCL2 family, and PIP3, p38 MAPK, and IL-10 signaling pathways were found in adult mBL relative to pediatric mBL. Interestingly, p38 MAPK signaling has been shown to up-regulate IL-10 gene expression in BL cells, in turn promoting lymphomagenesis.33 In contrast, pediatric mBL showed enrichment for inflammatory pathways and MYC targets relative to adult mBL tumors.
Differential pathway and gene set enrichment in adult and pediatric tumors
| Broad GeneSets . | No. of genes . | P (Goeman global test) . |
|---|---|---|
| Enrichment in Adult mGCB relative to Ped mGCB | ||
| BASSO_GERMINAL_CENTER_CD40_UP | 225 | .023 |
| TH1 TH2 PATHWAY | 28 | .009 |
| CELL_CYCLE_CHECKPOINT_II | 21 | .022 |
| CTLA4 PATHWAY | 41 | .009 |
| TARTE_BCELL | 71 | .024 |
| Enrichment in Adult mBL relative to Ped mBL | ||
| APOPTOSIS_KEGG | 118 | .0003 |
| BRENTANI_DEATH | 176 | .002 |
| APOPTOSIS_GENMAPP | 99 | .001 |
| APOPTOSIS | 168 | .001 |
| CASPASE PATHWAY | 54 | .0005 |
| SA_FAS_SIGNALING | 27 | .002 |
| DEATH PATHWAY | 86 | .002 |
| BAD PATHWAY | 60 | .004 |
| P38 MAPK PATHWAY | 104 | .009 |
| ST_P38_MAPK_PATHWAY | 99 | .02 |
| IL10 PATHWAY | 31 | .001 |
| IL18 PATHWAY | 10 | .001 |
| RAS PATHWAY | 53 | .016 |
| AKT PATHWAY | 32 | .026 |
| ST_JAK_STAT_PATHWAY | 23 | .003 |
| KIM_TH_CELLS_UP | 115 | .007 |
| ST_TUMOR_NECROSIS_FACTOR_PATHWAY | 67 | .009 |
| G1_TO_S_CELL_CYCLE_REACTOME | 142 | .008 |
| SIG_BCR_SIGNALING_PATHWAY | 139 | .005 |
| BCR PATHWAY | 98 | .019 |
| SIG_PIP3_SIGNALING_IN_B_LYMPHOCYTES | 97 | .003 |
| BASSO_GERMINAL_CENTER_CD40_UP | 225 | .004 |
| Enrichment in Pediatric mBL relative to Adult mBL | ||
| INFLAMMATORY_RESPONSE_PATHWAY | 70 | .002 |
| MYC_TARGETS | 109 | .0006 |
| Broad GeneSets . | No. of genes . | P (Goeman global test) . |
|---|---|---|
| Enrichment in Adult mGCB relative to Ped mGCB | ||
| BASSO_GERMINAL_CENTER_CD40_UP | 225 | .023 |
| TH1 TH2 PATHWAY | 28 | .009 |
| CELL_CYCLE_CHECKPOINT_II | 21 | .022 |
| CTLA4 PATHWAY | 41 | .009 |
| TARTE_BCELL | 71 | .024 |
| Enrichment in Adult mBL relative to Ped mBL | ||
| APOPTOSIS_KEGG | 118 | .0003 |
| BRENTANI_DEATH | 176 | .002 |
| APOPTOSIS_GENMAPP | 99 | .001 |
| APOPTOSIS | 168 | .001 |
| CASPASE PATHWAY | 54 | .0005 |
| SA_FAS_SIGNALING | 27 | .002 |
| DEATH PATHWAY | 86 | .002 |
| BAD PATHWAY | 60 | .004 |
| P38 MAPK PATHWAY | 104 | .009 |
| ST_P38_MAPK_PATHWAY | 99 | .02 |
| IL10 PATHWAY | 31 | .001 |
| IL18 PATHWAY | 10 | .001 |
| RAS PATHWAY | 53 | .016 |
| AKT PATHWAY | 32 | .026 |
| ST_JAK_STAT_PATHWAY | 23 | .003 |
| KIM_TH_CELLS_UP | 115 | .007 |
| ST_TUMOR_NECROSIS_FACTOR_PATHWAY | 67 | .009 |
| G1_TO_S_CELL_CYCLE_REACTOME | 142 | .008 |
| SIG_BCR_SIGNALING_PATHWAY | 139 | .005 |
| BCR PATHWAY | 98 | .019 |
| SIG_PIP3_SIGNALING_IN_B_LYMPHOCYTES | 97 | .003 |
| BASSO_GERMINAL_CENTER_CD40_UP | 225 | .004 |
| Enrichment in Pediatric mBL relative to Adult mBL | ||
| INFLAMMATORY_RESPONSE_PATHWAY | 70 | .002 |
| MYC_TARGETS | 109 | .0006 |
miRNA profiling
Expression profiles of 380 miRNAs were generated from a representative sample of 46 adult mDLBCL, 12 pediatric mDLBCL, 13 adult mBL, and 18 pediatric mBL specimens that were selected based on gene expression-defined molecular classification. Comparison of mBLs and mDLBCLs identified 35 differentially expressed miRNAs (supplemental Table 3). The 35 miRNA signature identified by SAM analysis included 15 miRNAs highly expressed in mBLs relative to mDLBCLs (BL signature), and 20 miRNA highly expressed in mDLBCLs relative to mBLs (DLBCL signature). The BL signature is dominated by MYC-regulated miRNAs, including the miR-17-92 cluster, along with the paralogous cluster on the X chromosome (miR-18b, miR-20b, and miR-106a). A subset of mDLBCL cases (20 of 58) showed high expression of the BL miRNA signature, suggesting high MYC or E2F activity. Hierarchical clustering of these 35 miRNAs segregated BL and DLBCL into 2 major distinct clusters, with each cluster containing an admixture of both pediatric and adult mBL or mDLBCL cases (Figure 1C). Of 89 cases, only 2 adult DLBCLs clustered with the BL cases and only one pediatric BL clustered with DLBCL cases, confirming that these are 2 distinct entities regardless of patient age. A subset of DLBCL cases, both pediatric and adult, expressed high levels of both the DLBCL and BL miRNA signatures, forming a distinct cluster of DLBCLs at the far right of Figure 1C. Comparison of miRNA expression between pediatric and adult tumors by SAM analysis (FDR = 0.1) revealed no significant differences in DLBCL and only a single significant miRNA in BL, whereby miR-9 expression was 5-fold lower in pediatric BL relative to adult BL.
MYC gene rearrangement analysis
FISH analysis was performed on all molecularly reclassified and concordant DLBCL cases that we had sufficient materials for examination. The results are shown in Table 4. Of the concordant cases, one of 7 had MYC rearrangement, whereas the discordant cases showed 4 of 9 evaluable cases with rearrangement. Gain or amplification affecting the MYC locus was frequent, affecting 3 of 5 concordant and 3 of 6 discordant cases without MYC rearrangement. Gain of chromosome 8 is rare in BL and pediatric DLBCL,19,34 indicating that these changes were not the result of whole chromosome gains. Thus, abnormalities of the MYC locus were frequent in these pediatric DLBCL cases with rearrangement in a total of 5 of 16 and gain or amplification in 6 of 11 nonrearranged cases. FISH and cytogenetic studies were performed on 26 cases of BL with 24 cases having evaluable results. Of these, 23 cases (96%) had MYC rearrangement. The remaining case showed amplification of the 8q24 locus.
Myc rearrangement and gain/amplification in pediatric DLBCL
| Case ID . | Pathologic diagnosis . | Molecular diagnosis by GEP . | Age, y . | Sex . | t(8;14) . | 8q24 CN . | |
|---|---|---|---|---|---|---|---|
| BL vs DLBCL . | DLBCL subgroup . | ||||||
| P01 | DLBCL | DLBCL | GCB | 9 | M | − | 3-4 |
| P02 | DLBCL | DLBCL | GCB | 10 | M | − | 3-5 |
| P03 | DLBCL | DLBCL | GCB | 13 | M | − | 3-4 |
| P04 | DLBCL | DLBCL | GCB | 18 | F | − | ND |
| P05 | DLBCL | DLBCL | PMBL | 15 | F | − | 2 |
| P06 | DLBCL | DLBCL | UC | 11 | M | − | 2 |
| P07 | DLBCL | DLBCL | UC | 18 | M | + | 3-4 |
| P08 | BL | DLBCL | ABC | 2 | M | + | 2 |
| P09 | BL | DLBCL | UC | 11 | F | + | 3 |
| P10 | BL | DLBCL | GCB | 6 | M | + | ND |
| P11 | BL | DLBCL | GCB | 4 | F | + | 2 |
| P12 | BL | DLBCL | GCB | 10 | M | − | 2 |
| P13 | BL | DLBCL | GCB | 10 | F | − | 3-4 |
| P14 | BL | DLBCL | GCB | 2 | M | − | 2 |
| P15 | BL | DLBCL | GCB | 5 | M | − | 3 |
| P16 | BL | DLBCL | GCB | 6 | M | ND | Amplification |
| P17 | BL | DLBCL | GCB | 12 | M | − | ND |
| Case ID . | Pathologic diagnosis . | Molecular diagnosis by GEP . | Age, y . | Sex . | t(8;14) . | 8q24 CN . | |
|---|---|---|---|---|---|---|---|
| BL vs DLBCL . | DLBCL subgroup . | ||||||
| P01 | DLBCL | DLBCL | GCB | 9 | M | − | 3-4 |
| P02 | DLBCL | DLBCL | GCB | 10 | M | − | 3-5 |
| P03 | DLBCL | DLBCL | GCB | 13 | M | − | 3-4 |
| P04 | DLBCL | DLBCL | GCB | 18 | F | − | ND |
| P05 | DLBCL | DLBCL | PMBL | 15 | F | − | 2 |
| P06 | DLBCL | DLBCL | UC | 11 | M | − | 2 |
| P07 | DLBCL | DLBCL | UC | 18 | M | + | 3-4 |
| P08 | BL | DLBCL | ABC | 2 | M | + | 2 |
| P09 | BL | DLBCL | UC | 11 | F | + | 3 |
| P10 | BL | DLBCL | GCB | 6 | M | + | ND |
| P11 | BL | DLBCL | GCB | 4 | F | + | 2 |
| P12 | BL | DLBCL | GCB | 10 | M | − | 2 |
| P13 | BL | DLBCL | GCB | 10 | F | − | 3-4 |
| P14 | BL | DLBCL | GCB | 2 | M | − | 2 |
| P15 | BL | DLBCL | GCB | 5 | M | − | 3 |
| P16 | BL | DLBCL | GCB | 6 | M | ND | Amplification |
| P17 | BL | DLBCL | GCB | 12 | M | − | ND |
The abnormal range for MYC rearrangement is 15%-100%. The abnormal range for MYC amplification is 10%-100%. The abnormal range for multiple copies of 8q24 is 10%-100%.
UC indicates unclassifiable DLBCL; and ND, not determined.
Chromosomal imbalances in pediatric lymphoma
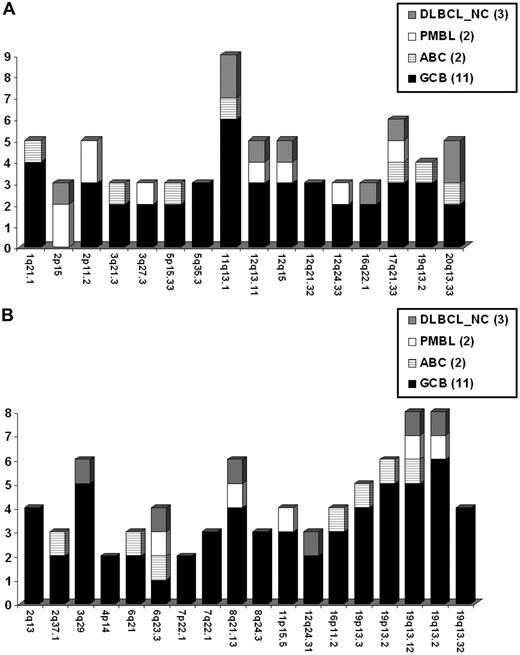
High-quality SNP data (SNP call rate ≥ 85) for DNA CN analysis were obtained from 18 of 21 pediatric cases of mDLBCL, including 11 GCB, 2 ABC, 2 PMBL, and 3 nonclassifiable mDLBCL (mDLBCL-NC). To determine the spectrum of CNAs in pediatric DLBCL, MCRs were compiled for aberrations occurring in 2 or more cases and included 16 recurrent gains (Figure 2A) and 18 recurrent losses (Figure 2B). Supplemental Figure 2 illustrates the genome wide aberrations for pediatric mDLBCL cases, coded by molecular subtype. Both PMBL cases harbored a gain of 2p that included the REL and BCL11A genes. Large aberrations previously found in DLBCL34,35 were also observed in the pediatric cases, including: 6q−, +7/7q+, +12, 17p−, and 17q+ (supplemental Figure 2). Losses of 19q13.32 and 4p14 minimal regions are novel and have not been reported in other DLBCL series. Recurrent regions of copy neutral LOH were also observed at 1q, 2, 6p, 9p, and 19p.
Pediatric mDLBCL DNA copy number alterations. Recurrent DNA CN gains (A) and losses (B) detected by array CGH in molecularly defined pediatric DLBCL. The cytoband is listed for each MCR occurring in 2 or more patient samples. Frequency of aberration is coded according to the molecular subtype classification.
Pediatric mDLBCL DNA copy number alterations. Recurrent DNA CN gains (A) and losses (B) detected by array CGH in molecularly defined pediatric DLBCL. The cytoband is listed for each MCR occurring in 2 or more patient samples. Frequency of aberration is coded according to the molecular subtype classification.
Supplemental Table 4 lists all RefSeq genes residing within each minimal region. Genes highlighted in bold demonstrated significant correlation between DNA CN and gene expression. However, because of small sample size and the heterogeneous groups of cases, only a small number of genes showed clear correlation of expression with gene CN. Extended MCRs, which included an additional 1 Mb on either side of the minimal region, were also examined. Table 5 lists candidate genes for select extended MCRs based on putative gene/protein function and/or correlation of gene expression with DNA CN (bolded genes).
Locus-specific impact of DNA CN on gene expression for candidate genes within select MCRs
| Cytoband . | Locus description . | Genes* . | . | . | . | . |
|---|---|---|---|---|---|---|
| Gains | ||||||
| 1q21.1 | MCL1 | OTUD7B | PIAS3 | BCL9 | NOTCH2NL | |
| 2p15 | Amplified in PMBL35 | ACTR2 | PELI1 | SLC1A4 | REL | BCL11A |
| 3q21.3 | CNBP | GATA2 | MCM2 | PIK3R4 | ||
| 3q27.3 | BCL6 | RFC4 | ST6GAL1 | TBCCD1 | ||
| 11q13.1 | BBS1 | ANKRD13D | RELA | ZNHIT2 | CCND1 | |
| 12q15 | Adult GCB35 | FRS2 | RAB3IP | IFNG | MDM1 | MDM2 |
| 16p11.2 | Novel in pediatric DLBCL | FUS | MAZ | BCL7C | CD19 | NFATC2IP |
| 19q13.2 | Adult ABC35 | AKT2 | LYPD4 | PAK4 | CD79A | NFKBIB |
| Losses | ||||||
| 2q13 | SEPT10 | BCL2L11 | ||||
| 4p14 | Novel in pediatric DLBCL | APBB2 | RHOH | TLR1/6/10 | ||
| 6q21 | Adult ABC35 | ATG5 | AIM1 | FOXO3 | LIN28B | PRDM1 |
| 6q23.3 | HBS1L | BCLAF1 | PERP | IFNGR1 | TNFAIP3 | |
| 12q24.31 | RHOF | BCL7A | DIABLO | |||
| 16p11.2 | TAOK2 | NFATC2IP | BCL7C | CD19 | TP53TG3 | |
| 19p13.3 | RFX2 | EBI3 | TNFAIP8L1 | TNFSF14 | TNFSF9 | |
| 19q13.32 | Novel in pediatric DLBCL | BAX | BBC3 | BCL3 | IRF2BP1 |
| Cytoband . | Locus description . | Genes* . | . | . | . | . |
|---|---|---|---|---|---|---|
| Gains | ||||||
| 1q21.1 | MCL1 | OTUD7B | PIAS3 | BCL9 | NOTCH2NL | |
| 2p15 | Amplified in PMBL35 | ACTR2 | PELI1 | SLC1A4 | REL | BCL11A |
| 3q21.3 | CNBP | GATA2 | MCM2 | PIK3R4 | ||
| 3q27.3 | BCL6 | RFC4 | ST6GAL1 | TBCCD1 | ||
| 11q13.1 | BBS1 | ANKRD13D | RELA | ZNHIT2 | CCND1 | |
| 12q15 | Adult GCB35 | FRS2 | RAB3IP | IFNG | MDM1 | MDM2 |
| 16p11.2 | Novel in pediatric DLBCL | FUS | MAZ | BCL7C | CD19 | NFATC2IP |
| 19q13.2 | Adult ABC35 | AKT2 | LYPD4 | PAK4 | CD79A | NFKBIB |
| Losses | ||||||
| 2q13 | SEPT10 | BCL2L11 | ||||
| 4p14 | Novel in pediatric DLBCL | APBB2 | RHOH | TLR1/6/10 | ||
| 6q21 | Adult ABC35 | ATG5 | AIM1 | FOXO3 | LIN28B | PRDM1 |
| 6q23.3 | HBS1L | BCLAF1 | PERP | IFNGR1 | TNFAIP3 | |
| 12q24.31 | RHOF | BCL7A | DIABLO | |||
| 16p11.2 | TAOK2 | NFATC2IP | BCL7C | CD19 | TP53TG3 | |
| 19p13.3 | RFX2 | EBI3 | TNFAIP8L1 | TNFSF14 | TNFSF9 | |
| 19q13.32 | Novel in pediatric DLBCL | BAX | BBC3 | BCL3 | IRF2BP1 |
Genes highlighted in bold showed a significant correlation between gene expression and gene CN. Locus description annotates MCR in the pediatric cases that were either novel or that have been previously reported to be enriched in specific DLBCL subtypes.
Discussion
In this study, we performed molecular profiling of a cohort of pediatric aggressive B-cell NHLs, including 57 BL and 13 DLBCL defined by morphology. Pediatric patients with NHL have a significantly better prognosis than adult patients with the same histologic subtype,8 a finding that was also observed in this study (supplemental Figure 1). Between the ages of 15 and 29 years, the predominant histologic subtype shifts from BL to DLBCL, transitioning from a childhood to an adult NHL spectrum.1,3 Despite this shift in incidence, adolescent DLBCL patients in the 15- to 20-year age range fare better when treated with more aggressive regimens.4,7 Accordingly, 5 DLBCL cases within the 15- to 20-year age range in this study had been treated similar to adult DLBCL patients, receiving a CHOP-like regimen, and showed significantly decreased OS relative to pediatric DLBCL receiving aggressive therapy (supplemental Figure 1). Additional cases need to be studied to discern whether this reflects a true change in biology or merely a better prognosis with younger age and more aggressive treatment.
Identifying a homogeneous patient population is important in studying underlying tumor biology, yet the distinction between BL and DLBCL remains a diagnostic challenge. The 2008 WHO classification includes a provisional category of B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and BL, to account for the significant number of cases with morphologic, immunophenotypic and cytogenetic features intermediate between BL and DLBCL.12 In contrast to adults, pediatric DLBCL have been reported to demonstrate moderate to high proliferation rates (83%), increased MYC protein expression (84%), a higher incidence of MYC translocation (37%),36 and an increased frequency of the GC phenotype (75%), suggesting greater similarity between the molecular features of DLBCL and BL in pediatric cases.13 GEP has been used to more precisely classify BL and DLBCL both in adults15,16,37 and children.17-19,34 Using our molecular signatures to distinguish BL and DLBCL, we observed an approximate 20% reclassification rate for both morphologic BL (10 of 57 mDLBCL) and DLBCL (2 of 13 mBL). We also examined our cases with the Hummel classifier,16 and cases classified as mBL or mDLBCL are in complete concordance with our cases, but there are more unclassifiable cases using their classifier. The relatively high rate of reclassification for childhood cases may be the result of an increase in cases with overlapping features in pediatric BL and DLBCL, particularly the higher incidence of MYC deregulation and increased proliferation in childhood DLBCL. MYC rearrangement studies indeed demonstrated a higher incidence of rearrangement in childhood DLBCL than adult cases, consistent with Poirel et al,34 findings that showed Myc rearrangement in 33% of pediatric DLBCLs.34 In addition, there was also a high incidence of gain or amplification in the MYC locus supporting that deregulated expression of the MYC gene may be a prominent feature of pediatric DLBCL. The study of pediatric DLBCL and BL by Klapper et al reclassified a large number of cases, but there was more reclassification of DLBCL into mBL than in the reverse direction.17 There was also a lower incidence of MYC rearrangement in the mDLBCL cases. The differences with our series could be partly the result of the different tendency of classification by pathologists of the 2 tumors in the United States. The incidence of MYC rearrangement is higher in our mDLBCL cases, which may be partly related to the limitations inherent to the rather small number of cases in both series. There were also a larger number of unclassifiable cases in the series by Klapper et al,17 and some of these cases could have been classified into a specific category by our diagnostic algorithm. These issues need to be addressed in the future by studying a large series of cases using uniform techniques and criteria. MYC gene was rearranged in the majority (96%) of mBL cases as expected. Similar incidence has been observed in Poirel et al34 and Klapper et al.17
DLBCL cases reclassified as molecular BL showed high expression of MYC and MYC targets, high expression of “BL-high” GCB genes,15 and low expression of MHC-I and NF-κB target genes. Unsupervised hierarchical clustering divided mBL and mDLBCL cases into 2 distinct clusters, providing further support for these classifications. Pediatric and adult cases were intermixed, demonstrating significant overlapping features within each entity regardless of patient age. These data show that molecular classification provides a robust means of diagnosing BL and DLBCL, particularly in pediatric cases.
By immunohistochemical classification, pediatric DLBCL cases show a predominance of the GCB subtype; however, unlike adult GCB DLBCL, the t(14;18) translocation is reported to be rare or absent.13,14 In a subsequent study, Klapper et al were not able to demonstrate the predominance of the GCB subtype in their series of DLBCL,17 but, using a GEP signature that distinguishes the GCB and ABC subtypes of DLBCL,23 we found a 3:1 predominance of the GCB subtype in pediatric mDLBCL by gene expression. However, too few cases were available to ascertain whether the GCB subtype predominance accounts for the favorable outcome in pediatric relative to adult DLBCL. Two female pediatric PMBL cases were also identified using a gene signature that distinguishes PMBL from the other DLBCL subtypes.27 Amplification of the REL locus is frequently seen in PMBL38 and was found in both pediatric cases by array comparative genomic hybridization.
Comparative analysis of the GEP of childhood and adult DLBCL has not previously been undertaken. A sufficient number of pediatric GCB cases (n = 13) were available in this study for comparison with adult GCB mDLBCL. At the gene expression level, pediatric and adult GCB DLBCL were highly homogeneous, differing in only 132 transcripts by SAM analysis (supplemental Table 1). Pathway analysis revealed enrichment in expression of B-cell surface molecules, genes involved in BCR signaling, and altered T- and B-cell signaling pathways in adult relative to pediatric GCB DLBCL. Enhanced antigen-independent B-cell signaling and/or pathway alterations, which facilitate tonic BCR signaling, may be important in B-cell survival in adult DLBCL. Pediatric DLBCL may be less dependent on BCR signaling, suggesting alterations in pathways that compensate for this. FOXO1-dependent PI3K signaling, downstream of the BCR, was recently shown to promote survival of BCR-deficient mature B cells.39 In GCB DLBCL, constitutive or “tonic” BCR survival signals through BCL6 in the absence of receptor stimulation have been shown.40,41 Further studies on BCR signaling in pediatric DLBCL would be of interest, as too few pediatric cases were available in the present study to ascertain preferential activation of PI3K or the alternative NF-κB pathways in pediatric relative to adult GCB DLBCL.
Genome-wide miRNA expression profiling demonstrated distinct expression profiles for mDLBCL and mBL patients. Pediatric and adult cases, including the reclassified cases, were indistinguishable by miRNA expression profiling, further supporting the close relationship between adult and pediatric tumors (Figure 1C). One-third of mDLBCL cases also exhibited high expression of many of the miRNA characteristically expressed by BL. Many of these miRNAs are contributed by the miR17∼92 cluster that is up-regulated by MYC and certain other transcription factors, such as E2F1.42 These may represent mDLBCL cases with higher expression of MYC and/or other factors that up-regulate miR17∼92. Segregation of mDLBCL into distinct subgroups by miRNA expression has been previously reported, with marked differences in MYC-regulated miRNAs between groups.22 Consistent with previous reports,20 a number of miRNAs prominently repressed in mBL relative to mDLBCL were found, including miR-22, 23a, 29a, 29b, 29c, 34a, 125b, 150, 155, and 342. Some of these are known MYC targets,43 and their repression is probably related to high MYC activity. However, this group of miRNAs tends not to show the marked repression, even in mDLBCL cases with moderate high expression of miRs up-regulated in BL. It is possible that down-regulating the MYC miRNA targets have different requirements that are not present in the mDLBCL, so these miRs remain elevated in these cases (MYC needs corepressors to repress gene expression and inhibitors of MYC may also be present to modulate its activities). In the DLBCL signature, many of the highly expressed miRNAs were previously reported to be differentially expressed between DLBCL and GCB cells, with high miR-222 expression correlating with poor overall and progression-free survival.21 High miR-125b expression may have functional relevance in DLBCL through down-regulation of PRDM1 expression.21 Diminished miR-155 expression in BL43 and elevated miR-155 expression in DLBCL21 have been reported and were found to differentiate these 2 entities in the present study. Characterization of the targets of the differentially expressed miRNAs may further aid in the classification of these 2 entities and in understanding the different biologies.
A second aim of this study was to identify genetic aberrations in pediatric mDLBCL using high-resolution array comparative genomic hybridization, and to compare these alterations with those previously found in adult tumors because such a comparison has not previously been reported. Poirel et al reported karyotype abnormalities in pediatric DLBCL treated on the FAB LMB 96 trial.34 In that report, Poirel et al34 reported +1q, del(13q), +7q, der(3q), der(9p), del(17p), der(18q), +12q, der (11q), and del(6q), in children and adolescents with DLBCL. Similarities with the current array CGH study were demonstrated with losses at 3q and 6q and gains at 1q and 12q. However, the current array CGH study was significantly more precise in identifying many more gains and losses compared with the previous karyotype study in pediatric DLBCL.34 Specific aberrations reported in adult DLBCL were also found in the pediatric cases, including gain of the 2p15 REL locus reported in PMBL; gain of 12q15, enriched in the GCB subtype of adult DLBCL; and gain of 19q13.2 and loss of 6q21, enriched in the ABC subtype of adult DLBCL.35 Our analysis was more focused on the GCB subtype because it constitutes the majority of pediatric DLBCL. Two novel deletions were found in the pediatric cases: −4p14 and −19q13.32. The 19q13.32 locus harbors the BAX and BBC3 tumor suppressor genes. Whereas deletion of chromosome 4 and the 4p arm has been reported in lymphoma, a minimal region has not been delineated. Deletion of 4p14 was resolved to approximately 283 kb in this study. The RHOH gene in this interval is expressed in T and B cells and is required for lymphocyte receptor signaling.44 RHOH is also a target of aberrant somatic hypermutation in B cells in DLBCL and other B-cell leukemias and lymphomas.45 Expression of the APBB2 gene was significantly diminished in the 4p14 deleted cases relative to nondeleted cases. Consistent with a tumor suppressor role, APBB2 is proapoptotic and a regulator of NF-κB, p53, and WWOX,46 suggesting a potential functional role in lymphomagenesis.
There have been many discussions regarding the reasons underlying the better prognosis of pediatric versus adult aggressive B-cell lymphomas,3,32 be related to the intensive chemotherapy regimens generally used in childhood cases, to age-related factors, or to intrinsic differences in the biology between pediatric and adult tumors. We have demonstrated the predominance of the GCB subgroup in pediatric mDLBCL, which may partly explain the better prognosis, in particular for tumors not expressing BCL2.47 Although pediatric BL and DLBCL were found to be similar at the molecular level compared with the corresponding adult tumors, we identified subtle differences in gene expression and genetic alterations in this study, suggesting underlying differences in tumor biology between childhood and adult cases. Some genetic abnormalities seem to be unique to the pediatric mDLBCL (−4p14, −19q13.32, +16p11.2), and elucidation of the underlying target genes may provide insight into factors that may modulate outcome. However, we are uncertain of their significance in influencing survival at this time. Further studies, such as next-generation sequencing, may help to answer these important questions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Martin Bast for the clinical data coordination, Lisa Bough for technical assistance, and Pamela Althof and Jennifer Sanmann for technical assistance with FISH experiments.
This work was supported in part by the National Cancer Institute (grant 5U01/CA114778), National Institutes of Health (NIH; grant U01/CA84967), and Eppley Core Grant. The University of Nebraska Medical Center Microarray Core Facility is supported in part by the NIH (grant P20 RR016469) from the INBRE Program of the National Center for Research Resources. Research is also supported in part by the Chair's Grant (grant U10 CA98543-08) and Statistics and Data Center (grant U10 CA98413-08) of the Children's Oncology Group from the National Cancer Institute, NIH.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the NIH. A complete listing of grant support for research conducted by CCG and POG before initiation of the Children's Oncology Group grant in 2003 is available online at http://www.childrensoncologygroup.org/admin/grantinfo.htm.
National Institutes of Health
Authorship
Contribution: K.E.D., J.I., Y.S., C.L., Z.L., and Y.L. performed experiments; W.S. performed FISH analysis; K.E.D., J.I., L.S., and J.L. analyzed the data and interpreted the results; M.S.L., S.L.P., M.S.C., and T.G.G. contributed valuable tissues and clinical information; K.F., L.M.S., L.M.R., E.J., A.R., G.K.O., J.D., E.C., R.D.G., D.D.W., T.C.G., and W.C.C. provided tissues and performed pathologic analysis of the specimens; K.E.D., J.I., and W.C.C. wrote the manuscript; W.C.C. designed, supervised, and provided support for the study; and all authors approved of the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wing C. Chan, Center for Research in Lymphoma and Leukemia, Department of Pathology and Microbiology, 983135 Nebraska Medical Center, Omaha, NE 68198-3135; e-mail: jchan@unmc.edu.
References
Author notes
K.E.D. and J.I. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal