Abstract
The discovery of the JAK2V617F mutation in most patients with Ph-negative myeloproliferative neoplasms has led to the development of JAK2 kinase inhibitors. However, JAK2 inhibitor therapy has shown limited efficacy and dose-limiting hematopoietic toxicities in clinical trials. In the present study, we describe the effects of vorinostat, a small-molecule inhibitor of histone deacetylase, against cells expressing JAK2V617F and in an animal model of polycythemia vera (PV). We found that vorinostat markedly inhibited proliferation and induced apoptosis in cells expressing JAK2V617F. In addition, vorinostat significantly inhibited JAK2V617F-expressing mouse and human PV hematopoietic progenitors. Biochemical analyses revealed significant inhibition of phosphorylation of JAK2, Stat5, Stat3, Akt, and Erk1/2 in vorinostat-treated, JAK2V617F-expressing human erythroleukemia (HEL) cells. Expression of JAK2V617F and several other genes, including GATA1, KLF1, FOG1, SCL, C/EPBα, PU.1, and NF-E2, was significantly down-regulated, whereas the expression of SOCS1 and SOCS3 was up-regulated by vorinostat treatment. More importantly, we observed that vorinostat treatment normalized the peripheral blood counts and markedly reduced splenomegaly in Jak2V617F knock-in mice compared with placebo treatment. Vorinostat treatment also decreased the mutant allele burden in mice. Our results suggest that vorinostat may have therapeutic potential for the treatment of PV and other JAK2V617F-associated myeloproliferative neoplasms.
Introduction
Myeloproliferative neoplasms (MPNs) are a group of clonal hematopoietic malignancies that include chronic myeloid leukemia (CML), polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF).1,2 These diseases are characterized by excessive proliferation of myeloid/erythroid lineage cells. A somatic point mutation (V617F) in the JAK2 tyrosine kinase has been found in most patients with PV and in 50%-60% patients with ET and PMF.3-6 JAK2V617F is a constitutively active tyrosine kinase that can transform factor-dependent hematopoietic cell lines into cytokine-independent cells.3,4 Expression of the JAK2V617F mutant activates multiple downstream signaling pathways, such as Stat, Erk, and PI3K/Akt pathways.3,7,8
Current therapies for MPNs include phlebotomy and myelosuppressive therapy (eg, hydroxyurea and anagrelide) for PV and ET and transfusions and supportive care for PMF. However, these empiric treatments are unlikely to cure or offer remission to patients with MPNs, so there is a clear need for new therapies for MPNs. The discovery of the JAK2V617F mutation in PV, ET, and PMF has led to the development of inhibitors of JAK2. Several JAK2 inhibitors are undergoing clinical trials. Although JAK2 inhibitors are effective in reducing splenomegaly and improving constitutional symptoms, significant hematopoietic toxicities, including anemia and thrombocytopenia, are observed in the majority of patients after this treatment,9,10 which is consistent with the known function of JAK2 in normal hematopoiesis.11,12 Ruxolitinib, a JAK1/JAK2 inhibitor, has been approved for the treatment of myelofibrosis. However, a recent report on long-term outcomes with Ruxolitinib treatment found improvement in constitutional symptoms, but no significant benefit in survival for myelofibrosis patients.13 In addition, there is an increased rate of discontinuation of Ruxolitinib therapy because of severe hematopoietic toxicities or lack of response.13 It is also possible that drug resistance may emerge in some patients treated with JAK2 inhibitors, similar to what is observed with the ABL inhibitor imatinib in CML patients.14 Therefore, identifying additional new therapies targeting JAK2V617F or pathways downstream of JAK2V617F would be beneficial for the treatment of patients with MPNs.
Acetylation is an important posttranslational modification that serves as a key modulator of chromatin structure and gene transcription, and provides a mechanism for coupling extracellular signals with gene expression.15 This process is regulated by 2 classes of enzymes, the histone acetyltransferases and the histone deacetylases (HDACs), which catalyze the acetylation or deacetylation of histones, respectively. Inhibition of HDAC activity has been linked to hematopoietic stem cell proliferation and self-renewal.16-20 Aberrant acetylation of histones and other cellular proteins has been found in leukemia, lymphoma, and solid tumors.15,21 Pharmacologic inhibition of HDACs has shown promise in treating hematologic malignancies and other forms of cancer.15,21
Several HDAC inhibitors, including trichostatin A (TSA), valproic acid, depsipeptide, vorinostat, ITF2357 (givinostat), and panobinostat, have been shown to cause death of cancer cells in vitro and in vivo.15,21-24 Vorinostat (also known as SAHA or Zolinza), a small-molecule inhibitor of class I and II HDACs, has been shown to induce growth arrest and to promote apoptosis of a variety of cancer cells15,21,25,26 and is a Food and Drug Administration–approved drug for the treatment of refractory cutaneous T-cell lymphoma.27 Vorinostat has also demonstrated activity against leukemias and solid tumors in preclinical and phase 1 clinical studies.15,21,28,29 Increased HDAC activity has been found in patients with PMF.30 In vitro treatment of PMF CD34+ cells with 5-azacytidine plus TSA or vorinostat resulted in a significant decrease in the proportion of JAK2V617F homozygous colonies and a marked reduction of JAK2V617F+ SCID-repopulating cells.23,31 Moreover, a beneficial effect of HDAC inhibition was observed in a patient with JAK2V617F+ advanced myelofibrosis.32 Other HDAC inhibitors, including ITF2357 (givinostat) and panobinostat, also showed potent antiproliferative and proapoptotic activity against murine and human cells expressing JAK2V617F.24,33 Therefore, inhibition of HDAC could be useful in treating MPNs. In the present study, we tested the efficacy of vorinostat in an animal model of Jak2V617F+ MPN.7 We reported earlier that expression of Jak2V617F in knock-in mice reproducibly produced all the features of human PV.7 We have used this Jak2V617F knock-in mouse model to test the in vivo effects of vorinostat in the present study. Our results show that vorinostat treatment improves peripheral blood counts significantly and attenuates splenomegaly in knock-in mice expressing Jak2V617F.
Methods
Reagents and Abs
Vorinostat was kindly provided by Merck. Polyinosine-polycytosine (pI:pC) was from Amersham/GE Healthcare. p-JAK2, p-Stat5, p-Stat3, p-Akt, p-Erk1/2, JAK2, and caspase-3 Abs were from Cell Signaling Technologies; acetyl histone H4 Ab was from Millipore; acetyl tubulin Ab was from Enzo Life Sciences; poly(adenosine diphosphate)-ribose polymerase (PARP) Ab was from BD Pharmingen; Stat5, Stat3, Akt, Erk2, c-Myc, and Pim-1 Abs were from Santa Cruz Biotechnology; and HSP90 Ab was from StressGen Biotechnologies.
Cell cultures
HEL, K562 and murine BA/F3-EpoR-JAK2V617F cells were maintained in RPMI-1640 medium with 10% FBS plus penicillin/streptomycin. UKE-1 cells were maintained in RPMI-1640 medium plus penicillin/streptomycin supplemented with 10% FBS, 10% donor horse serum, and 1μM hydrocortisone. Primary erythroblasts were generated from the BM of MxCre;Jak2V617F/+ mice as described previously.34
Cell proliferation and viability assays
Viable cells were plated at 3000 cells/well (for BA/F3-EpoR-JAK2V617F cells) or 10 000 cells/well (for HEL, UKE-1, and K562 cells) in 96-well plates. Cells were treated with DMSO or vorinostat (0.0625-1.0μM) for 48 hours. Cell proliferation was measured with the WST assay using a Quick Cell Proliferation Assay Kit (BioVision).
For cell viability measurement, 5 × 105 cells were cultured in RPMI-1640 medium with 10% FBS in the presence of DMSO or vorinostat (0.5 or 1.0μM). Viable cell number was assessed by trypan blue exclusion every 24 hours for 5 days.
Apoptosis and cell-cycle analysis
The number of apoptotic cells was determined by annexin V staining. The cells were treated with DMSO or vorinostat for 24 hours, harvested, stained with allophycocyanin-conjugated annexin V and propidium iodide according to the manufacturer's instructions (eBiosciences), and then analyzed by flow cytometry.
For cell-cycle analysis, cells were treated with DMSO or 1.0μM vorinostat for 24 hours, fixed in 100% ethanol, and stained with propidium iodide. Cells were recorded using an LSRII flow cytometer and FACS Diva Version 6.2 software (BD Biosciences). The cell-cycle distribution was analyzed with ModFit software (Verity).
Colony-forming assay
BA/F3-EpoR-JAK2V617F, HEL, and K562 cells were plated (250 cells/dish) in duplicates in methylcellulose medium (M3234; StemCell Technologies) without any cytokine in the presence of DMSO or vorinostat (0.5 or 1.0μM), and colonies were counted on day 5. To detect erythropoietin (EPO)–independent CFU-erythroid (CFU-E) colonies, spleen cells from MxCre;Jak2V617F/+ mice were plated (1 × 105 cells/dish) in duplicate in methylcellulose medium (M3234; StemCell Technologies) without any cytokine in the presence of DMSO or vorinostat (0.5 or 1.0μM). CFU-E colonies were counted 2 days after staining with benzidine solution (Sigma-Aldrich).
Flow cytometry
BM and spleen cells from placebo and vorinostat-treated mice were stained with allophycocyanin-conjugated Ter119 and PE-conjugated CD71 mAbs (eBiosciences) for 20 minutes on ice. Flow cytometry was performed with an LSRII (BD Biosciences) and analyzed with FlowJo Version 8.8.6 software (TreeStar).
Immunoblotting and immunoprecipitation
For immunoblot analysis, cells were harvested after treatment with vorinostat (0.5 or 1.0μM) for 24 hours. Cells were lysed and equal amounts of proteins were separated by SDS-PAGE. Immunoblotting was performed using phosphospecific or total Abs as indicated. For loading controls, blots were reprobed with anti–total Erk2 Abs.
For immunoprecipitation, 1 mg of total protein was incubated with protein A Sepharose beads, along with either anti-Hsp90 or anti-JAK2 Ab, for 3 hours. The bound beads were washed 5 times in lysis buffer and separated by SDS-PAGE, followed by immunoblotting with anti-JAK2 or anti-Hsp90 Ab.
Quantitative real-time PCR
Total RNA was extracted from the HEL cells after treatment with vorinostat (0.5 or 1.0μM) using the RNeasy Mini Kit (QIAGEN). Reverse transcription was carried out with 200 ng of total RNA using the QuantiTect reverse transcription kit (QIAGEN). Quantitative real-time PCR for JAK2, SOCS1, SOCS3, GATA1, KLF1, FOG1, SCL, C/EBPα, PU.1, and NF-E2 was performed using the SYBR Green PCR Master mix (Applied Biosystems). The primers used for real-time PCR are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). 18S was used for normalization of the expression levels of JAK2, SOCS1, SOCS3, GATA1, KLF1, FOG1, SCL, C/EBPα, PU.1, and NF-E2. Quantitative real-time PCR was performed using a LightCycler 480 (Roche Applied Science) and analyzed with the associated software.
Primary MPN Cells
Peripheral blood samples were collected from PV and ET patients and healthy volunteers after informed consent was obtained according to the Declaration of Helsinki and the guidelines of the Institutional Review Board of the State University of New York (SUNY) Upstate Medical University. CD34+ cells were isolated using a magnetic-activated cell isolation kit (Miltenyi Biotec) according to the manufacturer's instructions. For the hematopoietic progenitor assay, purified CD34+ cells were plated (1 × 103 cells/dish) in the presence of vehicle (DMSO) or vorinostat in methylcellulose medium (H4034; StemCell Technologies) containing cytokines (SCF, G-CSF, GM-CSF, IL-3, and EPO), and colonies were counted after 14 days. In addition, CD34+ cells from JAK2V617F+ PV patients and healthy volunteers were cultured in StemSpan H3000 medium (StemCell Technologies) supplemented with cytokines to enrich for erythroid progenitor cells, as described previously.35 Primary MPN cells were then treated with DMSO or vorinostat in the presence of cytokines for 48 hours and cell proliferation was measured with the WST assay.
Animal studies with vorinostat
A conditional Jak2V617F knock-in mouse was generated as described previously.7 Expression of Jak2V617F was induced in MxCre;Jak2V617F/+ mice by injection with pI:pC.7 Peripheral blood counts were measured 4 weeks after pI:pC induction to confirm the establishment of PV disease in these mice. Vorinostat was prepared in 50% polyethylene glycol (PEG-400) solution and administered by IP injection. Two groups of MxCre;Jak2V617F/+ mice were treated: 1 group received placebo and another group received 200 mg/kg of vorinostat for 5 days in a week for a period of 2 weeks. At the study end point, mice were assessed by their peripheral blood counts. BM and spleen from placebo and vorinostat-treated mice were analyzed by flow cytometry and histopathology. To determine the effects of vorinostat on Jak2V617F allele burden, BM from pI:pC-induced Jak2V617F knock-in mice (CD45.2+) were mixed with the BM from wild-type (WT; CD45.1+) mice at a ratio of 3 to 1 (75% V617F vs 25% WT) and injected into lethally irradiated CD45.1+ recipient animals. Four weeks after transplantation, peripheral blood counts were measured to confirm the establishment of PV disease. Mice were treated with either placebo or vorinostat (200 mg/kg) for 2 weeks as described in the “Methods.” The ratio of V617F to WT progenitors was determined in the peripheral blood and the BM of the recipient animals by determining the percentage of CD45.2+ (calculated as CD45.2+/CD45.1+ plus CD45.2+) cells in the myeloid (Gr-1+) populations. All animal studies were approved by the Committee for the Humane Use of Animals of SUNY Upstate Medical University.
Blood and tissue analysis
Peripheral blood counts were measured using Hemavet 950FS (Drew Scientific). Blood smears were stained with Wright-Giemsa. For histopathologic analysis, mouse tissue specimens were fixed in 10% neutral buffered formalin and embedded in paraffin. Tissue sections (4 μm) were stained with H&E.
Statistical analysis
Results are expressed as means ± SEM, and data were analyzed by the 2-tailed Student t test. P < .05 was considered to be statistically significant.
Results
Vorinostat significantly inhibits the proliferation of cell lines expressing JAK2V617F
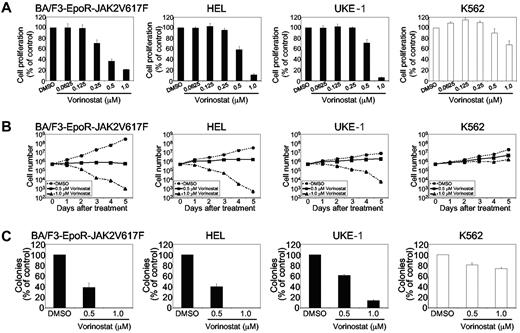
We first determined the effects of vorinostat on the proliferation of hematopoietic cell lines expressing JAK2V617F. BA/F3-EpoR cells stably expressing JAK2V617F (BA/F3-EpoR-JAK2V617F), HEL, or UKE-1 cells harboring JAK2V617F mutation, or WT JAK2-expressing BCR-ABL–transformed K562 cells were treated with various doses of vorinostat (0.0625-1.0μM) for 48 hours, and cell proliferation was assessed with the WST assay. Interestingly, the proliferation of JAK2V617F-expressing BA/F3-EpoR-JAK2V617F, HEL, and UKE-1 cells was significantly reduced after treatment with vorinostat in a dose-dependent manner (Figure 1A). In contrast, the proliferation of BCR-ABL+ K562 cells was only modestly affected by vorinostat and only at higher concentrations (Figure 1A).
Effects of vorinostat on cell proliferation and clonogenic outgrowth. (A) BA/F3-EpoR cells stably expressing JAK2V617F, HEL, and UKE-1 cells harboring the JAK2V617F mutation or BCR-ABL–transformed K562 cells expressing WT JAK2 were treated with various concentrations of vorinostat for 48 hours. Cell proliferation was measured with the WST assay. Columns represent the mean of 3 independent experiments; bars represent SEM. (B) Cells were treated with DMSO (control) or vorinostat, and viable cells were counted by trypan blue exclusion over 5 days. Results shown represent means ± SEM from 3 independent experiments. (C) BA/F3-EpoR-JAK2V617F, HEL, UKE-1, and K562 cells were left untreated or treated with vorinostat (0.5 or 1.0μM) and plated in methylcellulose medium (Methocult M3234) in the absence of cytokine. Cytokine-independent colonies were counted on day 5. Results shown represent means ± SEM from 3 independent experiments. Note that vorinostat significantly inhibited the clonogenic growth of JAK2V617F-transformed cells.
Effects of vorinostat on cell proliferation and clonogenic outgrowth. (A) BA/F3-EpoR cells stably expressing JAK2V617F, HEL, and UKE-1 cells harboring the JAK2V617F mutation or BCR-ABL–transformed K562 cells expressing WT JAK2 were treated with various concentrations of vorinostat for 48 hours. Cell proliferation was measured with the WST assay. Columns represent the mean of 3 independent experiments; bars represent SEM. (B) Cells were treated with DMSO (control) or vorinostat, and viable cells were counted by trypan blue exclusion over 5 days. Results shown represent means ± SEM from 3 independent experiments. (C) BA/F3-EpoR-JAK2V617F, HEL, UKE-1, and K562 cells were left untreated or treated with vorinostat (0.5 or 1.0μM) and plated in methylcellulose medium (Methocult M3234) in the absence of cytokine. Cytokine-independent colonies were counted on day 5. Results shown represent means ± SEM from 3 independent experiments. Note that vorinostat significantly inhibited the clonogenic growth of JAK2V617F-transformed cells.
We also tested the effects of vorinostat on cell viability. Similar to the effects on proliferation, cell viability was remarkably reduced in BA/F3-EpoR-JAK2V617F, HEL, and UKE-1 cells after vorinostat (1.0μM) treatment (Figure 1B). However, K562 cells continued to proliferate and the viable cell number was increased even after treatment with vorinostat (1.0μM) for 5 days (Figure 1B). These data demonstrate that JAK2V617F mutant–expressing cells are particularly sensitive to vorinostat treatment.
We next assessed the effects of vorinostat on the colony-forming ability of cells expressing JAK2V617F. The colony-forming assay was performed in methylcellulose medium in the absence of cytokine. Vorinostat treatment at 0.5μM reduced colony formation of JAK2V617F-expressing BA/F3-EpoR-JAK2V617F, UKE-1, and HEL cells by approximately 40%-60% (Figure 1C). Vorinostat at 1.0μM almost completely inhibited colony formation by BA/F3-EpoR-JAK2V617F, HEL, and UKE-1 cells (Figure 1C). However, vorinostat treatment resulted in only modest inhibition of colony formation of K562 cells, even at higher concentrations (1.0μM; Figure 1C). Therefore, vorinostat inhibits the clonogenic outgrowth of cells expressing JAK2V617F.
Vorinostat induces apoptosis and cell-cycle arrest in cells expressing JAK2V617F
To determine whether vorinostat-mediated inhibition of cell growth is associated with the induction of apoptosis, we performed annexin V staining after treatment of cells with various concentrations of vorinostat (0.25-1.0μM) for 24 hours. We observed that vorinostat treatment induced significant apoptosis in BA/F3-EpoR-JAK2V617F, HEL, and UKE-1 cells in a dose-dependent manner (Figure 2A). In contrast, vorinostat treatment exhibited much less of a cytotoxic effect against BA/F3-EpoR cells expressing JAK2WT or the BCR-ABL+ K562 cells (Figure 2A).
Vorinostat induces apoptosis in cells expressing JAK2V617F. BA/F3-EpoR cells expressing JAK2WT, JAK2V617F, and human HEL, UKE-1, and K562 cells were treated with DMSO or various concentrations of vorinostat (0.25-1.0μM) for 24 hours. (A) Apoptosis was determined by staining with propidium iodide and annexin V, followed by flow cytometric analysis. Results shown are representative of 3 independent experiments. Note that vorinostat treatment significantly increased apoptosis in JAK2V617F+ cells. (B) Cell-cycle distribution of HEL and K562 cells after 24 hours of exposure to vorinostat (1.0μM). Cell-cycle analysis was performed by propidium iodide staining and analyzed by ModFit software. Results from 3 independent experiments are shown as means ± SEM. Asterisks indicate significant differences (P < .05) between vehicle and vorinostat-treated cells. Note that vorinostat-treated HEL cells were arrested at the G0/G1 phase and there was a significant decrease in the percentage of cells in the S phase.
Vorinostat induces apoptosis in cells expressing JAK2V617F. BA/F3-EpoR cells expressing JAK2WT, JAK2V617F, and human HEL, UKE-1, and K562 cells were treated with DMSO or various concentrations of vorinostat (0.25-1.0μM) for 24 hours. (A) Apoptosis was determined by staining with propidium iodide and annexin V, followed by flow cytometric analysis. Results shown are representative of 3 independent experiments. Note that vorinostat treatment significantly increased apoptosis in JAK2V617F+ cells. (B) Cell-cycle distribution of HEL and K562 cells after 24 hours of exposure to vorinostat (1.0μM). Cell-cycle analysis was performed by propidium iodide staining and analyzed by ModFit software. Results from 3 independent experiments are shown as means ± SEM. Asterisks indicate significant differences (P < .05) between vehicle and vorinostat-treated cells. Note that vorinostat-treated HEL cells were arrested at the G0/G1 phase and there was a significant decrease in the percentage of cells in the S phase.
Cell-cycle analysis revealed that vorinostat (1.0μM) treatment caused a significant reduction in the number of cells in the S-phase, accompanied by an increase in the G0/G1 fraction in JAK2V617F-expressing HEL cells (Figure 2B). In contrast, vorinostat (1.0μM) treatment had no significant effect on the cell-cycle distribution in K562 cells (Figure 2B). These results suggest that vorinostat induces significant apoptosis and cell-cycle arrest in cells bearing the JAK2V617F mutation.
Vorinostat inhibits primary erythroblasts expressing JAK2V617F
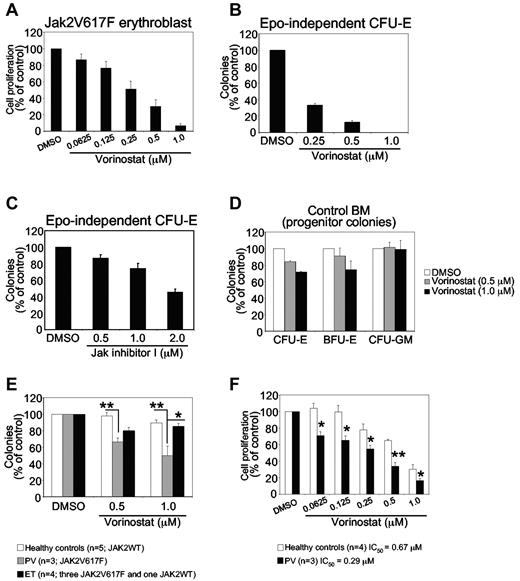
Expression of JAK2V617F in hematopoietic stem cells/progenitors results in a skewing toward erythroid lineage and an expansion of erythroid progenitors.7,36 Therefore, we investigated whether vorinostat could inhibit the growth of primary erythroblasts expressing JAK2V617F. Primary erythroblasts were derived from the BM of Jak2V617F knock-in mice as described previously.34 Jak2V617F-expressing primary erythroblasts were plated in a 96-well plate in the presence of vehicle (DMSO) or vorinostat at various concentrations (0.0625-1.0μM) for 48 hours, and proliferation was measured with the WST assay. The proliferation of murine erythroblasts expressing Jak2V617F was inhibited significantly by vorinostat in a dose-dependent manner (Figure 3A).
Effects of vorinostat on mouse and human primary hematopoietic progenitors or precursors expressing JAK2V617F. (A) Primary erythroblasts derived from the BM of Jak2V617F knock-in mice were treated with DMSO or vorinostat for 48 hours, and cell proliferation was measured with the WST assay. Note that the proliferation of erythroblasts expressing Jak2V617F was inhibited significantly by vorinostat treatment. (B-C) Spleen cells from Jak2V617F knock-in mice were plated in methylcellulose medium (Methocult M3234) without any cytokine in the presence of DMSO or vorinostat (0.25-1.0μM; B) or JAK inhibitor I (0.50-2.0μM; C). CFU-E colonies were scored after 2 days by staining with benzidine solution. Results are shown as percentage of vehicle (DMSO)–treated controls. Data are presented as means ± SEM from 3 independent experiments. (D) BM cells from a WT (control) mouse were plated in complete methylcellulose medium (Methocult M3434) in the presence of DMSO or vorinostat (0.5 or 1.0μM). CFU-E colonies were counted 2 days after plating, and BFU-E and CFU-GM colonies were counted on day 7. Results are shown as a percentage of vehicle (DMSO)–treated controls. Data are presented as means ± SEM from 3 independent experiments. Note that vorinostat treatment exhibited little or no toxicity against control BM progenitors. (E) Effects of vorinostat on MPN hematopoietic progenitors. CD34+ progenitor cells isolated from PV, ET, and normal healthy control blood samples were plated (1 × 103 cells/dish) in duplicate in the presence of DMSO or vorinostat in methylcellulose medium (H4034; StemCell Technologies) containing cytokines (SCF, G-CSF, GM-CSF, IL-3, and EPO). Hematopoietic progenitor colonies were counted after 14 days. Results are shown as means ± SEM of total colonies scored. Data are expressed as a percentage of vehicle (DMSO)–treated controls. Note that PV CD34+ progenitor cells were inhibited significantly by vorinostat treatment compared with ET or healthy control CD34+ progenitor cells. ET CD34+ progenitor cells were only modestly inhibited by vorinostat treatment compared with control progenitors. Asterisks indicate significant differences by Student t test. **P < .005; *P < .05. (F) Effects of vorinostat on the growth of primary MPN cells. Primary erythroid cells derived from PV or normal CD34+ cells were treated with DMSO or vorinostat in the presence of cytokines for 48 hours and cell proliferation was measured with the WST assay. Data are expressed as percentage of vehicle (DMSO)–treated controls. Asterisks indicate significant differences by Student t test. **P < .005; *P < .05. Note that primary erythroid cells from MPN patients were significantly more (> 2-fold) inhibited by vorinostat than were healthy control erythroid cells.
Effects of vorinostat on mouse and human primary hematopoietic progenitors or precursors expressing JAK2V617F. (A) Primary erythroblasts derived from the BM of Jak2V617F knock-in mice were treated with DMSO or vorinostat for 48 hours, and cell proliferation was measured with the WST assay. Note that the proliferation of erythroblasts expressing Jak2V617F was inhibited significantly by vorinostat treatment. (B-C) Spleen cells from Jak2V617F knock-in mice were plated in methylcellulose medium (Methocult M3234) without any cytokine in the presence of DMSO or vorinostat (0.25-1.0μM; B) or JAK inhibitor I (0.50-2.0μM; C). CFU-E colonies were scored after 2 days by staining with benzidine solution. Results are shown as percentage of vehicle (DMSO)–treated controls. Data are presented as means ± SEM from 3 independent experiments. (D) BM cells from a WT (control) mouse were plated in complete methylcellulose medium (Methocult M3434) in the presence of DMSO or vorinostat (0.5 or 1.0μM). CFU-E colonies were counted 2 days after plating, and BFU-E and CFU-GM colonies were counted on day 7. Results are shown as a percentage of vehicle (DMSO)–treated controls. Data are presented as means ± SEM from 3 independent experiments. Note that vorinostat treatment exhibited little or no toxicity against control BM progenitors. (E) Effects of vorinostat on MPN hematopoietic progenitors. CD34+ progenitor cells isolated from PV, ET, and normal healthy control blood samples were plated (1 × 103 cells/dish) in duplicate in the presence of DMSO or vorinostat in methylcellulose medium (H4034; StemCell Technologies) containing cytokines (SCF, G-CSF, GM-CSF, IL-3, and EPO). Hematopoietic progenitor colonies were counted after 14 days. Results are shown as means ± SEM of total colonies scored. Data are expressed as a percentage of vehicle (DMSO)–treated controls. Note that PV CD34+ progenitor cells were inhibited significantly by vorinostat treatment compared with ET or healthy control CD34+ progenitor cells. ET CD34+ progenitor cells were only modestly inhibited by vorinostat treatment compared with control progenitors. Asterisks indicate significant differences by Student t test. **P < .005; *P < .05. (F) Effects of vorinostat on the growth of primary MPN cells. Primary erythroid cells derived from PV or normal CD34+ cells were treated with DMSO or vorinostat in the presence of cytokines for 48 hours and cell proliferation was measured with the WST assay. Data are expressed as percentage of vehicle (DMSO)–treated controls. Asterisks indicate significant differences by Student t test. **P < .005; *P < .05. Note that primary erythroid cells from MPN patients were significantly more (> 2-fold) inhibited by vorinostat than were healthy control erythroid cells.
The expression of JAK2V617F results in EPO-independent erythroid colonies in the BM and spleens of mice and humans,7,37 a hallmark feature of PV. Therefore, we tested the effects of vorinostat on JAK2V617F-evoked, EPO-independent CFU-E colony formation in the spleens of mice expressing Jak2V617F. As shown in Figure 3B, vorinostat treatment at 0.25μM reduced EPO-independent CFU-E colonies by approximately 70%. At higher doses of vorinostat (0.5-1.0μM), EPO-independent CFU-E colonies were almost completely inhibited in the spleens of mice expressing Jak2V617F (MxCre;Jak2V617F/+; Figure 3B). Therefore, vorinostat markedly inhibited EPO-independent erythroid colony formation mediated by JAK2V617F. We also compared the effects of vorinostat with JAK inhibitor on EPO-independent erythroid colony formation mediated by JAK2V617F. Whereas vorinostat at 1μM completely inhibited EPO-independent CFU-E colony formation, treatment with JAK inhibitor I at a 2μM concentration, which almost completely inhibited JAK2 kinase activity (as described in Levine et al4 and data not shown), exhibited an approximately 50% reduction in EPO-independent CFU-E colonies in the spleens of Jak2V617F mice (Figure 3C). These results suggest that vorinostat could be more potent than JAK inhibitors in inhibiting EPO-independent erythroid colony formation mediated by JAK2V617F.
We also investigated whether vorinostat has any toxic effect on normal WT hematopoietic progenitors by comparing BM progenitors from WT mice treated with vorinostat (0.5-1.0μM) with those treated with vehicle by performing hematopoietic progenitor colony-forming assays in the presence of cytokine. Treatment with vorinostat (0.5-1.0μM) exhibited little effect on erythroid (CFU-E or blast-forming unit-erythroid [BFU-E]) and myeloid (CFU-granulocyte macrophage [CFU-GM]) colony formation in the BM of WT mice (Figure 3D). These results suggest that vorinostat preferentially inhibit the clonogenic growth of primary erythroid progenitors expressing Jak2V617F without exhibiting significant toxicity toward WT Jak2-expressing normal hematopoietic progenitors in mice.
Vorinostat inhibits the clonogenic growth of PV hematopoietic progenitors expressing JAK2V617F
We next examined the effects of vorinostat on the clonogenic growth of MPN primary hematopoietic progenitors. We isolated CD34+ cells from patients with JAK2V617F+ PV and ET and from WT JAK2-expressing healthy volunteers. Treatment with vorinostat (0.5-1.0μM) significantly reduced hematopoietic progenitor colonies in JAK2V617F+ PV CD34+ cells (Figure 3E). In contrast, no significant inhibition of hematopoietic progenitor colonies was observed in healthy control samples (Figure 3E). However, vorinostat treatment only modestly inhibited hematopoietic progenitor colonies in ET samples. Overall, the effect of vorinostat was significantly greater on hematopoietic progenitors from patients with PV than on those with ET (Figure 3E).
We also evaluated the effects of vorinostat on the growth of primary MPN cells. We cultured the PV and normal control CD34+ cells in a serum-free medium with cytokines that enrich erythroid cells.35 We found that erythroid cells from MPN patients were significantly more (> 2-fold) inhibited by vorinostat treatment than were healthy control erythroid cells (Figure 3F). Therefore, primary MPN cells are more sensitive to vorinostat than are normal cells.
Vorinostat down-regulates the expression of JAK2V617F and affects its downstream targets
We performed immunoblotting experiments to gain insight into the mechanism of inhibition of cells expressing JAK2V617F by vorinostat. Treatment with vorinostat significantly inhibited the phosphorylation of JAK2, Stat5, Stat3, Akt, and Erk1/2 in JAK2V617F+ HEL cells (Figure 4A). Immunoblotting for total JAK2 protein revealed that the expression of the JAK2 protein was significantly down-modulated by vorinostat in HEL cells (Figure 4A). Expression of Stat5, Stat3, and Akt was also reduced by vorinostat treatment in HEL cells, although Erk expression was unaffected by vorinostat treatment in these cells (Figure 4A). Immunoblotting with Abs against acetylated histone H4 and acetylated tubulin showed that vorinostat treatment resulted in hyperacetylation of both histone and nonhistone proteins in HEL cells (Figure 4A). A marked reduction in c-Myc and Pim-1 levels in JAK2V617F+ HEL cells was observed after vorinostat treatment (Figure 4B). In agreement with increased apoptosis in vorinostat-treated HEL cells, we also observed cleavage of caspase 3 and PARP in HEL cells after treatment with vorinostat (Figure 4B). These results suggest that vorinostat inhibits JAK2V617F-mutated cells by inhibiting the JAK2/Stat pathway.
Vorinostat down-regulates the expression of JAK2V617F and affects its downstream signaling. (A) HEL cells bearing the JAK2V617F mutant were treated with the indicated concentrations of vorinostat for 24 hours. Acetylation of histone H4 and tubulin was detected by anti–acetyl-histone H4 and acetyl-tubulin immunoblotting. Activation of JAK2, Stat5, Stat3, Akt, and Erk1/2 was detected by immunoblotting using phosphospecific Abs. Membranes were reprobed for total proteins. (B) HEL cells were left untreated or treated with indicated concentrations of vorinostat, and c-Myc, Pim-1, PARP, and caspase 3 levels were analyzed by immunoblotting. Levels of Erk2 served as the loading control. (C) HEL cells were treated with the indicated concentrations of vorinostat for 24 hours. Total RNA was extracted and analyzed by the quantitative real-time PCR. The relative expression of JAK2 was normalized to 18S expression. Results are shown as means ± SEM from 3 independent experiments. (D) HEL cells were treated with the indicated concentrations of vorinostat for 24 hours, and lysates were immunoprecipitated with anti-HSP90 or anti-JAK2 Ab and immunoblotted with anti-JAK2 or anti-HSP90 Ab. (E-F) HEL cells were treated with vorinostat (1.0μM) or DMSO for 24 hours. Total RNA was extracted and reverse transcribed. Relative expression of SOCS1 and SOCS3 mRNA was determined by quantitative real-time PCR and normalized to 18S expression (E). Relative expression of GATA1, KLF1, FOG1, SCL, C/EBPα, PU.1, and NF-E2 mRNA was determined by quantitative real-time PCR and normalized to 18S expression (F). Results shown represent means ± SEM from 3 independent experiments. Asterisks indicate significant differences by Student t test. *P < .05; **P < .005.
Vorinostat down-regulates the expression of JAK2V617F and affects its downstream signaling. (A) HEL cells bearing the JAK2V617F mutant were treated with the indicated concentrations of vorinostat for 24 hours. Acetylation of histone H4 and tubulin was detected by anti–acetyl-histone H4 and acetyl-tubulin immunoblotting. Activation of JAK2, Stat5, Stat3, Akt, and Erk1/2 was detected by immunoblotting using phosphospecific Abs. Membranes were reprobed for total proteins. (B) HEL cells were left untreated or treated with indicated concentrations of vorinostat, and c-Myc, Pim-1, PARP, and caspase 3 levels were analyzed by immunoblotting. Levels of Erk2 served as the loading control. (C) HEL cells were treated with the indicated concentrations of vorinostat for 24 hours. Total RNA was extracted and analyzed by the quantitative real-time PCR. The relative expression of JAK2 was normalized to 18S expression. Results are shown as means ± SEM from 3 independent experiments. (D) HEL cells were treated with the indicated concentrations of vorinostat for 24 hours, and lysates were immunoprecipitated with anti-HSP90 or anti-JAK2 Ab and immunoblotted with anti-JAK2 or anti-HSP90 Ab. (E-F) HEL cells were treated with vorinostat (1.0μM) or DMSO for 24 hours. Total RNA was extracted and reverse transcribed. Relative expression of SOCS1 and SOCS3 mRNA was determined by quantitative real-time PCR and normalized to 18S expression (E). Relative expression of GATA1, KLF1, FOG1, SCL, C/EBPα, PU.1, and NF-E2 mRNA was determined by quantitative real-time PCR and normalized to 18S expression (F). Results shown represent means ± SEM from 3 independent experiments. Asterisks indicate significant differences by Student t test. *P < .05; **P < .005.
To determine whether down-regulation of JAK2V617F in vorinostat-treated HEL cells occurs at the transcriptional or the posttranslational level, we analyzed the expression of JAK2 mRNA in HEL cells after treatment with vorinostat by quantitative real-time PCR. As shown in Figure 4C, treatment with vorinostat (0.5-1μM) resulted in an approximately 40%-50% decrease in JAK2 mRNA in HEL cells. However, treatment of HEL cells with vorinostat at 1μM resulted in greater inhibition of JAK2V617F protein expression (Figure 4A), indicating that down-modulation of JAK2 levels in HEL cells after vorinostat treatment may also occur at the protein level. Previous studies have suggested that heat-shock protein 90 (HSP90) serves as a molecular chaperone for protein tyrosine kinases, including JAK2.24,38,39 Therefore, we tested the interaction of HSP90 and JAK2V617F in HEL cells by immunoprecipitation with HSP90 or JAK2 Ab in the presence or absence of vorinostat. Immunoprecipitates of HSP90 and JAK2 showed an interaction between HSP90 and JAK2 in HEL cells, and this interaction was reduced significantly by vorinostat treatment (Figure 4D).
We also assessed the effects of vorinostat on the expression of suppressor of cytokine signaling (SOCS) molecules, which are known to negatively regulate the JAK-Stat pathway.40 As shown in Figure 4E, expression of SOCS1 and SOCS3 was significantly increased in HEL cells treated with vorinostat. These results provide evidence that vorinostat inhibits JAK2V617F-expressing hematopoietic cells by regulating the expression of the modulators of the JAK2/Stat pathway.
Because vorinostat treatment inhibited MPN hematopoietic progenitors expressing JAK2V617F (Figure 3), we evaluated the effects of vorinostat on the expression of several transcription factors that control hematopoiesis. Expression of transcription factors was analyzed in JAK2V617F-expressing HEL cells after treatment with vorinostat by quantitative real-time PCR. We found that expression of GATA1, KLF1, FOG1, SCL, C/EBPα, PU.1, and NF-E2 was significantly reduced in HEL cells treated with vorinostat (Figure 4F). GATA1, KLF1, FOG1, SCL, and NF-E2 are known to regulate erythropoiesis,41,42 whereas PU.1 and C/EBPα are important for granulopoiesis/monopoiesis.43 Therefore, vorinostat- evoked inhibition of JAK2V617F-expressing MPN cells could be due to reduced expression of these transcription factors.
Vorinostat induces significant hematologic response in a murine model of PV
Because vorinostat significantly inhibited cells expressing JAK2V617F, we investigated whether vorinostat could be useful in treating PV using our Jak2V617F knock-in mouse model.7 To assess the effects of vorinostat on Jak2V617F-induced PV, we first established PV disease in MxCre;Jak2V617F/+ mice by injecting pI:pC. Four weeks after induction, the disease was confirmed by peripheral blood counts. Animals were then randomized to begin treatment with placebo (vehicle) or vorinostat (200 mg/kg/d by IP injection) for 2 weeks. As expected, MxCre;Jak2V617F/+ mice receiving placebo exhibited elevated levels of RBCs, hemoglobin (Hb), hematocrit (HCT), and WBCs (Figure 5A-D). The increase in RBC, Hb, HCT, and WBC counts in MxCre;Jak2V617F/+ mice was significantly reduced by treatment with vorinostat (200 mg/kg/d) for 2 weeks (Figure 5A-D). However, the platelet count (Figure 5E) was not affected by vorinostat treatment. Vorinostat treatment also resulted in a significant decrease in spleen size compared with placebo-treated MxCre;Jak2V617F/+ mice (Figure 5F). Conversely, vorinostat treatment (200 mg/kg/d) did not cause any significant decrease in RBC, Hb, or HCT counts in control WT animals (supplemental Figure 1).
Vorinostat treatment normalizes peripheral blood counts and improves extramedullary hematopoiesis in Jak2V617F knock-in mice. Treatment with vorinostat at 200 mg/kg for 2 weeks resulted in significant improvement in RBC (A), Hb (B), HCT (C), and WBC (D) counts in Jak2V617F knock-in mice (n = 10) compared with placebo (vehicle)–treated mice (n = 10). (E) Platelet counts in the peripheral blood of placebo- and vorinostat-treated animals (n = 10). (F) Spleen weight was significantly reduced in Jak2V617F knock-in mice after 2 weeks of vorinostat treatment (n = 7).
Vorinostat treatment normalizes peripheral blood counts and improves extramedullary hematopoiesis in Jak2V617F knock-in mice. Treatment with vorinostat at 200 mg/kg for 2 weeks resulted in significant improvement in RBC (A), Hb (B), HCT (C), and WBC (D) counts in Jak2V617F knock-in mice (n = 10) compared with placebo (vehicle)–treated mice (n = 10). (E) Platelet counts in the peripheral blood of placebo- and vorinostat-treated animals (n = 10). (F) Spleen weight was significantly reduced in Jak2V617F knock-in mice after 2 weeks of vorinostat treatment (n = 7).
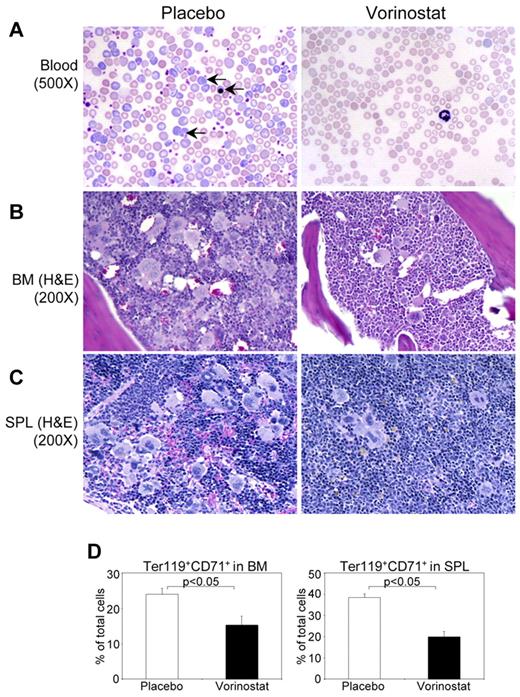
Histopathologic evaluation of the peripheral blood smears showed marked decreases in immature and mature forms of RBCs to normal levels in vorinostat-treated mice compared with placebo-treated animals (Figure 6A). BM from placebo-treated MxCre;Jak2V617F/+ mice showed hypercellularity with trilineage hyperplasia, which was significantly reduced by vorinostat treatment (Figure 6B). Whereas placebo-treated MxCre;Jak2V617F/+ mice exhibited effacement of normal splenic architecture with increased numbers of megakaryocytes and clusters of immature erythroid precursors, vorinostat therapy normalized the splenic architecture and reduced extramedullary hematopoiesis (Figure 6C). Consistent with histopathologic analyses, flow cytometric analysis of BM and spleen revealed a marked decrease in the proportion of erythroid precursors (CD71+/Ter119+) in mice treated with vorinostat compared with placebo-treated mice (Figure 6D). These results suggest that vorinostat could be efficacious in treating PV.
Histopathological characterization and flow cytometric analysis of BM and spleen of Jak2V617F knock-in mice treated with placebo or vorinostat. (A) Peripheral blood smears show normalization of RBCs and reticulocytes in vorinostat-treated Jak2V617F knock-in mice. Arrows indicate reticulocyte and immature nucleated RBCs. H&E staining of BM (B) and spleen (C) sections from placebo-treated Jak2V617F knock-in mice show hypercellularity with trilineage hyperplasia, which was markedly reduced by vorinostat treatment. (D) Percentage of erythroid precursors, determined by Ter119+ and CD71+ cells, was significantly reduced in both the BM and spleens of Jak2V617F knock-in mice treated with vorinostat compared with those of placebo-treated mice. Results shown represent means ± SEM from 7 mice in each group.
Histopathological characterization and flow cytometric analysis of BM and spleen of Jak2V617F knock-in mice treated with placebo or vorinostat. (A) Peripheral blood smears show normalization of RBCs and reticulocytes in vorinostat-treated Jak2V617F knock-in mice. Arrows indicate reticulocyte and immature nucleated RBCs. H&E staining of BM (B) and spleen (C) sections from placebo-treated Jak2V617F knock-in mice show hypercellularity with trilineage hyperplasia, which was markedly reduced by vorinostat treatment. (D) Percentage of erythroid precursors, determined by Ter119+ and CD71+ cells, was significantly reduced in both the BM and spleens of Jak2V617F knock-in mice treated with vorinostat compared with those of placebo-treated mice. Results shown represent means ± SEM from 7 mice in each group.
Vorinostat treatment decreases JAK2V617F mutant allele burden in a murine model
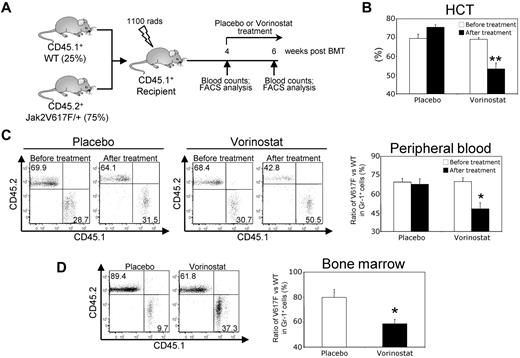
Because we observed that vorinostat treatment significantly improved blood parameters and reduced splenomegaly in Jak2V617F knock-in mice (Figure 5A-F), we investigated whether vorinostat treatment could reduce the Jak2V617F mutant allele burden. To address this question, we generated a chimeric mouse model by mixing BM from the Jak2V617F knock-in mice (CD45.2+) with the BM from WT (CD45.1+) mice at a ratio of 3 to 1 (75% V617F vs 25% WT) and injected it into lethally irradiated WT CD45.1+ recipient animals, as outlined in Figure 7A. As expected, the transplanted chimeric mice exhibited high HCT levels and a high percentage (approximately 70%) of CD45.2+ Jak2V617F–expressing cells in their peripheral blood within 4 weeks after transplantation (Figures 7B-C). Vorinostat treatment (200 mg/kg) for 2 weeks significantly reduced HCT levels in the transplanted animals (Figure 7B). In addition, vorinostat treatment caused significant reduction in Jak2V617F-expressing cells (determined by the ratio of CD45.2+/CD45.1+) in the peripheral blood and BM of the chimeric mice (Figure 7C-D). These results provide strong evidence that vorinostat treatment can reduce the mutant allele burden in vivo.
Vorinostat treatment reduces the mutant allele burden in mice. (A) Experimental design for analysis of mutant allele burden after vorinostat treatment. BM from pI:pC-induced MxCre;Jak2V617F/+ mice (CD45.2+) was mixed with the BM from WT (CD45.1+) mice at a ratio of 3 to 1 (75% V617F vs 25% WT) and injected into lethally irradiated WT CD45.1+ recipient animals. Four weeks after transplantation, peripheral blood counts were measured to confirm the establishment of PV disease and chimerism was assessed in the peripheral blood leukocytes by determining the ratio of CD45.2+/CD45.1+ cells using flow cytometry. Chimeric mice were treated with either placebo or vorinostat (200 mg/kg) for 2 weeks. The ratio of V617F (CD45.2+) to WT (CD45.1+) progenitors was determined by flow cytometry. (B) Treatment with vorinostat significantly reduced HCT levels in chimeric mice (n = 4). (C) Representative flow cytometry plots of the ratio of V617F (CD45.2+) to WT (CD45.1+) cells in peripheral blood leukocytes before and after treatment with placebo or vorinostat are shown (left). Bar graphs (right) show that the ratio of V617F to WT cells in Gr-1+ cells was dramatically decreased after vorinostat treatment (n = 4). (D) Representative flow cytometry plots of the ratio of V617F (CD45.2+) to WT (CD45.1+) cells in the BM of chimeric mice after treatment with placebo or vorinostat are shown (left). Bar graphs (right) show that the ratio of V617F to WT cells in Gr-1+ cells was significantly decreased in the BM after vorinostat treatment (n = 4). Results shown represent means ± SEM. Asterisks indicate significant differences by Student t test. *P < .05; **P < .005.
Vorinostat treatment reduces the mutant allele burden in mice. (A) Experimental design for analysis of mutant allele burden after vorinostat treatment. BM from pI:pC-induced MxCre;Jak2V617F/+ mice (CD45.2+) was mixed with the BM from WT (CD45.1+) mice at a ratio of 3 to 1 (75% V617F vs 25% WT) and injected into lethally irradiated WT CD45.1+ recipient animals. Four weeks after transplantation, peripheral blood counts were measured to confirm the establishment of PV disease and chimerism was assessed in the peripheral blood leukocytes by determining the ratio of CD45.2+/CD45.1+ cells using flow cytometry. Chimeric mice were treated with either placebo or vorinostat (200 mg/kg) for 2 weeks. The ratio of V617F (CD45.2+) to WT (CD45.1+) progenitors was determined by flow cytometry. (B) Treatment with vorinostat significantly reduced HCT levels in chimeric mice (n = 4). (C) Representative flow cytometry plots of the ratio of V617F (CD45.2+) to WT (CD45.1+) cells in peripheral blood leukocytes before and after treatment with placebo or vorinostat are shown (left). Bar graphs (right) show that the ratio of V617F to WT cells in Gr-1+ cells was dramatically decreased after vorinostat treatment (n = 4). (D) Representative flow cytometry plots of the ratio of V617F (CD45.2+) to WT (CD45.1+) cells in the BM of chimeric mice after treatment with placebo or vorinostat are shown (left). Bar graphs (right) show that the ratio of V617F to WT cells in Gr-1+ cells was significantly decreased in the BM after vorinostat treatment (n = 4). Results shown represent means ± SEM. Asterisks indicate significant differences by Student t test. *P < .05; **P < .005.
Discussion
The JAK2V617F mutation is the most common somatic mutation found in PV, ET, and PMF. Several small-molecule JAK2 inhibitors have been developed for the treatment of these MPNs, and some of these agents are currently undergoing clinical trials. Recent results have suggested that JAK2 inhibitor therapy can reduce splenomegaly and constitutional symptoms, but can cause significant hematologic toxicities in MPN patients.9,10,13 Complete remissions similar to those seen in CML with the BCR-ABL inhibitor imatinib cannot be achieved with the JAK2 inhibitors. Therefore, it is very likely that JAK2 inhibitor alone would not be sufficient to effectively treat MPN patients. Therefore, there is a clear need for the identification and development of alternative therapeutic approaches for the treatment of these patients.
In the present study, we evaluated the efficacy of the HDAC inhibitor vorinostat in hematopoietic cell lines and primary hematopoietic progenitors expressing JAK2V617F and in a murine model of PV. We tested vorinostat for several reasons. Vorinostat is a Food and Drug Administration–approved drug for the treatment of refractory cutaneous T-cell lymphoma and is currently under investigation in several other neoplasms. In the present study, we have demonstrated that vorinostat treatment inhibits significantly the growth of murine and human hematopoietic cells and progenitors expressing JAK2V617F. Other HDAC inhibitors, including ITF2357 (givinostat) and panobinostat, also showed activity against JAK2V617F-expressing cells.24,33 Treatment with vorinostat in vivo resulted in significant improvement in peripheral blood counts, with normalization of RBCs, Hb, and HCT in Jak2V617F knock-in mice (Figure 5A-D). We also observed marked improvements in BM and spleen architecture and a significant reduction in splenomegaly (Figures 5F and 6). Moreover, vorinostat treatment reduced the mutant allele burden in mice (Figure 7). Therefore, our data show that vorinostat may have therapeutic potential for the treatment of PV.
We did not observe anemia in control animals treated with vorinostat (supplemental Figure 1). We treated the control and Jak2V617F knock-in mice for only a short time. However, a toxicological study on the long-term effects of vorinostat treatment in normal rats and dogs showed decreased food consumption, weight loss, and some reduction in hematologic parameters in rats and primarily gastrointestinal toxicity with little or no reduction in WBC, RBC, and platelet counts in dogs after 26 weeks of treatment with vorinostat at high doses.44 It is possible that the toxicity profile of vorinostat in animals could be different from that in humans.
Biochemical analyses provide some clues to the inhibition of JAK2V617F-expressing cells by vorinostat. Phosphorylation of JAK2, Stat5, Stat3, Akt, and Erk1/2 was markedly inhibited by vorinostat in JAK2V617F+ HEL cells (Figure 4A). Interestingly, vorinostat treatment (1μM) almost completely inhibited the expression of JAK2V617F protein (Figure 4A). Therefore, down-regulation of JAK2V617F by vorinostat may result in an inhibition of JAK2V617F+ MPN cells. We observed a 50%-60% reduction in JAK2V617F mRNA after vorinostat treatment (Figure 4C), whereas JAK2V617F protein expression was profoundly inhibited (Figure 4A), suggesting that vorinostat inhibits JAK2V617F expression both at the mRNA and protein levels. One possible explanation for the reduction of JAK2V617F at the protein level is that vorinostat treatment may disrupt the binding of JAK2V617F to HSP90 and render the JAK2V617F susceptible to proteasomal degradation.24,45 It has been suggested that mutant oncoprotein kinases (eg, BCR-ABL and FLT3-ITD) are more dependent on a chaperone association with HSP90 than are unmutated kinases.38,39 Recent studies indicate that JAK2V617F is also an HSP90 client protein24,45 and, indeed, we observed dissociation of JAK2V617F with HSP90 after vorinostat treatment (Figure 4D); however, the mechanism by which vorinostat inhibits transcription of JAK2V617F is unclear.
Myc and Pim 1 are known as downstream targets of JAK2V617F and are required for JAK2V617F-mediated transformation.46 We observed marked reduction in c-Myc and Pim-1 in vorinostat-treated HEL cells (Figure 4B). In addition, significant cleavage of caspase 3 and PARP was observed in HEL cells treated with vorinostat (Figure 4B), which is consistent with increased apoptosis in HEL cells after vorinostat treatment.
A recent study has shown that hyperacetylation of SOCS1 and SOCS3 promoters by TSA treatment increases their expression in colorectal cancer cells.47 In the present study, we also observed significant increases in SOCS1 and SOCS3 expression in vorinostat-treated HEL cells (Figure 4E). Therefore, vorinostat treatment not only decreases the expression of JAK2V617F/Stat5/Stat3, but also increases the expression of the negative regulators (SOCS1 and SOCS3) of the JAK/Stat pathway.
We also observed significant decreases in the expression of several transcription factors, including GATA1, KLF1, FOG1, SCL, C/EBPα, PU.1, and NF-E2, in vorinostat-treated HEL cells (Figure 4F). GATA1, KLF1, FOG1, SCL, and NF-E2 control erythropoiesis,41,42 whereas PU.1 and C/EBPα are important for granulopoiesis/monopoiesis.43 Interestingly, up-regulation of NF-E2 and PU.1 expression was observed in MPN.48,49 In addition, it has been found recently that overexpression of NF-E2 induces MPN in mice and vorinostat treatment can reduce NF-E2 expression.50 Therefore, it is plausible that vorinostat may inhibit the growth of JAK2V617F-expressing hematopoietic progenitors by inhibiting the expression of transcription factors that control erythropoiesis and myelopoiesis. Future studies will determine how inhibition of HDACs by vorinostat regulates the expression of these transcription factors.
In the present study, the growth of JAK2V617F-expressing primary erythroid precursors from mice and humans was remarkably inhibited by vorinostat treatment (Figure 3). Interestingly, vorinostat was more potent than JAK inhibitor I at inhibiting erythroid progenitors expressing JAK2V617F (Figure 3B-C). We also observed that the effect of vorinostat was significantly greater on hematopoietic progenitors from PV patients than on those from ET patients (Figure 3E). Furthermore, we have demonstrated that vorinostat treatment provides significant hematologic response in a Jak2V617F knock-in mouse model of PV. More importantly, vorinostat treatment significantly reduced the mutant allele burden in mice (Figure 7). Our results suggest that vorinostat therapy may offer significant clinical benefit to patients with PV and possibly other JAK2V617F+ MPNs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Merck for providing vorinostat and Dr Diana Gilligan (SUNY Upstate Medical University) for procuring samples from MPN patients.
This work was supported by the National Institutes of Health (grants R01 HL095685 and R21 HL102618) and by a start-up fund from the SUNY Upstate Medical University (to G.M.).
National Institutes of Health
Authorship
Contribution: H.A. performed the research, analyzed the data, and wrote the manuscript; S.A. performed the research; A.G., A.B., and S.G. procured and assisted in performing studies on MPN primary cells; R.E.H. performed the histopathologic analysis and revised the manuscript; and G.M. designed and performed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Golam Mohi, Department of Pharmacology, SUNY Upstate Medical University, 750 East Adams St, Syracuse, NY 13210; e-mail: mohim@upstate.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal