Abstract
Cellular immune responses have the potential to elicit dramatic and sustained clinical remissions in lymphoma patients. Recent clinical trial data demonstrate that modification of T cells with chimeric antigen receptors (CARs) is a promising strategy. T cells containing CARs with costimulatory domains exhibit improved activity against tumors. We conducted a pilot clinical trial testing a “third-generation” CD20-specific CAR with CD28 and 4-1BB costimulatory domains in patients with relapsed indolent B-cell and mantle cell lymphomas. Four patients were enrolled, and 3 received T-cell infusions after cyclophosphamide lymphodepletion. Treatment was well tolerated, although one patient developed transient infusional symptoms. Two patients without evaluable disease remained progression-free for 12 and 24 months. The third patient had an objective partial remission and relapsed at 12 months after infusions. Modified T cells were detected by quantitative PCR at tumor sites and up to 1 year in peripheral blood, albeit at low levels. No evidence of host immune responses against infused cells was detected. In conclusion, adoptive immunotherapy with CD20-specific T cells was well tolerated and was associated with antitumor activity. We will pursue alternative gene transfer technologies and culture conditions in future studies to improve CAR expression and cell production efficiency. This study is registered at www.clinicaltrials.gov as NCT00621452.
Introduction
Adoptive T-cell therapy has emerged as a promising strategy for the treatment of cancer.1-7 The challenges of isolating T cells reactive toward tumor-associated antigens and using such cells in patients with a diversity of human leukocyte antigen (HLA) phenotypes have, to some extent, been overcome through the use of chimeric antigen receptors (CARs). These genetically engineered molecules typically consist of an extracellular antigen-binding domain derived from a monoclonal antibody (mAb), usually in the form of a single-chain variable fragment (scFv), linked via spacer and transmembrane domains to a T-cell signaling endodomain, most commonly the CD3ζ chain.8 CAR-redirected T cells have the capacity to recognize cell-surface tumor targets in an HLA-independent fashion, leading to antigen-specific T cell activation, proliferation, and cytokine production, and the capacity to eradicate tumors in mouse xenograft models.9-12
Non-Hodgkin lymphomas (NHLs), particularly the indolent B-cell lymphomas and mantle cell lymphoma (MCL), are appealing targets for CAR-based immunotherapy because of their susceptibility to T cell–mediated immune effects, as evidenced by long-term remissions after allogeneic stem cell transplantation (SCT).13,14 The CD20 antigen on the surface of B-NHL cells is a well-established immunotherapy target for lymphoma, with numerous randomized trials demonstrating improved clinical outcomes in patients treated with anti-CD20 Ab.
We previously reported the results of a phase 1 clinical trial investigating the use of anti-CD20 CAR+ autologous T cells to treat patients with relapsed indolent NHL and MCL.15 In that study, we demonstrated the safety and tolerability of this approach but found that the ex vivo expansion methods used were inefficient and that the antitumor activity and persistence of the modified T cells were modest. Numerous preclinical studies have demonstrated that CARs endowed with costimulatory endodomains, such as CD28, OX40, or 4-1BB (commonly referred to as “second generation” CARs), augment T-cell activity both in vitro and in vivo compared with first-generation CARs lacking costimulation.16-20 These enhanced CARs were designed to mimic normal physiology, where both a primary TCR signal and a second costimulatory signal are required for full activation of T cells. The benefit of CAR costimulation was recently confirmed in a clinical trial in which a CD28-containing CAR conferred superior in vivo expansion and persistence compared with a first-generation CAR.21 Potent antileukemic responses were seen in another recent trial in which a 4-1BB domain was incorporated into the CAR.6 Several groups have combined 2 costimulatory domains into the CAR construct and shown superior activity in preclinical studies.22-24 We previously tested multiple CAR configurations in vitro and found that a CAR containing CD28 and CD137 (4-1BB) domains was the most effective in terms of proliferation and cytokine production.23 We therefore selected this construct for clinical testing and report here the results of a small pilot trial testing this “third-generation” CAR.
Methods
Clinical trial protocol
This clinical protocol was approved by the Fred Hutchinson Cancer Research Center Institutional Review Board, University of Washington Institutional Biosafety Committee, and the National Institutes of Health Recombinant DNA Advisory Committee, and is supported by the United States Food and Drug Administration (BB-IND 13390). This study is registered at www.clinicaltrials.gov as NCT00621452. All patients provided written informed consent in accordance with the Declaration of Helsinki before enrolling on the study. Patients were eligible if they had a pathologically confirmed diagnosis of CD20+ MCL or indolent B-cell lymphoma, had relapsed or refractory disease after at least one prior chemotherapy, were deemed not to be candidates for (or refused) SCT, and had serologic evidence of prior Epstein-Barr virus (EBV) exposure (because the TM-LCL cell line used in T-cell culture is EBV-transformed). Patients were excluded if they had received fludarabine or cladribine within 2 years before apheresis (these drugs cause a protracted lymphopenia that could potentially impair the number or function of T cells collected during apheresis), anti-CD20 Abs or chemotherapy within 4 weeks of T-cell infusions; had lymph nodes larger than 5 cm or more than 5000 circulating lymphoma cells in the peripheral blood at the time of T-cell infusions, previous allogeneic SCT, or human anti–mouse Ab seropositivity.
Patients underwent apheresis to collect peripheral blood mononuclear cells (PBMCs) and could then receive cytoreductive chemotherapy for disease control or debulking during the 2- the 3-month period of T-cell expansion, at the discretion of the referring physician. Release criteria for ex vivo expanded T cells were as follows: sterility by United States Pharmacopeia, fungal, and mycoplasma testing, negative Gram stain, endotoxin less than 5 U/kg, more than 70% viability by Trypan Blue exclusion, CD3+ and TCRαβ+ by flow cytometry, IL-2–dependent growth, and CD20-specific cytotoxicity of more than 20% lysis at 100:1 E:T ratio. Patients subsequently received cyclophosphamide (CY) 1000 mg/m2 intravenously to achieve lymphodepletion 2 days before starting a series of 3 infusions of autologous CD20-specific T cells 2 to 5 days apart in escalating doses (108, 109, and 3.3 × 109 cells/m2), followed by 14 days of subcutaneous low-dose (250 000 IU/m2) IL-2 injections twice daily. An excisional biopsy of a palpable lymph node and a bone marrow biopsy were performed on each patient 24 to 48 hours after the final T-cell infusion. Another bone marrow biopsy at the 1-month time point was optional. Patients underwent clinical follow-up to evaluate toxicities related to therapy, which were assessed according to National Institutes of Health Common Terminology Criteria for Adverse Events Version 3.0 (http://ctep.cancer.gov). Clinical responses were assessed according to International Working Group criteria.25
Modification and expansion of CD20-specific T cells
CD20-specific T cells were generated as previously described.15,23 Patient PBMCs were activated with OKT3 and IL-2, and 4 days later were electroporated with the L29.19.1 plasmid,23 which encodes a Leu16 (murine anti–human CD20) scFv fused to a human IgG1 CH2-CH3 hinge, CD4 transmembrane, intracellular CD28 (with mutated dileucine motif)26 and CD137 (4-1BB) costimulatory domains, and CD3ζ, under the control of the CMV immediate-early promoter, hereafter denoted as the αCD20-28-BB-ζ CAR, as well as a neomycin resistance gene under the control of an SV40 promoter. Transfected cells were restimulated in 12- to 15-day cycles using a rapid expansion protocol15,27 and selected with G418 during the first, third, and fourth stimulation cycles. G418-resistant cells were transferred from flasks to culture bags until the target cell dose was reached. In some instances, batches of cells were cryopreserved during expansion and thawed before infusion.
Cytotoxicity assays
Standard 5-hour chromium release assays were performed as previously described,15 using the following targets: EL4 (negative control), EL4-CD20 (EL4 cells transfected to express CD20), Granta (CD20+ MCL line), and Daudi (CD20+ Burkitt lymphoma cell line).
Flow cytometry
Immunophenotypes of ex vivo expanded T cells were assessed by thawing and washing pre-infusion T cells, and incubated 30 minutes at 4°C with the following antibodies, all from BD Biosciences: anti-CD3–PE, anti-CD4–FITC, anti-CD8–FITC, anti-CD25–PE, anti-CD27–FITC, anti-CD28–FITC, anti-CD44–PE, anti-CD45RA–FITC, anti-CD45RO–FITC, anti-CD56–PE, anti-CD57–FITC, anti-CD62–PE, anti-CD183 (CXCR3)–PE, and anti-CD197 (CCR7)–FITC. Cell surface IL-15 and IL-15Rα were measured by labeling thawed, washed patient PBMCs from baseline and serial post-CY time points with either anti-CD3–PE or anti-CD14–PE (BD Biosciences) and either anti–IL-15–FITC (R&D Systems) or anti–IL-15Rα–FITC (eBioscience) and propidium iodide (BD Biosciences). Cells were washed with PBS plus 0.5% FCS, and approximately 20 000 events were acquired on a FACSCalibur machine (BD Biosciences). Data were analyzed using FlowJo Version 7.6.1 software (TreeStar). CAR expression was assessed by FACS using FITC-conjugated goat anti–mouse IgG F(ab′)2 or PE-conjugated goat anti–human IgG Fc (Jackson ImmunoResearch Laboratories) as previously described.15,23 Lymphocyte subsets in serially collected peripheral blood samples were analyzed using multiparameter flow cytometry as previously described.15
Quantitative PCR to detect modified T cells
Patient PBMCs collected at baseline and at serial time points after T-cell infusions were collected and separated by Ficoll centrifugation and then cryopreserved. Batched cells were thawed and genomic DNA was harvested using a DNeasy Blood and Tissue Kit (QIAGEN). The CAR transgene was detected by performing quantitative PCR as previously described,15 using either a primer amplifying a 111-bp fragment spanning the junction of the CD4 transmembrane region and adjacent CD28 domain (forward primer: 5′-TGGGCTAGGCATCTTCTTCA-3′, reverse primer: 5′-GCATAGGGCTGGTAATGCTT-3′, probe: 5′-FAM-CACAGTGACTACATGAACATGAC-TAMRA-3′), or a primer amplifying a 113-bp fragment spanning the junction of CD137 domain and adjacent CD3ζ chain (forward primer: 5′-GAAGAAGGAGGATGTGAACT-3′, reverse primer: 5′-TCCTCTCTTCGTCCTAGATT-3′, probe: 5′-FAM-AGGGCCAGAACCAGCTCTATAA-TAMRA-3′). Quantitative real-time PCR was performed in triplicate using TaqMan Universal PCR Master Mix (Applied Biosystems), in a 7900HT Fast Real-Time PCR System (Applied Biosystems). Copy numbers per microgram of genomic DNA, generated from a standard curve of 10-fold serial dilutions of purified plasmid, were used to calculate the percentage of CAR+ cells among PBMCs, assuming 1 copy/cell,28 based on: 1 μg genomic DNA/6.7 pg DNA per cell = 1.49 × 105 cells.
Immune response assays
Two humoral immune response assays were performed: an ELISA assay detecting Ab against the Leu16 murine anti-CD20 Ab and a FACS assay detecting Ab against HEK-293 cells transfected with the L29.19.1 vector. A cellular immune response assay was performed to detect T cells responding to stimulation with cells expressing the transgene products. Details of immune response assays are provided in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Telomere analysis
Patient PBMCs obtained at apheresis were thawed, washed, and separated over a Ficoll gradient, and positively CD3-selected by MACS (Miltenyi Biotec) according to the manufacturer's instructions. Ex vivo expanded pre-infusion CAR+ patient T cells were thawed and washed. Genomic DNA was isolated from CD3+ apheresis PBMCs and CAR+ T cells using a DNeasy Blood and Tissue kit (QIAGEN). Quantitative PCR was performed to measure the mean telomere length of each cell population.29 In brief, telomeric DNA and a single-copy internal control gene (36B4, acidic ribosomal phosphoprotein PO) were amplified in each sample. A 4-point standard curve (2-fold serial dilutions from 10 to 1.25 ng of DNA) was included in all PCRs to allow the transformation of cycle threshold into nanograms of DNA. All samples were run in triplicate, and the median was used for subsequent calculations. The amount of telomeric DNA (T) was expressed as a fraction of the amount of the single-copy control gene (S), yielding a relative measurement of telomere length (T/S ratio).
Cytokine measurements
Serum IL-2, IL-4, IL-6, IL-7, IL-10, IL-12p70, IL-15, IL-17, IL-21, IFN-γ, TNF-α, and MIP1-α levels from serial post-CY time points were batch-analyzed using a Luminex microbead sandwich immunoassay. Analyte concentrations were determined using a standard curve prepared with each assay. Luminex MicroPlex Microspheres diluted to 5000 beads/50 μL in assay buffer (1% BSA, 1× PBS, 0.1% Tween 20) were combined with 1:1 volumes of patient serum samples or standards in wells of filter plates (Millipore) in duplicate, and incubated with biotinylated detection antibodies, followed by streptavidin/PE conjugate (Prozyme). Plates were read on a Luminex 200 (Millipore) instrument and analyzed with Luminex xPonent Version 3.0 software.
Statistics
Telomere lengths were compared using a 1-sided paired t test.
Results
Study design and patient characteristics
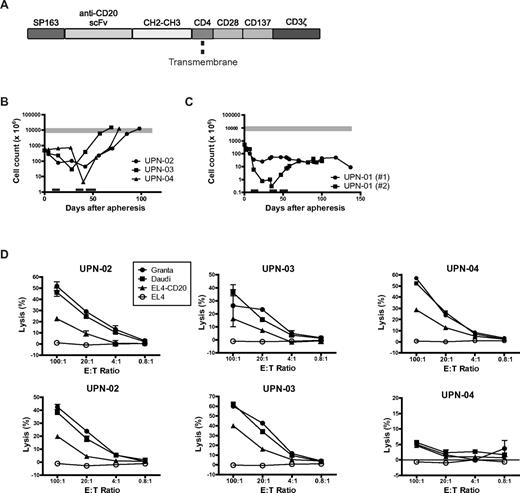
The primary objective of this study was to assess the safety and feasibility of adoptive therapy using autologous T cells genetically modified to express a CD20-specific CAR containing CD28 and 4-1BB costimulatory domains. PBMCs collected from study subjects by apheresis were activated by OKT3 and IL-2, and electroporated 3 to 4 days later with a DNA plasmid encoding a CD20-specific scFv-ζ CAR containing CD28 and 4-1BB intracellular costimulatory domains (denoted αCD20-28-BB-ζ; Figure 1A). Modified T cells were selected with 3 cycles of G418 and expanded using a rapid expansion protocol.23 The duration of cell culture before cell infusion was at least 69 days (Figure 1B and supplemental Table 1). Patients were treated with 1 g/m2 CY as lymphodepletion 2 days before the first of 3 infusions of modified T cells, 2 to 5 days apart, at incremental doses of 108, 109, and 3.3 × 109 cells/m2. After the last infusion, patients received 14 days of low-dose IL-2 injections and were then serially monitored for toxicity, response, and scientific endpoints.
Expansion and cytotoxicity of modified T cells. (A) Schematic representation of the αCD20-28-BB-ζ chimeric receptor, not to scale. (B-C) Growth curves of ex vivo expanded T cells for the 3 treated patients (B) and of the 2 attempts to expand cells from patient UPN-01 (C). PBMCs collected by apheresis were stimulated with anti-CD3 Ab and IL-2, transfected by electroporation by the plasmid encoding the CAR shown in panel A, selected with G418, and restimulated every 12 to 15 days in a rapid expansion protocol. Black bars represent periods of G418 selection; and gray bar, the target cell dose range. (D) CD20-specific cytotoxicity of G418-selected autologous patient T cells was assessed with 5-hour chromium-release assays using the following 51Cr-labeled target cells: Granta cells (MCL), Daudi cells (Burkitt NHL), EL4 cells transfected to express CD20, or the parental EL4 cell line lacking CD20 expression. The cells were tested after G418 selection (top panels) and 54, 27, and 40 days later for the 3 patients at the time of T-cell infusions (bottom panels). Data represent the mean of triplicate values, and error bars represent SEM.
Expansion and cytotoxicity of modified T cells. (A) Schematic representation of the αCD20-28-BB-ζ chimeric receptor, not to scale. (B-C) Growth curves of ex vivo expanded T cells for the 3 treated patients (B) and of the 2 attempts to expand cells from patient UPN-01 (C). PBMCs collected by apheresis were stimulated with anti-CD3 Ab and IL-2, transfected by electroporation by the plasmid encoding the CAR shown in panel A, selected with G418, and restimulated every 12 to 15 days in a rapid expansion protocol. Black bars represent periods of G418 selection; and gray bar, the target cell dose range. (D) CD20-specific cytotoxicity of G418-selected autologous patient T cells was assessed with 5-hour chromium-release assays using the following 51Cr-labeled target cells: Granta cells (MCL), Daudi cells (Burkitt NHL), EL4 cells transfected to express CD20, or the parental EL4 cell line lacking CD20 expression. The cells were tested after G418 selection (top panels) and 54, 27, and 40 days later for the 3 patients at the time of T-cell infusions (bottom panels). Data represent the mean of triplicate values, and error bars represent SEM.
Four patients were enrolled in this study, of whom 3 had relapsed MCL and one had relapsed follicular lymphoma. All patients had received at least 2 prior therapies, and 2 patients had a prior autologous SCT. Patient characteristics are summarized in Table 1, and a schematic illustration of the study protocol is given in supplemental Figure 1.
Patient characteristics
| Variable . | UPN-01 . | UPN-02 . | UPN-03 . | UPN-04 . |
|---|---|---|---|---|
| Age, y | 65 | 80 | 62 | 28 |
| Sex | Male | Male | Male | Male |
| Diagnosis | MCL | MCL | MCL | FL |
| Stage | IV | IV | IV | IV |
| Therapies before study entry | R, fenretinide, R-CHOP, ASCT | R-CHOP, fludarabine, colectomy, R, bortezomib, fenretinide | CHOP, ASCT | R-CHOP, R maintenance |
| Cytoreductive therapy during T-cell expansion | NA | Bendamustine, GCD, | None | None |
| 36 Gy RT to neck | ||||
| Last systemic therapy before T-cell infusions | ASCT | GCD | ASCT | R |
| Clinical response to last systemic therapy | PR | SD | CR | SD |
| Duration of response | 20 mo | Received RT 2 mo later | 12 y | 10 mo |
| Time between last treatment and first T-cell infusion | NA | 4 mo since GCD; 4 wks since RT | > 13 y | > 10 mo |
| Last anti-CD20 Ab therapy before T-cell infusions | NA | > 3 y | > 13 y | > 10 mo |
| Baseline B cell count (cells/μL) | NA | 1 | 277 | 40 |
| Variable . | UPN-01 . | UPN-02 . | UPN-03 . | UPN-04 . |
|---|---|---|---|---|
| Age, y | 65 | 80 | 62 | 28 |
| Sex | Male | Male | Male | Male |
| Diagnosis | MCL | MCL | MCL | FL |
| Stage | IV | IV | IV | IV |
| Therapies before study entry | R, fenretinide, R-CHOP, ASCT | R-CHOP, fludarabine, colectomy, R, bortezomib, fenretinide | CHOP, ASCT | R-CHOP, R maintenance |
| Cytoreductive therapy during T-cell expansion | NA | Bendamustine, GCD, | None | None |
| 36 Gy RT to neck | ||||
| Last systemic therapy before T-cell infusions | ASCT | GCD | ASCT | R |
| Clinical response to last systemic therapy | PR | SD | CR | SD |
| Duration of response | 20 mo | Received RT 2 mo later | 12 y | 10 mo |
| Time between last treatment and first T-cell infusion | NA | 4 mo since GCD; 4 wks since RT | > 13 y | > 10 mo |
| Last anti-CD20 Ab therapy before T-cell infusions | NA | > 3 y | > 13 y | > 10 mo |
| Baseline B cell count (cells/μL) | NA | 1 | 277 | 40 |
FL indicates follicular lymphoma; R, rituximab; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; ASCT, high-dose therapy followed by autologous stem cell transplantation; GCD, gemcitabine, carboplatin, and dexamethasone; RT, radiotherapy; PR, partial response; SD, stable disease; CR, complete response; and NA, not applicable.
Expansion and characterization of modified T cells
The target T-cell dose was successfully generated for 3 of the 4 enrolled patients. The cells for patient UPN-01 failed to expand adequately despite 2 attempts at electroporation and expansion, and the patient elected to withdraw from the study rather than receive a reduced cell dose. Growth curves are shown in Figure 1B and C, and cell doses are shown in supplemental Table 1.
T cells modified with the αCD20-28-BB-ζ CAR demonstrated CD20-specific cytotoxicity in vitro (Figure 1D) and G418 resistance, indicating expression of the plasmid-encoded transgenes. The αCD20-28-BB-ζ CAR was detectable by PCR, but expression was very low, below the limit of detection by flow cytometry and Western blotting (data not shown).
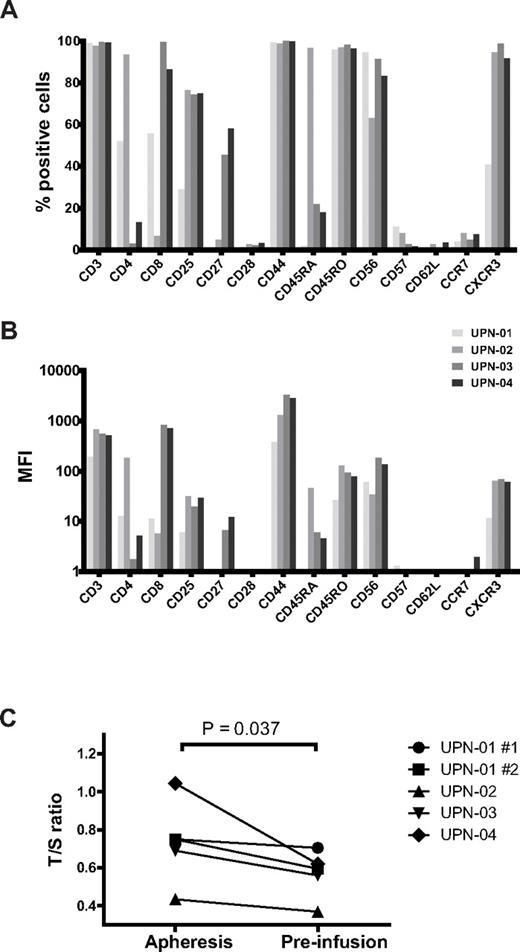
Detailed cell surface marker analysis was performed using flow cytometry to assess the phenotype of ex vivo expanded cells. Modified T cells expressed markers characteristic primarily of an activated effector phenotype (CD3+, CD45RO+, CXCR3+, CD25+, CD28−, CD62L−, CCR7−), but also some features of effector memory cells (CD27+ in 2 patients and CD57−; Figure 2A-B). The CD4+/CD8+ ratio varied, with T cells from patients UPN-03 and UPN-04 being predominantly CD8+, whereas those of UPN-02 were 93% CD4+ cells. The limited expansion product of patient UPN-01 consisted of even proportions of CD4+ and CD8+ cells.
Immunophenotype and relative telomere length of infused T cells. End of production T cells for patients UPN-02, UPN-03, and UPN-04, as well as cells from patient UPN-01 (after 9 stimulation cycles) were analyzed by flow cytometry after being thawed, washed, and stained with the antibodies shown. (A) Geometric mean fluorescence intensity (MFI) after subtracting the isotype control MFI and (B) percent positive cells. (C) Genomic DNA was harvested from CD3-selected apheresis PBMCs and from pre-infusion, ex vivo expanded CAR+ T cells for each patient. T cells from both attempts at expansion for UPN-01 were analyzed. Quantitative PCR was used to determine the amounts of telomeric DNA (T) and of a single-copy internal reference gene (S) for each sample. The relative mean telomere length of each cell population is represented by the T/S ratio. Data represent the mean of 2 assays, each performed in triplicate.
Immunophenotype and relative telomere length of infused T cells. End of production T cells for patients UPN-02, UPN-03, and UPN-04, as well as cells from patient UPN-01 (after 9 stimulation cycles) were analyzed by flow cytometry after being thawed, washed, and stained with the antibodies shown. (A) Geometric mean fluorescence intensity (MFI) after subtracting the isotype control MFI and (B) percent positive cells. (C) Genomic DNA was harvested from CD3-selected apheresis PBMCs and from pre-infusion, ex vivo expanded CAR+ T cells for each patient. T cells from both attempts at expansion for UPN-01 were analyzed. Quantitative PCR was used to determine the amounts of telomeric DNA (T) and of a single-copy internal reference gene (S) for each sample. The relative mean telomere length of each cell population is represented by the T/S ratio. Data represent the mean of 2 assays, each performed in triplicate.
The cytolytic potential of the modified T cells declined over time in one patient (Figure 1D), which may have been the result of functional exhaustion or diminution of CAR expression. Telomere length was shortened in all patients after ex vivo expansion compared with CD3+ cells collected at apheresis (Figure 2C). Together, these data support findings from other studies that prolonged in vitro culture with multiple rounds of stimulation is detrimental to T-cell function,30,31 although the degree of telomeric shortening was less than expected, and CD57, a marker of terminal differentiation, was not significantly expressed in any of the expanded T-cell products.
Safety and tolerability
The treatment regimen was generally well tolerated, with most adverse events being predictable grade 1 or 2 toxicities related to CY and IL-2 (Table 2). However, one patient (UPN-02) developed fever (39.2°C) and orthostatic hypotension immediately after the second T-cell infusion, and fever (39.9°C) and mild hypoxemia (90% by pulse oximetry) requiring supplemental O2 after the third infusion. In both instances, the toxicities resolved spontaneously after overnight observation in the hospital. The other 2 patients tolerated the T-cell infusions without incident. The most frequent toxicities related to CY were cytopenias, which reached grade 4 in patient UPN-02, a heavily pretreated 80-year-old patient, as well as alopecia and fatigue. The most frequent toxicities associated with IL-2 injections were dyspnea, fatigue, and skin injection site reactions. Patient UPN-04 developed a wound infection and cellulitis at the site of the excisional lymph node biopsy (right groin), which required hospitalization for intravenous antibiotics and was thus classified as a serious adverse event. He recovered without sequelae.
Adverse events at least possibly related to the treatment regimen
| Toxicity . | Grade* . | No. of patients experiencing adverse events at this grade . | “Possible” or greater attribution . |
|---|---|---|---|
| Leukopenia | 4 | 1 | CY |
| Lymphopenia | 4 | 1 | CY, IL-2 |
| Neutropenia | 4 | 1 | CY, IL-2 |
| Lymphopenia | 3 | 2 | CY |
| Thrombocytopenia | 3 | 1 | CY, IL-2 |
| Cellulitis | 3 | 1 | CY, IL-2 |
| Hypoxemia | 3 | 1 | T cells, IL-2 |
| Anemia | 2 | 1 | CY |
| Leukopenia | 2 | 2 | CY |
| Lymphopenia | 2 | 1 | CY |
| Neutropenia | 2 | 1 | CY |
| Thrombocytopenia | 2 | 1 | Apheresis |
| Orthostatic hypotension | 2 | 1 | T cells |
| Fatigue | 2 | 2 | CY, IL-2 |
| Fever | 2 | 1 | T cells, IL-2 |
| Night sweats | 2 | 1 | IL-2 |
| Injection site reaction | 2 | 1 | IL-2 |
| Headache | 2 | 1 | IL-2 |
| Anemia | 1 | 1 | CY, IL-2 |
| Lymphopenia | 1 | 1 | CY |
| Neutropenia | 1 | 2 | CY, IL-2 |
| Thrombocytopenia | 1 | 2 | CY, IL-2 |
| Chills | 1 | 1 | T cells, IL-2 |
| Fatigue | 1 | 2 | CY, IL-2 |
| Rigors | 1 | 1 | IL-2 |
| Alopecia | 1 | 3 | CY |
| Injection site reaction | 1 | 1 | IL-2 |
| Injection site redness | 1 | 1 | IL-2 |
| Diarrhea | 1 | 2 | CY, IL-2 |
| Nausea | 1 | 2 | CY |
| ALT elevation | 1 | 1 | CY |
| AST elevation | 1 | 1 | CY |
| Dyspnea | 1 | 2 | IL-2 |
| Flu-like syndrome | 1 | 1 | IL-2 |
| Toxicity . | Grade* . | No. of patients experiencing adverse events at this grade . | “Possible” or greater attribution . |
|---|---|---|---|
| Leukopenia | 4 | 1 | CY |
| Lymphopenia | 4 | 1 | CY, IL-2 |
| Neutropenia | 4 | 1 | CY, IL-2 |
| Lymphopenia | 3 | 2 | CY |
| Thrombocytopenia | 3 | 1 | CY, IL-2 |
| Cellulitis | 3 | 1 | CY, IL-2 |
| Hypoxemia | 3 | 1 | T cells, IL-2 |
| Anemia | 2 | 1 | CY |
| Leukopenia | 2 | 2 | CY |
| Lymphopenia | 2 | 1 | CY |
| Neutropenia | 2 | 1 | CY |
| Thrombocytopenia | 2 | 1 | Apheresis |
| Orthostatic hypotension | 2 | 1 | T cells |
| Fatigue | 2 | 2 | CY, IL-2 |
| Fever | 2 | 1 | T cells, IL-2 |
| Night sweats | 2 | 1 | IL-2 |
| Injection site reaction | 2 | 1 | IL-2 |
| Headache | 2 | 1 | IL-2 |
| Anemia | 1 | 1 | CY, IL-2 |
| Lymphopenia | 1 | 1 | CY |
| Neutropenia | 1 | 2 | CY, IL-2 |
| Thrombocytopenia | 1 | 2 | CY, IL-2 |
| Chills | 1 | 1 | T cells, IL-2 |
| Fatigue | 1 | 2 | CY, IL-2 |
| Rigors | 1 | 1 | IL-2 |
| Alopecia | 1 | 3 | CY |
| Injection site reaction | 1 | 1 | IL-2 |
| Injection site redness | 1 | 1 | IL-2 |
| Diarrhea | 1 | 2 | CY, IL-2 |
| Nausea | 1 | 2 | CY |
| ALT elevation | 1 | 1 | CY |
| AST elevation | 1 | 1 | CY |
| Dyspnea | 1 | 2 | IL-2 |
| Flu-like syndrome | 1 | 1 | IL-2 |
ALT indicates alanine aminotransferase; and AST, aspartate aminotransferase.
The maximum grade experienced for the corresponding toxicity for a given patient.
In vivo persistence of infused T cells and localization to tumor sites
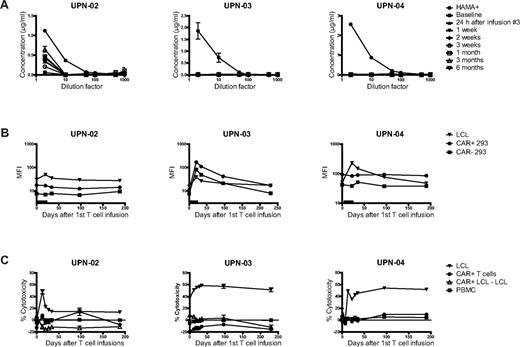
Quantitative real-time PCR using primers specific for the αCD20-28-BB-ζ CAR transgene was performed on genomic DNA harvested from patient PBMCs collected at various time points after T-cell infusions to assess the duration of in vivo persistence. The peak level of modified T cells detected was 3.2%, 3.0%, and 1.0% for the 3 patients, immediately after the infusions, and subsequently dropped to less than 1% (Figure 3A). Modified T cells were detectable for 12 months in patients UPN-02 and UPN-03, and for 9 months in UPN-04 (Figure 3B). The same quantitative PCR assay was used to assess trafficking of modified T cells to tumor sites, and these cells were detected at low levels 24 to 48 hours after the third T-cell infusion in malignant lymph nodes of all 3 treated patients and in the bone marrow of 2 of the 3 patients (Figure 3C-D). Patient UPN-03 underwent a marrow aspirate at 1 month after infusions, and modified T cells were detectable at a low level in the marrow at that time point as well.
Engraftment, persistence, and localization of modified T cells to tumor sites. Quantitative real-time PCR was performed on genomic DNA harvested from study subject PBMCs collected before and at serial time points after T-cell infusions, using primers specific for the transgene. CAR+ cells are shown as a percentage of total PBMCs for each of the treated patients over (A) the first month and (B) the first year. The same quantitative PCR assay was used to detect modified cells in genomic DNA harvested from surgically excised lymph nodes (C) or bone marrow aspirates (D) collected 24 to 48 hours after the third T-cell infusion. For patient UPN-03, 2 adjacent lymph nodes were excised and analyzed separately, and an additional bone marrow aspirate was performed 1 month after the third T-cell infusion. Data represent mean values (± SD) of at least 2 assays per time point, with each sample performed in triplicate. Arrows indicate infusions of genetically modified T cells; and black bar, the 14-day period of low-dose IL-2 injections.
Engraftment, persistence, and localization of modified T cells to tumor sites. Quantitative real-time PCR was performed on genomic DNA harvested from study subject PBMCs collected before and at serial time points after T-cell infusions, using primers specific for the transgene. CAR+ cells are shown as a percentage of total PBMCs for each of the treated patients over (A) the first month and (B) the first year. The same quantitative PCR assay was used to detect modified cells in genomic DNA harvested from surgically excised lymph nodes (C) or bone marrow aspirates (D) collected 24 to 48 hours after the third T-cell infusion. For patient UPN-03, 2 adjacent lymph nodes were excised and analyzed separately, and an additional bone marrow aspirate was performed 1 month after the third T-cell infusion. Data represent mean values (± SD) of at least 2 assays per time point, with each sample performed in triplicate. Arrows indicate infusions of genetically modified T cells; and black bar, the 14-day period of low-dose IL-2 injections.
Clinical responses to T-cell infusions
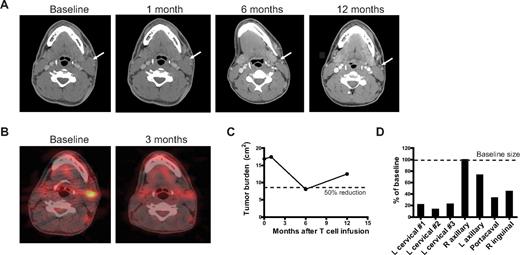
Patient UPN-02 had no evaluable disease after an excisional lymph node biopsy of a large cervical lymph node, which had been irradiated approximately 1 month before T-cell infusions, and had no evidence of disease progression until his 1 year evaluation after T-cell infusions. Patient UPN-03 had no evaluable disease after his excisional lymph node biopsy after T-cell infusions and did not progress until 2 years after completing study treatments. Patient UPN-04, a 28-year-old man with rituximab-refractory follicular lymphoma, initially had no response, but approximately 3.5 months after T-cell infusions, experienced rapid resolution of a 3-cm cervical lymph node on physical examination over 1 to 2 weeks. This corresponded to a partial response documented on his 6-month CT scan (Figure 4A-C), although his disease subsequently progressed as documented by a CT scan performed 1 year after T-cell infusions. The responses at his various tumor sites were heterogeneous, with some lymph nodes resolving to normal size, others responding less, and at least one node not responding at all (Figure 4D). He did not experience fevers or other constitutional symptoms around the time of his response.
Clinical response to T-cell infusions. Patient UPN-04 was treated with CY 1 g/m2 2 days before the first of 3 infusions of αCD20-28-BB-ζ CAR+ T cells, which were given 2 to 5 days apart and followed by 14 days of subcutaneous IL-2 injections. (A) Response in a cervical lymph node as imaged by CT scan of the neck at baseline and 1, 6, and 12 months after T-cell infusions. (B) PET-CT scan of the cervical lymph node at baseline and 3 months after T-cell infusions. (C) Change in tumor volume over time. The sum of the products of the diameters of the 7 largest lymph nodes seen on the baseline and 1-, 6-, and 12-month CT scans. (D) Heterogeneity of response in individual lymph nodes. The products of the diameters of the 7 largest lymph nodes from the 6-month CT scan are shown as a percentage of their baseline size. L indicates left; and R, right.
Clinical response to T-cell infusions. Patient UPN-04 was treated with CY 1 g/m2 2 days before the first of 3 infusions of αCD20-28-BB-ζ CAR+ T cells, which were given 2 to 5 days apart and followed by 14 days of subcutaneous IL-2 injections. (A) Response in a cervical lymph node as imaged by CT scan of the neck at baseline and 1, 6, and 12 months after T-cell infusions. (B) PET-CT scan of the cervical lymph node at baseline and 3 months after T-cell infusions. (C) Change in tumor volume over time. The sum of the products of the diameters of the 7 largest lymph nodes seen on the baseline and 1-, 6-, and 12-month CT scans. (D) Heterogeneity of response in individual lymph nodes. The products of the diameters of the 7 largest lymph nodes from the 6-month CT scan are shown as a percentage of their baseline size. L indicates left; and R, right.
It should be noted that B-cell aplasia, which is expected after CD20-targeted therapy,5 was not observed, although B-cell levels were very low at baseline for 2 of the 3 patients because of prior therapy (Figure 6C; and supplemental Figure 2).
Effects of CY lymphodepletion and IL-2 on lymphocyte subsets and cytokine levels. Multiparameter flow cytometry was performed with patient peripheral blood samples collected at baseline and at serial time points after T-cell infusions to quantify (A) all lymphocytes and CD4+ and CD8+ T cells, expressed as percentage change from baseline and (B) absolute numbers of Treg cells (CD4+/FoxP3+) and the ratios of Treg to CD4+ T cells and CD8+ T cells for UPN-02 (●), UPN-03 (■), and UPN-04 (▴). (C) CD19+ and CD20+ B cells, expressed as percentage change from baseline. (D) Levels of multiple cytokines were measured in patient serum samples collected at serial time points after CY administration using a Luminex microbead immunoassay and expressed as fold change from baseline (day −15, day −9, and day 0 before CY for UPN-02, UPN-03, and UPN-04, respectively). The range and baseline values (listed in parentheses sequentially for patients UPN-02, UPN-03, and UPN-04) in picograms per milliliter are as follows: IL-2: < 8 to 413 (90.9, 32.7, < 8); IL-4: < 2 to 298 (50.2, 10.6, < 2); IL-6: < 2 to 174 (58.0, 11.7, 2.73); IL-7: 4.53 to 336 (49.7, 14.9, 6.41); IL-10: < 1 to 526 (41.3, 29.4, < 1); IL-12p70: < 8 to 2011 (328, 16.3, < 8); IL-15: 1.61 to 76.4 (21.5, 4.69, 1.74); IL-17: < 15 to 412 (27.3, < 15, < 15); IL-21: < 15 to 4997 (685, 151, < 15); IFN-γ: 1.6 to 23.1 (8.18, 2.52, 1.70); TNF-α: < 3.6 to 164 (28.6, 8.58, < 3.6); and MIP1-α: < 300 to 868 (335, < 300, < 300). Arrows indicate infusions of genetically modified T cells; and black bar, the 14-day period of low-dose IL-2 injections.
Effects of CY lymphodepletion and IL-2 on lymphocyte subsets and cytokine levels. Multiparameter flow cytometry was performed with patient peripheral blood samples collected at baseline and at serial time points after T-cell infusions to quantify (A) all lymphocytes and CD4+ and CD8+ T cells, expressed as percentage change from baseline and (B) absolute numbers of Treg cells (CD4+/FoxP3+) and the ratios of Treg to CD4+ T cells and CD8+ T cells for UPN-02 (●), UPN-03 (■), and UPN-04 (▴). (C) CD19+ and CD20+ B cells, expressed as percentage change from baseline. (D) Levels of multiple cytokines were measured in patient serum samples collected at serial time points after CY administration using a Luminex microbead immunoassay and expressed as fold change from baseline (day −15, day −9, and day 0 before CY for UPN-02, UPN-03, and UPN-04, respectively). The range and baseline values (listed in parentheses sequentially for patients UPN-02, UPN-03, and UPN-04) in picograms per milliliter are as follows: IL-2: < 8 to 413 (90.9, 32.7, < 8); IL-4: < 2 to 298 (50.2, 10.6, < 2); IL-6: < 2 to 174 (58.0, 11.7, 2.73); IL-7: 4.53 to 336 (49.7, 14.9, 6.41); IL-10: < 1 to 526 (41.3, 29.4, < 1); IL-12p70: < 8 to 2011 (328, 16.3, < 8); IL-15: 1.61 to 76.4 (21.5, 4.69, 1.74); IL-17: < 15 to 412 (27.3, < 15, < 15); IL-21: < 15 to 4997 (685, 151, < 15); IFN-γ: 1.6 to 23.1 (8.18, 2.52, 1.70); TNF-α: < 3.6 to 164 (28.6, 8.58, < 3.6); and MIP1-α: < 300 to 868 (335, < 300, < 300). Arrows indicate infusions of genetically modified T cells; and black bar, the 14-day period of low-dose IL-2 injections.
Immune responses against modified cells
Several gene products of the transferred vector, including the murine scFv, NeoR gene, and junctional epitopes, are potentially immunogenic, and we therefore assessed study subjects for evidence of humoral or cellular immune responses against the infused T cells. We evaluated patient serum samples for the presence of Ab against the transgene products using 2 assays: an ELISA testing Ab binding to the murine Leu16 from which the αCD20-28-BB-ζ scFv is derived, expected to be the most immunogenic epitope (Figure 5A); and a flow cytometry-based assay using HEK-293 cells transfected with the L29.19.1 plasmid (Figure 5B). We observed no convincing evidence of humoral immune responses to the infused cells. We tested for cellular immune responses by coculturing patient PBMCs collected at serial time points with either irradiated CAR-transduced LCL (UPN-02 and UPN-03) or irradiated CAR+ T cells (UPN-04) in 2 1-week stimulations, followed by a chromium release assay against labeled autologous CAR+ T cells as targets. We found no evidence of cellular immune responses against the modified T cells (Figure 5C).
Immune responses to modified T cells. Two assays were performed to detect Ab against the transgene in patient serum samples collected before and at serial time points after T-cell infusions. (A) An ELISA was performed testing for the presence of Ab binding to Leu16 mouse anti–human CD20 Ab (from which the αCD20-28-BB-ζ CAR is derived). Baseline patient serum was used as a negative control. Data represent the mean ± SEM of duplicate values. (B) A flow cytometric assay was performed in which serial patient serum samples were incubated with HEK-293 cells genetically modified to express the αCD20-28-BB-ζ CAR, untransfected HEK-293 cells (negative control), or autologous EBV-LCL (positive control), followed by FITC-conjugated goat anti–human F(ab′)2 Ab. The median fluorescence intensity (MFI) for each sample at various time points at the 1:2 dilution is shown for each cell line. The black bar indicates the 14-day period of low-dose IL-2 injections. (C) The presence of cellular immune responses to infused T cells was assessed by stimulating serially collected patient PBMCs with irradiated autologous EBV-LCL modified to express the αCD20-28-BB-ζ CAR and NeoR gene products (UPN-02 and UPN-03) or irradiated autologous CAR+ T cells (UPN-04) at a 2:1 responder/stimulator ratio. After two 1-week stimulations, these PBMCs were used as effectors in 51Cr-release assays in which target cells were pre-infusion modified T cells, untransfected autologous EBV-LCL, CAR+ autologous EBV-LCL, or untransfected autologous PBMCs at E:T ratios of 25:1 (UPN-03 and UPN-04) or 12.5:1 (UPN-02). The lysis of CAR+ LCL is expressed as the difference between CAR+ and CAR− LCL for patients UPN-02 and UPN-03. The mean ± SEM of triplicate wells is shown.
Immune responses to modified T cells. Two assays were performed to detect Ab against the transgene in patient serum samples collected before and at serial time points after T-cell infusions. (A) An ELISA was performed testing for the presence of Ab binding to Leu16 mouse anti–human CD20 Ab (from which the αCD20-28-BB-ζ CAR is derived). Baseline patient serum was used as a negative control. Data represent the mean ± SEM of duplicate values. (B) A flow cytometric assay was performed in which serial patient serum samples were incubated with HEK-293 cells genetically modified to express the αCD20-28-BB-ζ CAR, untransfected HEK-293 cells (negative control), or autologous EBV-LCL (positive control), followed by FITC-conjugated goat anti–human F(ab′)2 Ab. The median fluorescence intensity (MFI) for each sample at various time points at the 1:2 dilution is shown for each cell line. The black bar indicates the 14-day period of low-dose IL-2 injections. (C) The presence of cellular immune responses to infused T cells was assessed by stimulating serially collected patient PBMCs with irradiated autologous EBV-LCL modified to express the αCD20-28-BB-ζ CAR and NeoR gene products (UPN-02 and UPN-03) or irradiated autologous CAR+ T cells (UPN-04) at a 2:1 responder/stimulator ratio. After two 1-week stimulations, these PBMCs were used as effectors in 51Cr-release assays in which target cells were pre-infusion modified T cells, untransfected autologous EBV-LCL, CAR+ autologous EBV-LCL, or untransfected autologous PBMCs at E:T ratios of 25:1 (UPN-03 and UPN-04) or 12.5:1 (UPN-02). The lysis of CAR+ LCL is expressed as the difference between CAR+ and CAR− LCL for patients UPN-02 and UPN-03. The mean ± SEM of triplicate wells is shown.
Effect of patient conditioning with CY lymphodepletion and IL-2
Multiple prior studies have shown a benefit of lympodepletion before adoptive T-cell therapy, hypothesized to occur via multiple potential mechanisms including increased availability of homeostatic cytokines resulting from depletion of T cells acting as cytokine sinks,32 elimination of regulatory T cells (Tregs),33,34 enhanced susceptibility of tumor cells to T-cell lysis,35 and, in the case of adoptive therapy targeting B-cell antigens, removal of competing CD19- or CD20-expressing cells, which can potentially lead to depletion of CAR+ T cells targeting those antigens.2,36 The optimal regimen and dose intensity of lymphodepleting therapy are not known. We chose an intermediate dose of CY (1000 mg/m2). Previous studies at our center have suggested that T-cell persistence may be improved with administration of low-dose IL-2,15,37 which led us to administer 250 000 U/m2 of IL-2 subcutaneously twice daily for 2 weeks in this study. We evaluated the effect of these adjuvant therapies on lymphocyte subsets, including Tregs, using multiparameter flow cytometry, and on serum cytokine levels.
This dose of CY resulted in significant lymphopenia of all subsets tested (Figure 6A-C). At the nadir, the absolute CD3+ counts were reduced in the 3 patients by 93%, 80%, and 77%, respectively. The absolute Treg level was reduced by 79%, 96%, and 87% at the nadir for each patient after CY administration. B-cell counts were decreased by 80%, 59%, and 85%, respectively. The lymphodepletion was transient, with T-cell counts recovering to baseline levels after approximately 12 days. Subsequently, during IL-2 therapy, lymphocyte counts increased predictably to higher than baseline levels.
IL-2 is known to promote Treg proliferation, and as expected, we observed increased Treg levels after IL-2 injections. In patient UPN-03, the ratio of Tregs to CD4+ and CD8+ cells did not change from baseline, but in UPN-02 and UPN-04, preferential expansion of Tregs was observed, which, in the case of UPN-04, persisted for at least 1 year (Figure 6B).
Serum cytokine levels increased in all patients, although the timing varied, correlating with T-cell infusions and lymphodepletion in UPN-02 and with IL-2 injections in UPN-03 and UPN-04 (Figure 6D). No serum was available from UPN-04 at the time of his clinical response, so it is unknown whether his cytokine levels increased at that time as observed in a recent CD19-CAR study.6,38 The homeostatic cytokines IL-7 and IL-15 are of particular interest, and increases in serum levels followed the same pattern as the other cytokines tested. Because IL-15 is trans-presented at the cell surface in conjunction with IL-15Rα,39 serum levels may not be as meaningful as cell surface IL-15 and IL-15Rα. We therefore measured surface IL-15 and IL-15Rα using flow cytometry on PBMCs collected at various time points after CY administration and found a small but measurable increase in IL-15 and IL-15Rα on the surface of both CD3+ T cells and CD14+ monocytes during the period of lymphopenia for all 3 patients (supplemental Figure 3).
Discussion
The treatment regimen used in this study was generally safe and well tolerated. One patient developed a transient infusional febrile syndrome of fever, mild hypoxemia, and orthostatic hypotension, all of which resolved after overnight observation. Similar infusional symptoms have been observed in other CAR trials.2,38 The other 2 treated patients had no symptoms attributable to T-cell infusions. Most of the adverse events observed were associated with CY and IL-2 and were predictable and manageable. These safety results should be interpreted with caution because of the small sample size. It is also possible that a third-generation CAR could engender significant toxicity if expressed at higher levels or in T cells with better engraftment potential, as suggested by a recent study using CD19-41BB CAR-bearing T cells modified using a lentiviral vector.6,38
The cell production methods used in this protocol resulted in levels of CAR expression that were below the level of detection by flow cytometry and Western blotting. The cause for low expression of the CAR may relate to inefficient gene transfer by electroporation and separate promoters for NeoR and CAR genes, such that G418 resistance does not necessarily select cells with the highest CAR expression. In addition, exposure of the T cells during expansion to CD20+ LCL and feeder cells might induce apoptosis of CARhi cells and favor the outgrowth of T cells with lower CAR expression (S. Terakura and S.R.R., unpublished data, June 2011).40
Although higher CAR expression would undoubtedly be advantageous, our results suggest that even low CAR expression can produce significant effects, as evidenced by in vitro cytotoxicity against lymphoma cell lines and at least one likely in vivo tumor response. This is supported by past reports of antigen-specific cytotoxicity by CARs expressed below the limit of detection by flow cytometry41,42 and with the observation that as few as 3 TCR-peptide-MHC complexes are sufficient to induce killing of target cells.43 Furthermore, because activity of CAR+ T cells depends on levels of both antigen and CAR,40 high CD20 expression on lymphoma cells may “compensate” somewhat for the low CAR expression.
The clinical results of this therapy were promising: 2 patients without evaluable disease remained free of progression for 12 and 24 months, and the third patient had an objective partial response. The contribution of the modified T cells to the progression-free periods of patients UPN-02 and UPN-03 cannot be determined in the absence of measurable disease; however, the median time to progression for relapsed MCL in most clinical trials is approximately 6 months. For patient UPN-04, the timing of the response was highly suggestive of a T cell-mediated effect, as there was initial mild progression after CY, but more than 3 months later there was a dramatic reduction in several lymph nodes. Although a spontaneous remission cannot be ruled out, adoptively transferred T cells were detected by quantitative PCR in a lymph node after infusions and were present in the patient's peripheral blood before and after the response, albeit at a low level. It is unclear why the response varied among different lymph nodes and why only a partial remission was achieved. Potential explanations include factors in the microenvironment varying from node to node, such as inhibition of effector activity by Tregs, suppressive cytokines, such as TGF-β, IL-10, or other inhibitory signals, or selective outgrowth of low or negative antigen-expressing cells. In addition, T cell factors, such as inadequate levels of localization to lymph nodes, exhaustion, T-cell subset-specific intrinsic limitations, or failure to achieve activation signal threshold because of low CAR expression, may have resulted in failure to clear the tumor. Future adoptive T-cell therapy clinical trial protocols should consider including a biopsy at the time of relapse to help elucidate mechanisms of immune escape.
The development of B-cell aplasia is an expected occurrence in CAR trials targeting B-cell antigens, which we did not observe. There was no significant reduction in peripheral B cells even in the patient who achieved a partial remission, although this phenomenon was observed in another recent trial as well.2 It is possible that T cells with low CAR expression are not able to mount a significant response against very low levels of circulating B cells, in contrast to a lymph node with a high density of CD20+ lymphoma cells. This is consistent with previous observations that a sufficiently high level of antigen expression is required to provoke a response in T cells with low CAR expression.40,41
The recent dramatic responses observed by Kalos et al38 have naturally led to scrutiny of other CAR trials in an attempt to discern which of the many variables in CAR therapy are the most important. Many differences exist between that study and ours: target antigen, disease target, vector, culture methods, costimulatory domains, lymphodepletion regimen, and use of IL-2. It is difficult to know which of these variables could have been altered in our trial to obtain stronger clinical responses, but we speculate that the low level of surface membrane CAR expression in our study and protracted culture time, potentially leading to a degree of T-cell exhaustion, may be 2 of the most important factors.
We were able to detect the presence of genetically modified T cells in the peripheral blood up to 12 months after infusions by quantitative PCR, although the low levels precluded analysis of the CAR expression, phenotype, and functionality of these cells. The peak level of cells we detected around the time of T-cell infusions was in the 3% to 4% range, comparable with other recently published CAR studies.2,5,21 Higher levels of circulating CAR+ cells were observed by Kalos et al in 2 patients achieving complete remissions,38 suggesting that more robust engraftment may be desirable. We also detected modified T cells in malignant lymph nodes in all 3 patients at 24 hours after the final T-cell infusion. Although the levels of tumor-infiltrating CAR+ cells were very low, a later time point may have been more relevant for sampling given the more than 3-month delay until response in patient UPN-04.
This trial was not designed to discern whether the inclusion of costimulatory domains improves on the functionality of first-generation CAR+ T cells. The improvement in persistence from 2 months in our previous study to 12 months, along with apparently enhanced antitumor activity, suggests a possible beneficial effect of the costimulatory domains, although this could also be partially the result of CY lymphodepletion. It is worth noting that the in vitro cytotoxicity of the T-cell product for UPN-02, which consisted primarily of CD4+ T cells, was equivalent to that of the other patients with CD8+-predominant products. Robust cytolytic activity can be acquired by CD4+ cells cultured in vitro with IL-244 and has been observed with ex vivo cultured CD4+ CAR T cells in clinical trials as well.2
As with our previous study, we found no evidence of cellular or humoral immune responses against the infused cells. This contrasts with other recent CAR studies that found antitransgene immune responses directed against the NeoR gene or scFv epitopes.45,46 This may be partially explained by the low level of transgene expression observed in our study, but it is probably the result of the impaired immunity of lymphoma patients that mitigates against rejection of genetically modified T cells. The small number of patients treated on this trial makes it difficult to draw definitive conclusions about the immunogenicity of our CAR protein, but it is worth striving to minimize potential immunogenicity in designing future CARs by using humanized Abs, minimizing junctional regions, and avoiding xenogeneic genes, such as those encoding for antibiotic resistance.
The optimal lymphodepletion regimen for adoptive T-cell therapy is not known. Recent clinical trial results suggest that transfer of T cells with high activity and capacity for in vivo proliferation may obviate the need for high-intensity lymphodepletion strategies.6,38 The intermediate dose of CY we used led to effective (∼ 80%-90%) levels of lymphodepletion of all T-cell subsets, including Tregs, with minimal toxicity, and may be a reasonable regimen to consider in future trials. Low-dose IL-2 injections have been found to prolong T-cell persistence, but as demonstrated in this and other studies, IL-2 also leads to expansion of Tregs; therefore, other cytokines, such as IL-15, may be more advantageous.
One of the primary endpoints of this study was to assess feasibility. The generation of CD20-specific T cells using the methods described was technically feasible but difficult to sustain. Gene transfer by electroporation of naked plasmid DNA is highly inefficient and necessitates antibiotic selection of transfectants during expansion. This process results in prolonged and intensive use of many resources (eg, personnel, cGMP facilities) and is not practical for scaling up to treat larger numbers of patients. Furthermore, it probably impairs T-cell functionality and potential for in vivo expansion, as evidenced by a decrease in cytotoxicity in one patient, shortened telomeres, and low levels of engraftment in vivo. In addition, taking into account patients treated in our previous trial,15 cells failed to expand adequately in 4 of 13 patients. No characteristics emerge as clear risk factors for expansion failure among these patients, aside from expanding T cells as clones as opposed to bulk cultures (supplemental Tables 2 and 3). In view of these problems, in future trials we plan to use lentiviral vectors for gene delivery and shorter ex vivo culture times, which we anticipate will prove to be much more efficient and feasible, and will probably result in higher CAR expression and enhanced antilymphoma activity.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sally Lundberg, RN, Jennifer Davies, and the patients who enrolled in the study.
This work was supported by the National Institutes of Health R21 grant CA117131, the Lymphoma Research Foundation (B.G.T. and O.W.P), a Damon Runyon–Pfizer Clinical Investigator Award (B.G.T.), an ASCO Young Investigator Award (B.G.T.), National Institutes of Health grant 1 UL1 RR025014 of the University of Washington Clinical Research Center, and donations from David and Patricia Giuliani and the Edson Foundation.
National Institutes of Health
Authorship
Contribution: B.G.T., M.C.J., S.R.R., and O.W.P. designed the clinical trial and associated experiments; B.G.T., J.W., X.Q., and J.M.P. performed experiments; B.G.T., C.G.L., and O.W.P. oversaw cell processing and quality control testing; Y.L. constructed the initial CAR plasmid; B.G.T., A.K.G., D.G.M., and O.W.P. enrolled patients on study and took care of patients; B.G.T. wrote the manuscript; B.G.T., M.C.J., J.W., A.K.G., D.G.M., Y.L., L.E.B., A.R., S.J.F., P.D.G., S.R.R., and O.W.P. interpreted data; and B.G.T., M.C.J., S.R.R., A.K.G., and O.W.P. contributed to the revisions of the manuscript.
Conflict-of-interest disclosure: M.C.J. is the inventor of a licensed patent related to the CD20 CAR plasmid and a cofounder and equity holder in ZetaRx Biosciences Inc, which has licensed the patent. The remaining authors declare no competing financial interests.
Correspondence: Brian G. Till, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D3-190, Seattle, WA 98109; e-mail: tillb@fhcrc.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal