Abstract
Combinations of bortezomib (V) and dexamethasone (D) with either lenalidomide (R) or cyclophosphamide (C) have shown significant efficacy. This randomized phase 2 trial evaluated VDC, VDR, and VDCR in previously untreated multiple myeloma (MM). Patients received V 1.3 mg/m2 (days 1, 4, 8, 11) and D 40 mg (days 1, 8, 15), with either C 500 mg/m2 (days 1, 8) and R 15 mg (days 1-14; VDCR), R 25 mg (days 1-14; VDR), C 500 mg/m2 (days 1, 8; VDC) or C 500 mg/m2 (days 1, 8, 15; VDC-mod) in 3-week cycles (maximum 8 cycles), followed by maintenance with V 1.3 mg/m2 (days 1, 8, 15, 22) for four 6-week cycles (all arms) ≥ very good partial response was seen in 58%, 51%, 41%, and 53% (complete response rate of 25%, 24%, 22%, and 47%) of patients (VDCR, VDR, VCD, and VCD-mod, respectively); the corresponding 1-year progression-free survival was 86%, 83%, 93%, and 100%, respectively. Common adverse events included hematologic toxicities, peripheral neuropathy, fatigue, and gastrointestinal disturbances. All regimens were highly active and well tolerated in previously untreated MM, and, based on this trial, VDR and VCD-mod are preferred for clinical practice and further comparative testing. No substantial advantage was noted with VDCR over the 3-drug combinations. This trial is registered at www.clinicaltrials.gov (NCT00507442).
Introduction
The initial therapy of multiple myeloma (MM) has changed significantly in the past decade because of the introduction of new drugs such as the proteasome inhibitor bortezomib (V) and immunomodulatory drugs (thalidomide, lenalidomide [R]).1 Combinations of bortezomib or lenalidomide with dexamethasone (D) have improved response rates and prolonged response duration compared with older approaches such as vincristine/doxorubicin/dexamethasone regimens.2-5 Based on these studies, bortezomib and lenalidomide have been incorporated into various multidrug combinations in an effort to improve efficacy.6-13 Three- and 4-drug combinations of these agents with melphalan, cyclophosphamide (C), or doxorubicin are being studied in phase 2 and 3 clinical trials.6,8,14 In particular, addition of thalidomide, lenalidomide, or cyclophosphamide to bortezomib and dexamethasone has been associated with high response rates and longer progression-free survival (PFS).6,8,14 Bortezomib and dexamethasone have also been combined with lenalidomide (VDR or RVD) or cyclophosphamide (VDC) in single institution phase 2 trials, with high overall and complete response (CR) rates.8,14 These findings raise the question of whether a combination of all 4 drugs as initial therapy will further improve response rates and outcomes.
We designed the EVOLUTION study to examine the tolerability and efficacy of combining bortezomib, cyclophosphamide, lenalidomide, and dexamethasone (VDCR) and to study the combination concurrently with 2 commonly used 3-drug combinations (VDR and VDC) in a randomized multicenter setting. We first determined the maximum tolerated dose (MTD) of cyclophosphamide in combination with VDR in a phase 1 trial, where we observed high efficacy for the 4-drug combination.15 We then evaluated the 3 regimens (VDCR, VDR, and VDC) in the randomized phase 2 part of the study, with the goal of selecting one or 2 of the regimens for future phase 3 trials. Based on an interim analysis and phase 2 data8 that became available during the course of the study, a modified VDC regimen was added.
Methods
Patients
Patients 18 years of age or older with previously untreated symptomatic MM, with measurable disease16 and a Karnofsky Performance Status ≥ 50% were enrolled, regardless of their eligibility for autologous stem cell transplantation (ASCT). Diagnosis of MM was made using standard criteria.16 Exclusion criteria included grade ≥ 2 peripheral neuropathy; serum creatinine ≥ 2.5 mg/dL (moderate severe renal dysfunction allowed); absolute neutrophil count (ANC) < 1000/μL; platelets < 70 000/μL; aspartate aminotransferase/alanine aminotransferase > 2 times upper limit of normal (ULN); or total bilirubin > 3 times ULN. Patients with myocardial infarction within the previous 6 months, New York Heart Association class III or IV heart failure, uncontrolled angina, or significant arrhythmias were also excluded. All patients provided written informed consent. Review boards at each site approved the study, which was conducted in accordance with the Declaration of Helsinki.
Study design
This phase 2 study was conducted at 24 centers and enrolled patients from June 2008 to September 2009; data cutoff was April 13, 2011. The primary objective was to determine the combined rate of CR plus very good partial response (VGPR) for the VDCR, VDR, and VDC regimens. Secondary objectives included safety and tolerability, overall response rates (ORR), time to response, time to progression (TTP), PFS, and overall survival (OS). In addition, we explored the feasibility of performing minimal residual disease (MRD) assessment by multiparameter flow cytometry.
Study treatment
Patients were initially randomized to VDCR, VDR, or VDC. Treatment consisted of eight 3-week cycles of induction therapy followed by four 6-week cycles of bortezomib maintenance therapy. The planned doses and dose reductions for toxicities are detailed in Tables 1 and 2. After the interim analysis, the VDC arm was modified to add a third dose of cyclophosphamide at 500 mg/m2 on day 15 (VDC-mod). This change was made despite the VDC arm meeting the prespecified criteria for success at interim analysis, as the response rates observed were lower compared with those from a phase 2 study investigating the same regimen.8 Patients could undergo stem cell mobilization any time after 2 cycles and undergo ASCT any time after 4 cycles.
Treatment arms and drug dosages for toxicity
| Induction, eight 3-wk cycles . | Bortezomib, 1.3 mg/m2, days 1, 4, 8, 11 . | Dexamethasone, 40 mg, days 1, 8, 15 . | Cyclophosphamide, 500 mg/m2,* days 1, 8 . | Lenalidomide, days 1-14 . |
|---|---|---|---|---|
| VDCR | ✓ | ✓ | ✓ | ✓ (15 mg) |
| VDR | ✓ | ✓ | ✓ (25 mg) | |
| VDC | ✓ | ✓ | ✓ | |
| VDC-mod | ✓ | ✓ | ✓ (+day 15) | |
| Maintenance (four 6-wk cycles) | Bortezomib 1.3 mg/m2 (days 1, 8, 15, 22) | |||
| Induction, eight 3-wk cycles . | Bortezomib, 1.3 mg/m2, days 1, 4, 8, 11 . | Dexamethasone, 40 mg, days 1, 8, 15 . | Cyclophosphamide, 500 mg/m2,* days 1, 8 . | Lenalidomide, days 1-14 . |
|---|---|---|---|---|
| VDCR | ✓ | ✓ | ✓ | ✓ (15 mg) |
| VDR | ✓ | ✓ | ✓ (25 mg) | |
| VDC | ✓ | ✓ | ✓ | |
| VDC-mod | ✓ | ✓ | ✓ (+day 15) | |
| Maintenance (four 6-wk cycles) | Bortezomib 1.3 mg/m2 (days 1, 8, 15, 22) | |||
V indicates bortezomib; D, dexamethasone; R, lenalidomide; C, cyclophosphamide; and VDC-mod, as in VDC plus a day 15 dose of C.
Cyclophosphamide was given as a single oral dose, rounded to the nearest 50 mg.
Treatment arms and dose modifications for toxicity
| . | Bortezomib 1.3 mg/m2, days 1, 4, 8, 11 . | Dexamethasone 40 mg, days 1, 8, 15 . | Cyclophosphamide 500 mg/m2,* days 1, 8 . | Lenalidomide, days 1-14 . |
|---|---|---|---|---|
| First-dose reduction | 1.0 mg/m2 days 1, 4, 8, 11 | 20 mg days 1, 8, 15 | 400 mg/m2 days 1, 8 (15**) | 20 mg† days 1-14 |
| Second-dose reduction | 0.7 mg/m2 days 1, 4, 8, 11 | 8 mg days 1, 8, 15 | 300 mg/m2 days 1, 8 (15**) | 15 mg† days 1-14 |
| Third-dose reduction | Discontinue | Discontinue | 200 mg/m2 days 1, 8 (15**) | 10 mg† days 1-14 |
| Fourth-dose reduction | 100 mg/m2 days 1, 8 (15**) | 5 mg† days 1-14 | ||
| Fifth-dose reduction | Discontinue | Discontinue |
| . | Bortezomib 1.3 mg/m2, days 1, 4, 8, 11 . | Dexamethasone 40 mg, days 1, 8, 15 . | Cyclophosphamide 500 mg/m2,* days 1, 8 . | Lenalidomide, days 1-14 . |
|---|---|---|---|---|
| First-dose reduction | 1.0 mg/m2 days 1, 4, 8, 11 | 20 mg days 1, 8, 15 | 400 mg/m2 days 1, 8 (15**) | 20 mg† days 1-14 |
| Second-dose reduction | 0.7 mg/m2 days 1, 4, 8, 11 | 8 mg days 1, 8, 15 | 300 mg/m2 days 1, 8 (15**) | 15 mg† days 1-14 |
| Third-dose reduction | Discontinue | Discontinue | 200 mg/m2 days 1, 8 (15**) | 10 mg† days 1-14 |
| Fourth-dose reduction | 100 mg/m2 days 1, 8 (15**) | 5 mg† days 1-14 | ||
| Fifth-dose reduction | Discontinue | Discontinue |
V indicates bortezomib; D, dexamethasone; R, lenalidomide; C, cyclophosphamide; and VDC-mod, VDC plus a day 15 dose of C.
Cyclophosphamide was given as a single oral dose, rounded to the nearest 50 mg.
Day 15 only for VDC-mod arm.
Values shown for VDR arm; in the VDCR arm, lenalidomide dose reductions were 10, 5, and discontinue.
Patients could receive supportive therapy including bisphosphonates and transfusions as necessary. Prophylactic aspirin (325 mg daily) was required; warfarin or low-molecular-weight heparin could be substituted at investigator discretion based on thrombosis risk. Prophylactic antibiotics for Pneumocystis jirovecii and acyclovir for herpes zoster were recommended. Erythropoietin was permitted but not recommended, given the increased risk of lenalidomide-associated thromboembolism.17 Granulocyte colony-stimulating factor (G-CSF) prophylaxis after grade 4 neutropenia for > 7 days or febrile neutropenia was permitted after day 8 of the second and subsequent cycles.
Assessments
Adverse events (AEs) were graded according to NCI-CTCAE Version 3.0. Response was assessed before every other treatment cycle from cycle 3 onwards. Response categories were based on the International Myeloma Working Group uniform response criteria,18 with the addition of near CR (defined as meeting criteria for CR but with positive serum and/or urine immunofixation). A central laboratory was used for disease assessments and MRD evaluation. Response criteria were applied by automated computer algorithm to determine response, and further verified by the principal investigator and medical monitor. Use of the algorithm assured consistent, rigorous assessment of responses across all patients.
For MRD evaluation, marrow aspirates were obtained at baseline and at the time of suspected CR. Aspirates were placed in a fixative (Cytochek; for maintaining surface expression of antigens) and were analyzed within 48 hours using a panel of antibodies against CD138, CD38, CD45, CD19, CD56, κ and λ on a FACSCanto flow cytometer.18 An algorithmic gating strategy was used using CD38/CD138 for initial detection of plasma cells, followed by assessment of κ/λ expression on detected plasma cells, and finally CD56 and/or CD19 expression to detect aberrant plasma cells in the absence of light-chain restriction. If no clonal plasma cells were detected at thresholds of < 20 events or < 0.01% of total nucleated events, the sample was considered MRD negative.
Statistical methods
Based on the primary objective (CR + VGPR), sample size was estimated separately for each of the 3 treatment arms using a one-sided test at the significance level of α = 0.15, power of 80%, a null hypothesis CR + VGPR rate of 30%, and an alternative hypothesis CR + VGPR rate of 45%. Based on Simon optimal 2-stage design,19 39 patients per arm (or 117 in total) were required. To obtain 117 response-evaluable patients (39 per arm), enrollment of ∼ 126 patients was targeted (42 per arm). After the modification to add a VDC-mod arm, 16 response-evaluable patients were required in this arm to provide estimates of response rate with a certain degree of confidence. For example, if 8 (50%) of the 16 patients achieve VGPR or better, the one-sided 80% exact binomial confidence interval (CI) is 37%, 100%. Hence, with a 50% response rate, we would be 80% confident the rate for ≥ VGPR is at least 37%. Given the noncomparative nature of the study, no formal statistical comparisons between the 4 treatment arms were made. Randomization was stratified on the basis of the International Staging System (ISS) and whether, in the opinion of the treating physician, the patient was eligible for ASCT. Patients enrolled at the MTD in the phase 1 part were analyzed together with patients enrolled in the VDCR arm in phase 2.15
All patients who received at least 1 dose of any study drug were included in the safety analyses. A modified intent-to-treat (mITT) population, defined as all patients who were randomized and received at least one dose of any study drug, was used for the analyses of TTP and survival. The response-evaluable population was defined as a subset of the mITT population with measurable disease at baseline and with at least one postbaseline response assessment. This group was used for all response analyses.
Results
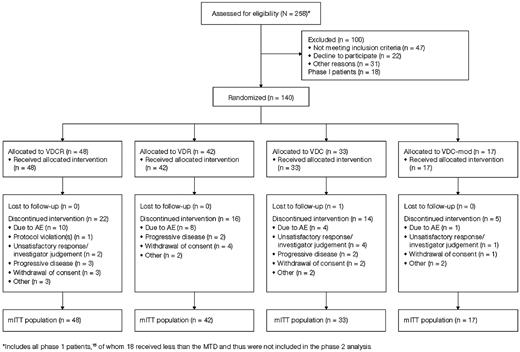
One hundred forty patients were enrolled, including 7 in the VDCR arm treated at MTD in phase 1; patient disposition is shown in Figure 1. The median follow-up from randomization was 20 months (range: 0-30); 20, 20, 22 and 15 months, respectively, for the VDCR, VDR, VDC, and VDC-mod arms. No patients are currently receiving treatment. Baseline characteristics were similar between the treatment arms (Table 3). The median age was 61 years (range: 40-81) with 31% > 65 years of age. The majority of patients were considered transplant eligible. Overall, 36%, 41%, and 23% of patients, respectively, had ISS stage I, II, or III disease. High-risk MM was present in 17% of patients based on the presence of del 13/−13q14 by conventional cytogenetics, any of t(4;14), t(14;16), or −17p13 by conventional cytogenetics or FISH, or hypodiploidy by conventional metaphase cytogenetic analysis.1
Baseline characteristics
| Characteristic . | VDCR n = 48 . | VDR n = 42 . | VDC n = 33 . | VDC-mod n = 17 . |
|---|---|---|---|---|
| Median age (range), y | 61.5 (41-81) | 60 (42-75) | 62 (40-75) | 63 (40-72) |
| Age over 65 y, n (%) | 15 (31) | 13 (31) | 11 (33) | 5 (29) |
| Male sex, n (%) | 29 (60) | 24 (57) | 19 (58) | 7 (41) |
| Myeloma type, n (%) | ||||
| IgG | 33 (69) | 27 (64) | 22 (67) | 8 (47) |
| IgA | 8 (17) | 9 (21) | 7 (21) | 2 (12) |
| Light chain | 7 (15) | 6 (14) | 3 (9) | 6 (35) |
| Other | 0 | 0 | 1 (3) | 1 (6) |
| International Staging System stage, n (%) | ||||
| I | 16 (33) | 16 (38) | 11 (33) | 8 (47) |
| II | 22 (46) | 18 (43) | 11 (33) | 6 (35) |
| III | 10 (21) | 8 (19) | 11 (33) | 3 (18) |
| Karnofsky Performance Status, n (%) | ||||
| 50%-60% | 2 (4) | 1 (2) | 1 (3) | 1 (6) |
| 70%-80% | 14 (29) | 16 (38) | 9 (27) | 7 (41) |
| 90%-100% | 32 (67) | 25 (60) | 23 (70) | 9 (53) |
| Serum creatinine > 1.5 mg/dL, % | 10 | 5 | 12 | 0 |
| Median bone marrow plasma cell, % (range) | ||||
| Aspirate | 28 (0-95) | 27 (2-90) | 41 (1-93) | 30 (4-75) |
| Biopsy | 33 (0-90) | 40 (1-80) | 47 (2-90) | 38 (13-90) |
| Lactate dehydrogenase, > 190 U/L, % | 49 | 44 | 43 | 38 |
| Bone disease present, % (skeletal abnormality) | 72 | 90 | 94 | 94 |
| Eligible for autologous stem cell transplantation, n (%) | 46 (96) | 41 (98) | 31 (94) | 14 (82) |
| High-risk multiple myeloma,*n (%) | 7 (15) | 7 (17) | 7 (23) | 3 (18) |
| Del 13 (metaphase cytogenetics) | 2 (5) | 1 (3) | 1 (4) | 1 (6) |
| t(4;14) | 3 (6) | 1 (2) | 2 (6) | 1 (6) |
| t(14;16) | 0 | 0 | 0 | 0 |
| −17p13 | 3 (6) | 4 (10) | 5 (16) | 1 (6) |
| Characteristic . | VDCR n = 48 . | VDR n = 42 . | VDC n = 33 . | VDC-mod n = 17 . |
|---|---|---|---|---|
| Median age (range), y | 61.5 (41-81) | 60 (42-75) | 62 (40-75) | 63 (40-72) |
| Age over 65 y, n (%) | 15 (31) | 13 (31) | 11 (33) | 5 (29) |
| Male sex, n (%) | 29 (60) | 24 (57) | 19 (58) | 7 (41) |
| Myeloma type, n (%) | ||||
| IgG | 33 (69) | 27 (64) | 22 (67) | 8 (47) |
| IgA | 8 (17) | 9 (21) | 7 (21) | 2 (12) |
| Light chain | 7 (15) | 6 (14) | 3 (9) | 6 (35) |
| Other | 0 | 0 | 1 (3) | 1 (6) |
| International Staging System stage, n (%) | ||||
| I | 16 (33) | 16 (38) | 11 (33) | 8 (47) |
| II | 22 (46) | 18 (43) | 11 (33) | 6 (35) |
| III | 10 (21) | 8 (19) | 11 (33) | 3 (18) |
| Karnofsky Performance Status, n (%) | ||||
| 50%-60% | 2 (4) | 1 (2) | 1 (3) | 1 (6) |
| 70%-80% | 14 (29) | 16 (38) | 9 (27) | 7 (41) |
| 90%-100% | 32 (67) | 25 (60) | 23 (70) | 9 (53) |
| Serum creatinine > 1.5 mg/dL, % | 10 | 5 | 12 | 0 |
| Median bone marrow plasma cell, % (range) | ||||
| Aspirate | 28 (0-95) | 27 (2-90) | 41 (1-93) | 30 (4-75) |
| Biopsy | 33 (0-90) | 40 (1-80) | 47 (2-90) | 38 (13-90) |
| Lactate dehydrogenase, > 190 U/L, % | 49 | 44 | 43 | 38 |
| Bone disease present, % (skeletal abnormality) | 72 | 90 | 94 | 94 |
| Eligible for autologous stem cell transplantation, n (%) | 46 (96) | 41 (98) | 31 (94) | 14 (82) |
| High-risk multiple myeloma,*n (%) | 7 (15) | 7 (17) | 7 (23) | 3 (18) |
| Del 13 (metaphase cytogenetics) | 2 (5) | 1 (3) | 1 (4) | 1 (6) |
| t(4;14) | 3 (6) | 1 (2) | 2 (6) | 1 (6) |
| t(14;16) | 0 | 0 | 0 | 0 |
| −17p13 | 3 (6) | 4 (10) | 5 (16) | 1 (6) |
V indicates bortezomib; D, dexamethasone; R, lenalidomide; C, cyclophosphamide; and VDC-mod, VDC plus a day 15 dose of C.
High-risk defined as any of the following: del 13/−13q14 or hypodiploidy by conventional metaphase cytogenetics; t(4;14), t(14;16), or −17p13 by conventional metaphase cytogenetics or FISH.
Response
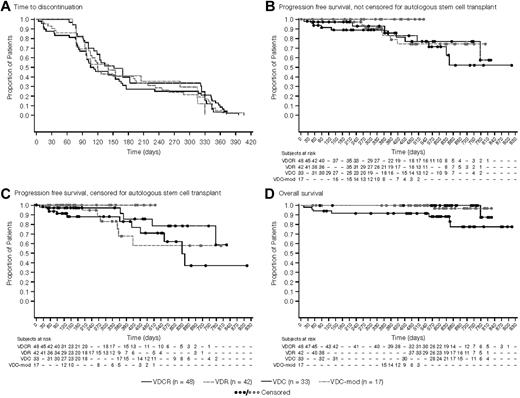
The median number of cycles administered was 6 (range: 1-12), with similar distribution across arms. Median time-to-treatment discontinuation was ∼ 6 months in all treatment groups (Figure 2A). Overall, 33%-45% of patients across the 4 arms completed all 8 cycles of induction, and 19%-30% of patients completed maintenance as planned (Table 4). The majority of patients who went off-study before starting any maintenance therapy did so to undergo ASCT.
Kaplan-Meier analyses of treatment outcomes. (A) Time-to-study discontinuation among the 4 treatment arms, irrespective of the reason for discontinuation (mITT population). The median time to discontinuation was nearly 6 months for all treatment groups with the most common reason being a decision to proceed to stem cell transplantation. (B) PFS from randomization, irrespective of whether the patient proceeded to stem cell transplantation (mITT population). (C) PFS from randomization, with patients proceeding to stem cell transplantation censored at the time of transplantation (mITT population). (D) Overall survival from randomization in the 4 treatment groups (mITT population).
Kaplan-Meier analyses of treatment outcomes. (A) Time-to-study discontinuation among the 4 treatment arms, irrespective of the reason for discontinuation (mITT population). The median time to discontinuation was nearly 6 months for all treatment groups with the most common reason being a decision to proceed to stem cell transplantation. (B) PFS from randomization, irrespective of whether the patient proceeded to stem cell transplantation (mITT population). (C) PFS from randomization, with patients proceeding to stem cell transplantation censored at the time of transplantation (mITT population). (D) Overall survival from randomization in the 4 treatment groups (mITT population).
Treatment exposure and response
| . | VDCR n = 48 . | VDR n = 42 . | VDC n = 33 . | VDC-mod n = 17 . |
|---|---|---|---|---|
| Patient experience on study | ||||
| Median follow-up, mo | 20 | 20 | 22 | 15 |
| Median cycles, n (range) | 5 (1-12) | 6 (1-12) | 6 (3-12) | 6 (3-12) |
| Completed induction, n (%) | 16 (33) | 17 (40) | 15 (45) | 7 (41) |
| Completed maintenance, n (%) | 12 (25) | 8 (19) | 10 (30) | 5 (29) |
| Confirmed response at cycle 4* | n = 40 | n = 41 | n = 32 | n = 17 |
| Complete response | 2 (5) | 3 (7) | 1 (3) | 2 (12) |
| sCR | 1 (3) | 1 (2) | 0 | 2 (12) |
| VGPR or better | 13 (33) | 13 (32) | 4 (13) | 7 (41) |
| ORR (PR or better) | 32 (80) | 30 (73) | 20 (63) | 14 (82) |
| Progression | 0 | 0 | 0 | 0 |
| Best response across all cycles | ||||
| Complete response | 10 (25) | 10 (24) | 7 (22) | 8 (47) |
| sCR | 6 (15) | 7 (17) | 3 (9) | 5 (29) |
| VGPR or better | 23 (58) | 21 (51) | 13 (41) | 9 (53) |
| ORR (PR or better) | 35 (88) | 35 (85) | 24 (75) | 17 (100) |
| Progression | 1 (3) | 1 (2) | 1 (3) | 0 |
| Best response across all cycles among patients ≤ 65 y | n = 28 | n = 28 | n = 21 | n = 12 |
| Complete response | 6 (21) | 6 (21) | 2 (10) | 7 (58) |
| VGPR or better | 15 (54) | 17 (61) | 5 (24) | 8 (67) |
| ORR (PR or better) | 24 (86) | 26 (93) | 14 (67) | 12 (100) |
| . | VDCR n = 48 . | VDR n = 42 . | VDC n = 33 . | VDC-mod n = 17 . |
|---|---|---|---|---|
| Patient experience on study | ||||
| Median follow-up, mo | 20 | 20 | 22 | 15 |
| Median cycles, n (range) | 5 (1-12) | 6 (1-12) | 6 (3-12) | 6 (3-12) |
| Completed induction, n (%) | 16 (33) | 17 (40) | 15 (45) | 7 (41) |
| Completed maintenance, n (%) | 12 (25) | 8 (19) | 10 (30) | 5 (29) |
| Confirmed response at cycle 4* | n = 40 | n = 41 | n = 32 | n = 17 |
| Complete response | 2 (5) | 3 (7) | 1 (3) | 2 (12) |
| sCR | 1 (3) | 1 (2) | 0 | 2 (12) |
| VGPR or better | 13 (33) | 13 (32) | 4 (13) | 7 (41) |
| ORR (PR or better) | 32 (80) | 30 (73) | 20 (63) | 14 (82) |
| Progression | 0 | 0 | 0 | 0 |
| Best response across all cycles | ||||
| Complete response | 10 (25) | 10 (24) | 7 (22) | 8 (47) |
| sCR | 6 (15) | 7 (17) | 3 (9) | 5 (29) |
| VGPR or better | 23 (58) | 21 (51) | 13 (41) | 9 (53) |
| ORR (PR or better) | 35 (88) | 35 (85) | 24 (75) | 17 (100) |
| Progression | 1 (3) | 1 (2) | 1 (3) | 0 |
| Best response across all cycles among patients ≤ 65 y | n = 28 | n = 28 | n = 21 | n = 12 |
| Complete response | 6 (21) | 6 (21) | 2 (10) | 7 (58) |
| VGPR or better | 15 (54) | 17 (61) | 5 (24) | 8 (67) |
| ORR (PR or better) | 24 (86) | 26 (93) | 14 (67) | 12 (100) |
V indicates bortezomib; D, dexamethasone; R, lenalidomide; C, cyclophosphamide; and VDC-mod, VDC plus a day 15 dose of C; CR, complete response; SCT, stem cell transplantation; sCR, stringent complete response; VGPR, very good partial response; PR, partial response; and ORR, objective response rate.
Ten patients were not evaluable for response.
Among the 140 patients enrolled, 130 patients were evaluable for response. As patients were allowed to go off-study for an ASCT after 4 cycles, responses were first evaluated at 4 cycles. Overall, 122 patients received at least 4 cycles; 39, 36, 31, and 16, respectively, in the VDCR, VDR, VDC, and VDC-mod arms. After 4 cycles, 80%, 73%, 63%, and 82% of patients in the VDCR, VDR, VDC, and VDC-mod arms had a confirmed response including VGPR or better in 33%, 32%, 13%, and 41%, respectively (Table 4). Across all treatment cycles, the ORR was 88%, 85%, 75%, and 100% for the VDCR, VDR, VDC, and VDC-mod arms including VGPR or better in 58%, 51%, 41%, and 53%, respectively (Table 4). For those patients going to transplantation, the best response obtained on the study regimen before transplantation was included. The median time-to-best response was 105, 91, 118, and 85 days in the VDCR, VDR, VDC, and VDC-mod arms, respectively. In addition, we evaluated the response rates among the group of patients aged ≤ 65 years and, as shown in Table 4, the results were similar to the entire group.
Tolerability
At least one grade ≥ 3 AE was seen in ∼ 80% of patients in each arm. AEs leading to discontinuation were seen in 21%, 19%, 12%, and 6% in the VDCR, VDR, VDC, and VDC-mod arms, respectively. The median time-to-study discontinuation because of any toxicity was 3 months across the entire study, with 10, 8, 4, and 1 patients discontinuing because of AEs in the VDCR, VDR, VDC, and VDC-mod arms, respectively. Hematologic toxicity was frequent, with neutropenia being the most common in the cyclophosphamide-containing arms. The most common nonhematologic toxicities included peripheral neuropathy, fatigue, nausea, constipation and diarrhea. The most common grade ≥ 3 AEs are shown in Table 5. Grade 1/2 peripheral neuropathy was seen in 27, 23, 21, and 8 patients in the VDCR, VDR, VDC, and VDC-mod arms, respectively. No secondary malignancies were reported. There were 2 deaths during the study, both were considered related to study treatment and were because of renal failure; both patients were in the VDCR group and one patient also had renal insufficiency and amyloidosis at the time of diagnosis.
Major adverse events of grade 3 or above
| . | VDCR n = 48 . | VDR n = 42 . | VDC n = 33 . | VDC-mod n = 17 . |
|---|---|---|---|---|
| Hematological AEs, n (%) | ||||
| Neutropenia | 21 (44) | 4 (10) | 10 (30) | 4 (24) |
| Febrile neutropenia | 4 (8) | 1 (2) | 2 (6) | 0 |
| Thrombocytopenia | 7 (15) | 5 (12) | 4 (12) | 0 |
| Leukopenia | 6 (13) | 0 | 3 (9) | 1 (6) |
| Anemia | 4 (8) | 3 (7) | 0 | 2 (12) |
| Lymphopenia | 4 (8) | 1 (2) | 4 (12) | 0 |
| Nonhematological AEs | ||||
| Pneumonia | 2 (4) | 2 (5) | 0 | 1 (6) |
| Neuropathy | 6 (13) | 7 (17) | 3 (9) | 3 (18) |
| Fatigue | 8 (17) | 3 (7) | 1 (3) | 0 |
| Diarrhea | 3 (6) | 1 (2) | 1 (3) | 1 (6) |
| Nausea | 0 | 1 (2) | 0 | 0 |
| Thromboembolism | 1 (2) | 0 | 0 | 0 |
| Constipation | 0 | 0 | 0 | 0 |
| Hyperglycemia | 0 | 0 | 0 | 0 |
| Summary | ||||
| At least one grade 3 or above AE | 40 (83) | 32 (76) | 26 (79) | 15 (88) |
| At least one drug-related grade 3 or above AE | 38 (79) | 25 (60) | 20 (61) | 12 (71) |
| At least one grade 3 or above hematological AE | 28 (58) | 11 (26) | 17 (52) | 5 (29) |
| AE resulting in discontinuation | 10 (21) | 8 (19) | 4 (12) | 1 (6) |
| . | VDCR n = 48 . | VDR n = 42 . | VDC n = 33 . | VDC-mod n = 17 . |
|---|---|---|---|---|
| Hematological AEs, n (%) | ||||
| Neutropenia | 21 (44) | 4 (10) | 10 (30) | 4 (24) |
| Febrile neutropenia | 4 (8) | 1 (2) | 2 (6) | 0 |
| Thrombocytopenia | 7 (15) | 5 (12) | 4 (12) | 0 |
| Leukopenia | 6 (13) | 0 | 3 (9) | 1 (6) |
| Anemia | 4 (8) | 3 (7) | 0 | 2 (12) |
| Lymphopenia | 4 (8) | 1 (2) | 4 (12) | 0 |
| Nonhematological AEs | ||||
| Pneumonia | 2 (4) | 2 (5) | 0 | 1 (6) |
| Neuropathy | 6 (13) | 7 (17) | 3 (9) | 3 (18) |
| Fatigue | 8 (17) | 3 (7) | 1 (3) | 0 |
| Diarrhea | 3 (6) | 1 (2) | 1 (3) | 1 (6) |
| Nausea | 0 | 1 (2) | 0 | 0 |
| Thromboembolism | 1 (2) | 0 | 0 | 0 |
| Constipation | 0 | 0 | 0 | 0 |
| Hyperglycemia | 0 | 0 | 0 | 0 |
| Summary | ||||
| At least one grade 3 or above AE | 40 (83) | 32 (76) | 26 (79) | 15 (88) |
| At least one drug-related grade 3 or above AE | 38 (79) | 25 (60) | 20 (61) | 12 (71) |
| At least one grade 3 or above hematological AE | 28 (58) | 11 (26) | 17 (52) | 5 (29) |
| AE resulting in discontinuation | 10 (21) | 8 (19) | 4 (12) | 1 (6) |
V indicates bortezomib; D, dexamethasone; R, lenalidomide; C, cyclophosphamide; VDC-mod, VDC plus a day 15 dose of C; and AE, adverse event.
Stem cell mobilization and transplantation
Seventy-five patients underwent a stem cell mobilization attempt. Median CD34 yields and failure rates are shown in Table 6. At data cutoff, 59 (42%) patients had proceeded to ASCT. The engraftment data appear similar across the groups and were as expected. 20, 19, 10 and 10 patients went to transplant in the VDCR, VDR, VDC, and VDC-mod arms, respectively, with 18, 18, 9, and 9 patients leaving the study for transplantation after 4 cycles in each of the study arms. Overall, the median time-to-initiation of transplantation was 5 months (95% CI 4, 13) across the entire study.
Stem cell mobilization and transplantation
| . | VDCR n = 48 . | VDR n = 42 . | VDC n = 33 . | VDC-mod n = 17 . |
|---|---|---|---|---|
| Stem cell mobilization | ||||
| Patients undergoing stem cell mobilization with data available, n (%) | 25 (50) | 26 (62) | 14 (42) | 10 (59) |
| Median CD34+ cell yield, × 106/kg, n (range) | 6.8 (0.3-21) | 7.8 (2.2-25.9) | 7.95 (3.1-17.6) | 7.75 (2.1-20) |
| Patients with < 2.5 × 106/kg CD34+ cells during first attempt, n (%)* | 4 (8) | 3 (7) | 0 | 2 (12) |
| Stem cell transplantation | ||||
| Patients undergoing ASCT with data available, n (%) | 20 (42) | 19 (45) | 10 (30) | 10 (59) |
| Median time to recovery of ANC > 500 cells/mm3, d (range) | 11 (7-15) | 11 (0-13) | 11 (0-16) | 11 (0-13) |
| Median time to recovery of platelets > 20 000 cells/mm3, d (range) | 12 (0-37) | 10.5 (0-21) | 11.5 (0-31) | 10 (0-23) |
| . | VDCR n = 48 . | VDR n = 42 . | VDC n = 33 . | VDC-mod n = 17 . |
|---|---|---|---|---|
| Stem cell mobilization | ||||
| Patients undergoing stem cell mobilization with data available, n (%) | 25 (50) | 26 (62) | 14 (42) | 10 (59) |
| Median CD34+ cell yield, × 106/kg, n (range) | 6.8 (0.3-21) | 7.8 (2.2-25.9) | 7.95 (3.1-17.6) | 7.75 (2.1-20) |
| Patients with < 2.5 × 106/kg CD34+ cells during first attempt, n (%)* | 4 (8) | 3 (7) | 0 | 2 (12) |
| Stem cell transplantation | ||||
| Patients undergoing ASCT with data available, n (%) | 20 (42) | 19 (45) | 10 (30) | 10 (59) |
| Median time to recovery of ANC > 500 cells/mm3, d (range) | 11 (7-15) | 11 (0-13) | 11 (0-16) | 11 (0-13) |
| Median time to recovery of platelets > 20 000 cells/mm3, d (range) | 12 (0-37) | 10.5 (0-21) | 11.5 (0-31) | 10 (0-23) |
V indicates bortezomib; D, dexamethasone; R, lenalidomide; C, cyclophosphamide; VDC-mod, VDC plus a day 15 dose of C; ANC, absolute neutrophil count; and ASCT, autologous stem cell transplantation.
At second mobilization attempt, 5 patients received cyclophosphamide + G-CSF and 2 patients (both VDR arm) received plerixafor + G-CSF.
Survival outcomes
One-year PFS was 86%, 83%, 93%, and 100% for the VDCR, VDR, VDC, and VDC-mod arms, respectively (Figure 2B). After censoring patients who proceeded to ASCT at the time of last response assessment, the 1-year PFS was 83%, 68%, 97%, and 100% for the VDCR, VDR, VDC, and VDC-mod arms, respectively (Figure 2C). The proportion of patients without disease progression at 1 year was 91%, 68%, 97%, and 100% for the VDCR, VDR, VDC, and VDC-mod treatment groups, respectively. The 1-year OS estimate was 92% for the VDCR arm and 100% for the other 3 arms (Figure 2D). Survival outcomes among the 96 patients aged ≤ 65 years were similar to the overall group, with a 1-year OS estimate of 94% for the VDCR arm and 100% for the other 3 arms.
We also examined the PFS and OS among the patients who elected to go to transplantation during the induction phase (after 4-8 cycles) and those who stayed on therapy or went to transplantation only after completing study treatments or for disease relapse. The 1-year PFS was 100%, 100%, 88%, and 100% for the VDCR, VDR, VDC, and VDC-mod arms, respectively. The 1-year OS estimate was 100% for all 4 arms. In addition, we also examined the impact of cytogenetic/FISH risk stratification on PFS. The 1-year PFS for the high-risk patients (n = 24) was 100% and 85% for the standard-risk patients, and was similar across the study arms.
Minimal residual disease
Including phase 1,15 155 (98%) patients provided a baseline bone marrow sample for MRD assessment, demonstrating the feasibility of MRD assessments by multiparameter flow cytometry in the multicenter clinical trial setting. Among the 35 patients with a documented CR, 28 (80%) provided samples for MRD assessment. Overall, 13 (46%) of these patients were considered MRD negative; including 5 (50%), 6 (85%), 0 and 2 (29%) patients in the VDCR, VDR, VCD, and VCD-mod arms, respectively. The impact of MRD negativity on PFS and OS was not analyzed given the small number of patients and low event rate.
Discussion
Availability of new drugs with novel mechanisms of action has transformed the treatment landscape for MM, leading to improved survival.20,21 Given the success seen in lymphoma and acute lymphoblastic leukemia with multidrug combinations, current research in MM is devoted to identifying the best approach to combine the available drugs to effect long-term disease control and, if possible, a cure. In the phase 2 trials, the most promising were those combining either lenalidomide or cyclophosphamide with bortezomib and dexamethasone.8,14,22
The VDR combination was studied in previously untreated MM by Richardson et al in a phase 2 study, with all patients achieving at least a partial response after a median of 10 cycles, including 74% of patients with ≥ VGPR and 37% with CR.14 The estimated PFS with or without ASCT at 18 months was 75%; in line with 1-year uncensored PFS of 83% reported here. The ORR and VGPR and CR rates appear lower than in the study by Richardson et al, with slightly higher rates of grade 3 neuropathy; however, the small numbers of patients and the differences in baseline characteristics preclude any direct comparisons. Furthermore, there were some differences in treatment regimen between the studies. This includes the use of dexamethasone on the day of, and the day after, bortezomib compared with weekly dexamethasone in the current study. In addition, patients could receive maintenance with bortezomib and lenalidomide after 8 cycles of induction, unlike the current study, in which only bortezomib was given as maintenance.
Similarly, the combination of bortezomib and dexamethasone with cyclophosphamide has been associated with high response rates in previously untreated patients.23 In a study by Reeder and colleagues, an ORR of 88%, with VGPR and CR rates of 61% and 39%, respectively, was observed after 4 cycles.8 Toxicities included neuropathy, and the safety profile was similar to the present study. However, Reeder et al used a 4-week regimen with cyclophosphamide administered every week with high-dose dexamethasone in a 4-days-on, 4-days-off manner. In the current trial, we used a higher dose of cyclophosphamide for 3 of 4 weeks initially. This was subsequently modified to add an extra dose of cyclophosphamide resulting in its continuous weekly administration. While the number of patients is lower compared with the other arms, the VDC-mod arm had the highest ORR and VGPR rates, supporting its future exploration in larger trials. Importantly, this combination may present a substantial cost saving compared with regimens containing both lenalidomide and bortezomib.
An important goal of this trial was to evaluate the feasibility and activity of the combination of all 4 drugs. Clearly, VDCR was an active regimen with high response rates and deep responses. While the current study design is not powered for a direct comparison of the regimens, the responses seen with VDCR appear to be similar to those seen with the VDR or VDC-mod arms. However, the toxicities with VDCR appear to be more than the other arms, especially hematologic toxicity. One might attribute the lower response rates to the lower dose of lenalidomide (15 mg) used in the VDCR regimen compared with 25 mg in VDR. However, it is unlikely that a higher dose of lenalidomide would be tolerated given the hematologic toxicity profile observed. It is also conceivable that combination of an alkylator with lenalidomide does not provide the degree of benefit seen when combined with bortezomib. Combinations of bortezomib with either cyclophosphamide or melphalan have been associated with high response rates and, in the context of melphalan, with improved OS compared with melphalan prednisone in elderly patients.8,20 In contrast, addition of melphalan to lenalidomide did not lead to any PFS improvement in a previous study13 and the combination of cyclophosphamide with lenalidomide and dexamethasone was no more active than lenalidomide and dexamethasone alone.10
In the current study, 9%-18% of patients reported grade ≥ 3 neuropathy, which is a recognized toxicity of bortezomib. However, recent data indicate that a once-weekly schedule of bortezomib24,25 or administration via subcutaneous injection26 results in significantly lower rates of peripheral neuropathy compared with standard twice-weekly IV dosing. However, we note that at higher weekly bortezomib doses (1.5 mg/m2), this reduction is not as evident.23 As minimizing treatment-related toxicity is particularly important when combining multiple agents, future studies of bortezomib in combination should further explore dosing options.
In conclusion, the current study, by virtue of its randomized phase 2 design, allowed us to assess the relative efficacy and toxicities of VDCR, VDR, VDC, and VDC-mod in similar groups of patients studied concurrently and assessed in a uniform fashion. The results do not show a substantial advantage for the 4-drug regimen. However, both VDR and the VCD-mod arms showed significant activity with high rates of deep responses and are appropriate regimens for clinical practice, warranting further evaluation in a phase 3 setting.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Jonathan Glass for his involvement in the study. They also gratefully acknowledge all the patients who participated in the study, all the investigators, nursing staff, and research support staff involved in the study, and the research team at Millennium Pharmaceuticals Inc. The authors wrote the initial draft of the manuscript and all material content. The acknowledge Emma Brougham and editing assistance from Catherine Crookes of FireKite during the development of this publication, which was funded by Millennium Pharmaceuticals Inc.
This work was supported by research funding from Janssen Global Services and Millennium Pharmaceuticals Inc.
Authorship
Contribution: S.K., P.G.R., and I.J.W. were involved in the concept and design of the study, collection and assembly of data, and data analysis/interpretation, and provided study materials/patients; S.K. wrote the first draft of the manuscript; I.F., P.H., S.J.N., J.W., F. T., and S.V.R. were involved in the collection and assembly of data; N.C. was involved in the collection and assembly of data and data analysis/interpretation; A.K.S. was involved in data analysis/interpretation and provided study materials/patients; R.R. was involved in the concept and design of the study and collection and assembly of data; J.E. was involved in data analysis/interpretation; G.M. was involved in the concept and design of the study and data analysis/interpretation; H.S. was involved in the concept and design of the study, collection and assembly of data, and data analysis/interpretation; and all authors participated in the writing, review, and approval of the manuscript.
Conflict-of-interest disclosure: S.K. has acted as a consultant/advisor for Merck, and has received research funding from Millennium Pharmaceuticals Inc, Celgene, Novartis, Genzyme and Bayer. I.F. and N.C. have received research funding from Millennium Pharmaceuticals Inc. P.G.R. has held consultant/advisory roles for Millennium Pharmaceuticals Inc, Celgene, and Johnson & Johnson. S.J.N. has received research funding and honoraria from, and has acted as an advisor for, Amgen, Millennium Takeda, Celgene, and Cephalon, and has also received honoraria from, and has acted as an advisor for, Ortho-Centicor. A.K.S. has held consultant/advisory roles for, and received honoraria from, Onyx, Celgene, and Millennium Pharmaceuticals Inc, and has received research funding from Millennium Pharmaceuticals Inc. R.R. has held consultant/advisory roles for Onyx, Celgene, and Millennium Pharmaceuticals Inc, and has received honoraria from Millennium Pharmaceuticals Inc. J.W. has been a consultant for Millennium Pharmaceuticals Inc, has received honoraria for participation in speakers' bureaus for Celgene, Novartis, OrthoBiotech and Millennium Pharmaceuticals Inc, and has participated in advisory boards for Genentech and Multiple Myeloma Research Consortium. J.E., G.M., H.S., and I.J.W. are all employees of Millennium Pharmaceuticals Inc. The remaining authors declare no competing financial interests.
The current affiliation for F.T. is Department of Lymphoma/Myeloma, MD Anderson Cancer Center, Houston, TX.
Correspondence: Shaji Kumar, MD, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: kumar.shaji@mayo.edu.