Abstract
The mechanisms mediating hematopoietic stem and progenitor cell (HSPC) mobilization by G-CSF are complex. We have found previously that G-CSF–enforced mobilization is controlled by peripheral sympathetic nerves via norepinephrine (NE) signaling. In the present study, we show that G-CSF likely alters sympathetic tone directly and that methods to increase adrenergic activity in the BM microenvironment enhance progenitor mobilization. Peripheral sympathetic nerve neurons express the G-CSF receptor and ex vivo stimulation of peripheral sympathetic nerve neurons with G-CSF reduced NE reuptake significantly, suggesting that G-CSF potentiates the sympathetic tone by increasing NE availability. Based on these data, we investigated the NE reuptake inhibitor desipramine in HSPC mobilization. Whereas desipramine did not by itself elicit circulating HSPCs, it increased G-CSF–triggered mobilization efficiency significantly and rescued mobilization in a model mimicking “poor mobilizers.” Therefore, these data suggest that blockade of NE reuptake may be a novel therapeutic target to increase stem cell yield in patients.
Introduction
Hematopoietic stem and progenitor cells (HSPCs) traffic continuously between the blood and BM under homeostasis.1,2 G-CSF can enhance this phenomenon and therefore has emerged as one of the most potent and safe agents to mobilize HSPCs for stem cell transplantation, but its mechanisms are complex. Studies have attributed its mobilizing activity to the release of neutrophil-derived proteolytic enzymes that leads to the cleavage of CXCL12 and other retention signals.3,4 However, this model has been challenged by the normal mobilization found in the absence of serine protease and metalloproteinase activity,5 suggesting functional redundancy with other proteases or the involvement of additional mechanisms. Adrenergic signals play a key role in the release of HSPCs either when enforced by G-CSF6 or during steady state.1 Egress is associated with the down-regulation in Nestin+ niche cells of Cxcl12 mRNA expression and other genes (eg, Kitl, Angpt1, and Vcam1) that contribute to HSPC retention in the BM.7 However, expression of the G-CSF receptor (encoded by Csf3r) on transplantable hematopoietic cells has been reported to be required for mobilization,8 and recent studies suggest that this cell may be a macrophage9-11 with activity that promotes hematopoietic stem cell (HSC) retention by the niche.9 Because the administration of either G-CSF or β3 adrenergic agonists has the same effect on the expression of these retention factors by the niche,7 it suggests that increased sympathetic tone may enhance mobilization.
Methods
Eight-week-old male C57BL/6J mice were purchased from the National Cancer Institute (Frederick Cancer Research Center, Frederick, MD). All mice were housed at the Center for Comparative Medicine and Surgery at Mount Sinai School of Medicine or the institute for Animal Studies at Albert Einstein College of Medicine, and the animal care and use committees of both institutions approved the experimental procedures performed on mice. For HSPC mobilization, mice received 8 consecutive injections of G-CSF (125 μg/kg twice a day subcutaneously) or saline. Desipramine (10 mg/kg intraperitoneally), reboxetine (5 mg/kg intraperitoneally), or saline treatment was started 4 days before G-CSF treatment and continued throughout the experiment. For AMD3100-induced mobilization, mice were treated with desipramine (10 mg/kg intraperitoneally) or saline. On day 8, mice were injected with AMD3100 (5 mg/kg subcutaneously) 1 hour before blood was harvested by retroorbital bleeding. All other experimental methods are described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results and discussion
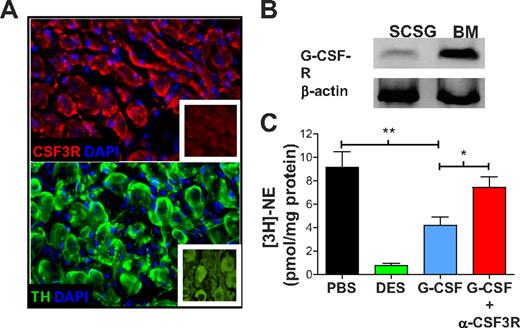
We performed immunofluorescence analyses in murine superior cervical sympathetic ganglia (SCSG) to assess the expression of Csf3r in the peripheral sympathetic nerves. SCSG neurons have large cell bodies and express the catecholaminergic enzyme tyrosine hydroxylase (Figure 1A). These SCSG neurons also expressed Csf3r (Figure 1A) and Csf3r mRNA specifically (Figure 1B). This is consistent with other studies that have shown Csf3r expression in neocortical neurons and that G-CSF promotes neuronal survival after ischemic brain injury.12
The G-CSF receptor is expressed on peripheral sympathetic neurons and its stimulation reduces norepinephrine (NE) uptake. (A) Immunofluorescence staining for the G-CSF receptor (G-CSF-R; Cy3 top panel) or tyrosine hydroxylase (TH; Alexa 647, bottom panel) in sections of murine SCSG neurons. Images were acquired with a 40×/1.4 oil objective in a Zeiss AX10 microscope and a Coolsnap HQ2 camera (Fisher) at room temperature. Images were aquired using Slidebook 5.0 (3I). Purified rabbit immunoglobulin (TH) and isotype control (G-CSFR) are shown in insets. (B) G-CSF-R mRNA expression was assessed by RT-PCR in SCG neurons and whole BM. (C) SCSGs were cultured in medium containing PBS (n = 6), desipramine (DES; n = 6) at a concentration of 10μM, or 50 ng of G-CSF alone (n = 5) or in combination with anti–G-CSFR Ab (1 μg/mL; n = 3). *P < .05; **P < .005.
The G-CSF receptor is expressed on peripheral sympathetic neurons and its stimulation reduces norepinephrine (NE) uptake. (A) Immunofluorescence staining for the G-CSF receptor (G-CSF-R; Cy3 top panel) or tyrosine hydroxylase (TH; Alexa 647, bottom panel) in sections of murine SCSG neurons. Images were acquired with a 40×/1.4 oil objective in a Zeiss AX10 microscope and a Coolsnap HQ2 camera (Fisher) at room temperature. Images were aquired using Slidebook 5.0 (3I). Purified rabbit immunoglobulin (TH) and isotype control (G-CSFR) are shown in insets. (B) G-CSF-R mRNA expression was assessed by RT-PCR in SCG neurons and whole BM. (C) SCSGs were cultured in medium containing PBS (n = 6), desipramine (DES; n = 6) at a concentration of 10μM, or 50 ng of G-CSF alone (n = 5) or in combination with anti–G-CSFR Ab (1 μg/mL; n = 3). *P < .05; **P < .005.
The activity of the natural neurotransmitter of the sympathetic nervous system, norepinephrine (NE), is tightly regulated by a balance of secretion and reuptake by nerve terminals.13 To determine whether Csf3r on sympathetic neurons was functional, we evaluated whether G-CSF could influence NE release. In these studies, isolated SCSGs were loaded with tritiated NE ([3H]-NE), and the ability of G-CSF to increase the release of radiolabeled NE in these organ cultures was assessed. Whereas control KCl induced profound NE release, there was no difference between unstimulated and G-CSF–treated cultures in [3H]-NE content in the medium (supplemental Figure 1). We then evaluated the influence of G-CSF on the reuptake of NE by the peripheral sympathetic nerves by culturing SCSGs in medium containing [3H]-NE in the presence or absence of G-CSF. We found that incubation with G-CSF reduced [3H]-NE reuptake significantly compared with the unstimulated control (Figure 1C). Preincubation with an Ab that blocked Csf3r abrogated this effect, indicating that the effect was specific (Figure 1C). These data therefore suggest that G-CSF enhances the sympathetic tone by altering the ability of sympathetic neurons to take up NE, thereby increasing catecholamines available to target cells. The drug desipramine, an NE reuptake inhibitor, completely blocked NE uptake in the SCSG organ culture (Figure 1C), which led us to investigate whether in vivo blockade of NE reuptake could enhance G-CSF mobilization efficiency.
Mice were treated with desipramine and G-CSF, as shown in Figure 2A. Desipramine alone did not mobilize HSPCs (supplemental Figure 2A-B), an observation consistent with previous studies indicating that β-adrenergic agonists are not sufficient to induce robust mobilization6 and that other G-CSF–mediated signals cooperate with adrenergic receptors.1,6,9 When administered in conjunction with G-CSF, desipramine increased the number of clonogenic progenitors (CFU-Cs) and Lin−Sca-1+c-kit+Flt3− (LSKF) cells (which are highly enriched in HSCs) in the peripheral blood significantly compared with mice treated with G-CSF alone (Figure 2B-D). The combination of G-CSF and desipramine increased the number of functional HSCs, as measured by the number of long-term culture-initiating cells in the blood (Figure 2E and supplemental Table 1). We detected no difference in BM cellularity, CFU-Cs, or LSKF cell numbers in the BM (supplemental Figure 2C-E), indicating that the increase in progenitor mobilization does not result from increased numbers in the BM. Consistent with the fact that desipramine treatment enhanced G-CSF–induced sympathetic outflow, it did not affect AMD3100-induced mobilization (supplemental Figure 2F-G). This notion is further supported by the fact that another selective NE reuptake blocker, reboxetine, enhanced G-CSF–induced mobilization to a similar extent (supplemental Figure 2H-I).
The NE reuptake inhibitor desipramine enhances G-CSF–triggered HSC/progenitor mobilization. (A) Treatment scheme of 8-week-old male C57BL/6 mice for HSPC mobilization using G-CSF in combination with desipramine. (B-C) Absolute numbers of CFU-Cs (B) or LSKF cells (C) in the peripheral blood of mice mobilized with G-CSF alone (G) or in combination with desipramine (G+D), respectively (n = 4-5). (D) Gating strategy and representative dot plots to determine LSKF numbers in the peripheral blood of mice mobilized with G-CSF alone or in combination with desipramine, respectively. (E) Long-term culture-initiating cells per milliliter in the blood of mice mobilized as in panel A (n = 4-5). (F) Experimental design to assess the effect of desipramine on G-CSF–induced mobilization in poor mobilizer mice. (G) LSKF cells per femur (n = 12-14) in poor mobilizer mice. (H-I) CFU-Cs (H) and LSKF cells (I) after mobilization in the peripheral blood of poor mobilizer mice (n = 8-7). Dashed lines indicate the mobilization values in nonirradiated mice as determined in panels B and C. *P < .05; **P < .005.
The NE reuptake inhibitor desipramine enhances G-CSF–triggered HSC/progenitor mobilization. (A) Treatment scheme of 8-week-old male C57BL/6 mice for HSPC mobilization using G-CSF in combination with desipramine. (B-C) Absolute numbers of CFU-Cs (B) or LSKF cells (C) in the peripheral blood of mice mobilized with G-CSF alone (G) or in combination with desipramine (G+D), respectively (n = 4-5). (D) Gating strategy and representative dot plots to determine LSKF numbers in the peripheral blood of mice mobilized with G-CSF alone or in combination with desipramine, respectively. (E) Long-term culture-initiating cells per milliliter in the blood of mice mobilized as in panel A (n = 4-5). (F) Experimental design to assess the effect of desipramine on G-CSF–induced mobilization in poor mobilizer mice. (G) LSKF cells per femur (n = 12-14) in poor mobilizer mice. (H-I) CFU-Cs (H) and LSKF cells (I) after mobilization in the peripheral blood of poor mobilizer mice (n = 8-7). Dashed lines indicate the mobilization values in nonirradiated mice as determined in panels B and C. *P < .05; **P < .005.
We reasoned that patients who mobilize poorly might benefit from increased sympathetic tone more than healthy individuals. To test this idea in a preclinical setting, we modeled “poor mobilizers,” which commonly result from prior cytotoxic therapy,14 by subjecting mice to a lethal dose of irradiation and BM transplantation. After a 4-month recovery period, mice were treated with desipramine and mobilized with G-CSF (Figure 2F). Desipramine treatment did not change the number of HSPCs in the BM (Figure 2G); however, transplanted animals exhibited lower numbers of G-CSF–mobilized progenitors and LSKF cells than did healthy mice (Figure 2H-I). Remarkably, desipramine approximately doubled HSPC mobilization in these mice (Figure 2H-I) to a level closer to the values of healthy mice (compare “D+G” with dashed red lines in Figure 2H-I). Because a 2-fold increase in mobilization is sufficient to obtain adequate HSPC yields in approximately 50% of poor mobilizers,15 this approach has the potential to be clinically useful in this setting. Although desipramine may not be as potent as AMD3100, which induces an approximately 3-fold increase in mobilization when administered together with G-CSF,16 it provides an inexpensive alternative that should be investigated further in a clinical trial. Inhibition of NE reuptake might be used not only to reduce the number of patients with poor HSPC yield, but also to increase HSPC yields in healthy individuals. Transplantation with increased numbers of HSPCs may accelerate engraftment17 and reduce transplantation-related morbidity. Further, because AMD3100 and desipramine seem to act through different pathways (supplemental Figure 2F-G), it is possible that the combination of G-CSF, desipramine, and AMD3100 may enhance mobilization even further.
The data from the present study indicate that G-CSF likely increases the sympathetic tone in the BM microenvironment by engaging neurally expressed Csf3r directly and by inhibiting NE reuptake. Modulation of the sympathetic outflow by desipramine increases the efficiency of HSPC harvests and may thus provide a therapeutic alternative.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Colette Prophete for mouse husbandry and technical assistance.
D.L. was a fellow of the Fundación Ramón Areces. I.B. is the recipient of a 2010 American Society of Hematology–European Hematology Association research-exchange award. This work was supported by the National Institutes of Health (R01DK056638 and R01HL097819 to P.S.F.).
National Institutes of Health
Authorship
Contribution: D.L. and I.B. performed the experiments, analyzed the data, and wrote the manuscript; M.B. and Y.K. performed the experiments; C.M. performed the experiments and analyzed the data; S.M.-F. contributed to experiments on norepinephrine reuptake and release; and P.S.F. designed the research, supervised the study, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for S.M.-F. is Centro Nacional de Investigaciones Cardiovasculares Carlos CNIC, Madrid, Spain.
Correspondence: Paul S. Frenette, Ruth L. and David S. Gottesman Institute for Stem Cell Biology and Regenerative Medicine, Albert Einstein College of Medicine, 1301 Morris Park Ave, Rm 101B, Bronx, NY 10461; e-mail: paul.frenette@einstein.yu.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal