Abstract
Immune responses to foreign and self-Ags can be controlled by regulatory T cells (Tregs) expressing CD4 and IL-2Rα chain (CD25). Defects in Tregs lead to autoimmunity, whereas induction of Ag-specific CD4+CD25+ Tregs restores tolerance. Ag-specific CD4+CD25+ FOXP3+Tregs activated by the T helper type 2 (Th2) cytokine, IL-4, and specific alloantigen promote allograft tolerance. These Tregs expressed the specific IL-5Rα and in the presence of IL-5 proliferate to specific but not third-party Ag. These findings suggest that recombinant IL-5 (rIL-5) therapy may promote Ag-specific Tregs to mediate tolerance. This study showed normal CD4+CD25+ Tregs cultured with IL-4 and an autoantigen expressed Il-5rα. Treatment of experimental autoimmune neuritis with rIL-5 markedly reduced clinical paralysis, weight loss, demyelination, and infiltration of CD4+ (Th1 and Th17) CD8+ T cells and macrophages in nerves. Clinical improvement was associated with expansion of CD4+CD25+FOXP3+ Tregs that expressed Il-5rα and proliferated only to specific autoantigen that was enhanced by rIL-5. Depletion of CD25+ Tregs or blocking of IL-4 abolished the benefits of rIL-5. Thus, rIL-5 promoted Ag-specific Tregs, activated by autoantigen and IL-4, to control autoimmunity. These findings may explain how Th2 responses, especially to parasitic infestation, induce immune tolerance. rIL-5 therapy may be able to induce Ag-specific tolerance in autoimmunity.
Introduction
Both Ag-specific1 and naive regulatory T cells (Tregs)2-4 that control immune responses are mainly CD4+CD25+ T cells5 expressing transcription factor FOXP3.6 Autoimmunity occurs with the breakdown in immune tolerance to self-Ag and can be because of a failure of natural Tregs (nTregs) produced by the thymus which prevent spontaneous autoimmune activation of CD4+CD25− T effector cells by inhibiting APCs.5 nTregs maintain immune homeostasis and are polyclonally expanded by IL-2. nTregs can suppress all immune responses, because they are not Ag specific. To fully suppress high ratios to effector lineage, CD4+CD25− T cells are required, usually > 1:1; whereas the natural ratio of these cells in peripheral lymphoid tissues is tightly regulated to < 1:10.7,8
There is ample evidence for Ag-specific Treg induction in vivo, including T-cell transfer of tolerance to specific autoantigen induced by immunization with autoantigen without complete Freund adjuvant (CFA),9 the parabiosis of tolerance to autoimmunity from normal hosts,10 and the epitope specificity of tolerance induction with an autoantigen.11
Ag-specific CD4+CD25+ Tregs have phenotypic and functional differences from the nTreg, recently reviewed by Hall et al.12 Activated Tregs do not migrate from blood to lymph but express chemokine receptors and other ligands that promote their migration to sites of inflammation, where they control local inflammation.12 Further, their action is not to inhibit APCs via CTLA4, but to inhibit or eliminate activated effector T cells and macrophages, by a variety of mechanisms.12
To date, most studies focused on nTregs that suppress in a non-Ag–specific manner and must be present at high ratios with effector T cells to fully suppress an immune response. In autoimmune disease it would be desirable to induce Ag-specific Tregs that can suppress only the specific immune response at low ratios (< 1:10) to effector cells.13
Specific immune tolerance, as occurs in adult rodents that accept an allograft long term, is mediated by Ag-specific CD4+CD25+ Tregs that suppress at ratios < 1:10.1,14,15 These alloantigen-specific Tregs are difficult to identify, because their survival depends on stimulation by both Ag1,16 and T cell–derived cytokines.17 IL-217 or IL-4 do not fully maintain activated Ag-specific Tregs, but other cytokines such as IL-5 can.3
In our studies, the initial activation of nTregs to alloantigen-specific Tregs occurred when they were cultured with specific alloantigens and either the T helper type 1 (Th1) cytokine IL-2 or the Th2 cytokine IL-4, but not other Th1 or Th2 cytokines.3 Alloactivation of nTregs with IL-2 induces the receptor for the late Th1 cytokine IFN-γ (Ifnγr) but not the receptor for the Th2 cytokine IL-5 (Il-5rα).3 Alloactivation of nTregs in the presence of IL-4, as opposed to IL-2, induces Il-5rα but does not induce Ifngr.3 IL-5Rα is not expressed by any other T cells, including Th2 cells,3 but is expressed on eosinophils, basophils, and in some species B cells.3 We called the Th1-induced lineage of Ag-induced Tregs, Ts1, because the term Tr1 had already been used. The Th2-induced Tregs were thus called Ts2 cells. The Ts2 phenotype was CD4+CD25+FOXP3+TCR-α,β, expressing Il-5rα, Ifn-γ, Il-4, Il-10, Tgf-β, but not Il-2 or Il-5, Ifnγr.3
The selective induction of Il-5rα on Ag-activated Tregs that have been stimulated by IL-4, not IL-2, raised the possibility that treatment with rIL-5 might promote activation of Ag-specific Tregs that could control immune responses, including autoimmunity. This hypothesis was examined in a model of autoimmune-mediated demyelination, experimental autoimmune neuritis (EAN), that is induced in Lewis rats by immunization with peripheral nerve myelin (PNM) in CFA.18,19 In this model immunization induces Th1, Th2, and Th17 cytokines in the lymph node draining the site of immunization19 (G.T.T., and S.J.H., unpublished data). Thus, IL-4 is produced and could induce nTregs activated with autoantigen to express Il-5rα during the induction of EAN. In this study, we tested our hypothesis that therapy with rat rIL-5 would induce proliferation and expansion of the IL-4–activated Ag-specific Tregs and that these cells would control autoimmune inflammation. We first examined if autoantigen and rIL-4 induced nTregs to express Il-5rα, and whether human Tregs can be induced to express Il-5rα by culture with IL-4. We found therapy with rIL-5 could reduce the clinical severity of EAN as well as inflammation and demyelination of the nerves. Further, the rIL-5 therapy was shown to expand autoantigen-specific Il-5rα–expressing CD4+CD25+ T cells, and that these cells requiring IL-4 for their induction were critical for the reduction in autoimmune inflammation.
Methods
Induction of EAN
Animals were bred and maintained at the Animal House Liverpool Hospital. Female Lewis rats (10-12 weeks old) were immunized with bovine PNM in CFA, as described.18,19 Animals were monitored daily for weight loss, and clinical disease was scored as 1+ for limp tail, 2+ for hind leg weakness, 3+ for paraplegia, and 4+ for quadriplegia. The University of New South Wales Animal Ethics Committee approved the experiments.
IL-5 treatment
rIL-5 was produced as serum-free culture supernatant from transfected Chinese hamster ovary (CHO)–K1 cells, as described.20 Biologic activity was assayed by proliferation of an IL-5–dependent cell line, B13 (clone provided by Dr C. Sanderson, Molecular Immunology, Curtin University of Technology, Perth, Australia [retired]). One unit was defined as the amount required for 50% of maximum proliferation, as described.21
Unless specified, treatment groups received 5000 U of rIL-5 per day by intraperitoneal injection for 10 days.21 Sham-treated control rats received an equivalent volume of serum-free tissue culture supernatant from nontransfected CHO-K1 cells. Some experiments used a rat rIL-5 clone provided by Dr X. Y. He (Liverpool Hospital, Liverpool, Australia).20,21
mAb treatment
mAbs were prepared as described.15 The activity of rIL-5 or IL-4 was blocked by coadministration intraperitoneally of 7 mg/kg anti–IL-5 mAb TRFK5 (IgG1; anti–human IL-5; Dr W. Sewell, Center for Immunology, Darlinghurst, Australia)15 or MRCOx81 (anti–rat IL-4).22 CD25+ cells were depleted with NDS61 (Dr M. Dallman, Nuffield Department of Surgery, Oxford, United Kingdom) an anti-CD25 mAb given intraperitoneally at 7 mg/kg on days 4, 3, 2, and 0 before immunization, as described23 (personal e-mail communication, Dr G. Tellides, Department of Surgery, Yale University, New Haven, CT, June 30, 2009).
Blood counts and sample collection
Blood was collected every 7 days from tail vein for blood counts, assayed with a veterinary program on CELLDYN 3500 (Abbott Diagnostics). Lymphocyte subset analysis and anti–bovine PNM Ab assays were also performed, as described.24 Total Ig and IgG1, IgG2a, IgG2b, and IgG2c isotypes were assayed with mAb to rat Ig subclasses.19
At day 14 and day 21 after immunization, extra sample animals from treated, and control groups were killed and perfused with PBS as described.24 Peripheral nerve samples from the cauda equina and lymph node and spleen cells were collected, and mRNA was extracted as described.25 Samples of nerves for histology were prepared by routine fixation for staining with toluene blue for light microscopy or for electron microscopy, as described.19,24 Light microscopy used an Olympus BH-2 (Olympus) with a Olympus S Plan Apo ×20 NA-0.70 objective at 21°. Imaging medium used was Kodak KAI 2000 CCD sensor 1600 × 1200 pixels, 24-bit uncompressed RGB color TIFF format images using a SPOT RT Slider, with SPOT advanced Control aquisition software (Diagnostic Instruments Inc). Electron microscopy used a FEI Morgagni 268D transmission electron microscope (FEI Co) with a magnetic lens F = 1.6 mm/Cs = 1.6 mm/Cc = 1.5 mm used at 80 Kv, all at 20°. An Olympus CCD sensor 1376 × 1032 pixels, 24-bit RGB color jpeg was used to format images. A SIS Megaview III CCD camera (Olympus) used AnalySIS PRO TEM (Olympus) acquisition software. Subsequent image analysis used manual image segmentation followed by pixel area determination used Image-Pro Plus Version 3.2 (Media Cybernetics). Conversion of color images to grayscale, cropping to square format, linear changes to brightness, and contrast and unsharp mask sharpening with Adobe Photoshop CS2 Version 9.0 (Adobe Systems Inc). The quantification of demyelination was done with semithin epoxy sections stained with methylene blue, and image analysis was based on area segmentation with Image-Pro Plus (Media Cybernetics). Samples of nerve were frozen for immunostaining with the use of an indirect immunoperoxidase technique and monoclonal to rat CD3 (G4.18), CD4 (MRCOX35), CD8 (MRCOX8), CD25 (MRCOX39), macrophages (ED1; BD PharMingen), and FOXP3 (eBiosciences), as described.26,27
PCR analysis of mRNA
Primers for rat Il-2, Il-4, Il-5, Ifn-γ, Tnf-α, Gapdh, Il-5rα, Ifnγr, Foxp3, Il-17A, T-bet, Gata3, and Tcr-α, Τcr-β were as described.3 Primers for human β-actin were forward, 5′-GAAACTACCTTCAACTCCATC-3′, and reverse, 5′-CTAGAAGCATTTGCGTGGAC-3′, and for IL-5Rα (R&D Systems). Real-time PCR was performed with a Rotorgene(Corbett Research) and SYBR Green I detection. Sensimix Taq polymerase (BioLine) was used according to manufacturer's instructions. Copy numbers in experimental samples were derived from a known standard curve performed in parallel and were normalized against Gapdh expression for rats and β-actin for humans.
In vitro proliferation assays with CD4+CD25+ T cells
Lymphoid cells were prepared from lymph nodes and spleen, in media with 10% rat serum, and enriched for CD4+ T cells as described.26 CD4+CD25+ T-cell subset enrichment, immunostaining of cells, and proliferation assays were essentially as described.3 The enriched CD4+CD25+ T-cell populations were stained with anti–rat mAb to CD3, CD4, CD8, CD25, and FOXP3, as described.7 These enriched CD4+CD25+ T cells were > 99% CD4+ and CD3+, > 98% CD25+, and ∼ 80% FOXP3+. Stimulator cells were thymus cells from Lewis rats given 10 Gr irradiation.3 Stimulator cells were preincubated with either PNM or renal tubular Ag (RTA), as an irrelevant control Ag, prepared as described.27 Stimulator cells that had not been preincubated with an Ag were used as a control. The methods for culture, addition of cytokines such as rIL-5 (200 U/mL), and assessment of proliferation with 3H thymidine incorporation were as described.3,7
Human Tregs
PBMCs were separated from human buffy coats (Red Cross Blood Transfusion Service) as described.28 CD4+CD25+ T cells were isolated with a Human CD4+CD25+ Regulatory T-Cell Isolation Kit (Miltenyi Biotec) according to the manufacturer's instructions. Triple immunostaining of lymphocytes was performed with a standard technique, as described.28 mAbs used were CD3-FITC (UCHT1), CD4-Cy (RPA-T4), CD8-FITC (RPA-T8), CD25-PE (M-A 251), (BD PharMingen), and FOXP3 (eBioscience).
Culture of human Tregs used was RPMI supplemented with 2 mmol l-glutamine, 10 mmol HEPES, 10% human serum, 100 ng/mL penicillin, and 100 U/mL streptomycin. Bulk culture was set up in 25-cm3 flasks with 1 × 106 CD4+CD25+ T cells/mL and 2 × 106 allogeneic PBMCs/mL that had been irradiated with 9 Gy. The final culture volume was 10 mL. Separate cultures with human recombinant IL-2 or IL-4 (R&D Systems) at 200 U/mL were cultured for 3 days before being harvested for immunostaining and real-time RT-PCR studies.
Statistics
Data were expressed as mean ± SD, and comparisons were made with a 2-tailed Student t test (Microsoft Excel).
Results
In vitro activation of rat and human CD4+CD25+ T cells with IL-4 and Ag induces expression of Il-5rα
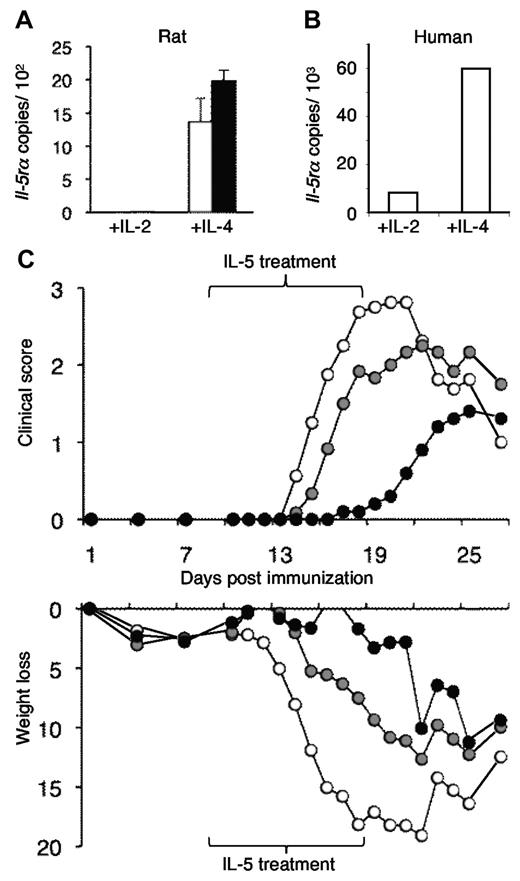
We described that alloantigen and IL-4 activated nTregs to express mRNA for IL-5Rα (Il-5rα).3 To establish that autoantigen in the presence of IL-4 would also activate nTregs to express Il-5rα, we cultured normal Lewis rat CD4+CD25+ Tregs prepared from lymph nodes and spleen with PNM and either IL-4 or IL-2. Both IL-2 and IL-4 induced significant proliferation, with PNM primed and unprimed APCs, whereas there was minimal proliferation without either IL-2 or IL-4 (data not shown). PNM with IL-4 induced mRNA for Il-5rα, whereas PNM with IL-2 (Figure 1A) or without cytokine did not (data not shown). The IL-4 with PNM-activated nTregs also had increased expression of Ifn-γ and reduced expression of Il-5, as we described in IL-4 and alloantigen-activated Tregs.3 Thus, IL-4 and an autoantigen induced the same phenotype in nTregs as IL-4 and alloantigen, a Ts2 phenotype.3
Demonstration that IL-4 and PNM induce Ag-specific Tregs that express mRNA for the IL-5Rα and that IL-5 can suppress induction of EAN. (A) IL-5 receptor α mRNA (Il-5rα) was induced in CD4+CD25+ Tregs from normal Lewis rats that were cultured with (■) and without (□) PNM, the Ag used to induce EAN and IL-4. Culture with PNM and IL-2 did not induce Il-5rα. This is similar to induction of Il-5rα in Tregs by culture with alloantigen and IL-4 but not with IL-2.3 (B) Human CD4+CD25+ T cells cultured with IL-4 and alloantigen also expressed Il-5rα but not if cultured with IL-2. This confirmed that Il-5rα can be induced in human Tregs activated by IL-4. (C) Compared with controls with EAN (○), IL-5 therapy reduced the severity of EAN induced in Lewis rats by immunization with PNM in CFA.18,19 Top panel shows treatment with IL-5, either 5000 U/d (●) or 500 U/d (gray circle) from 9 to 18 days after immunization, delayed onset of EAN (n = 6 per group); 5000 U/d fully suppressed EAN, with P < .001 on days 14-21, P < .03 on days 13 and 21. This dose was used in all subsequent experiments. With 500 U of IL-5, clinical severity was less at days 13-16 and day 18 (P < .05). Clinical disease was scored as 1+ for limp tail, 2+ for hind leg weakness, 3+ for paraplegia, and 4+ for quadriplegia.18,19 Bottom panel shows weight loss was also reduced in IL-5–treated animals. There is early weight loss after immunization, which continues in controls. Weight loss is arrested with treatment with 5000 U of IL-5 and partially reduced with treatment with 500 U of IL-5.
Demonstration that IL-4 and PNM induce Ag-specific Tregs that express mRNA for the IL-5Rα and that IL-5 can suppress induction of EAN. (A) IL-5 receptor α mRNA (Il-5rα) was induced in CD4+CD25+ Tregs from normal Lewis rats that were cultured with (■) and without (□) PNM, the Ag used to induce EAN and IL-4. Culture with PNM and IL-2 did not induce Il-5rα. This is similar to induction of Il-5rα in Tregs by culture with alloantigen and IL-4 but not with IL-2.3 (B) Human CD4+CD25+ T cells cultured with IL-4 and alloantigen also expressed Il-5rα but not if cultured with IL-2. This confirmed that Il-5rα can be induced in human Tregs activated by IL-4. (C) Compared with controls with EAN (○), IL-5 therapy reduced the severity of EAN induced in Lewis rats by immunization with PNM in CFA.18,19 Top panel shows treatment with IL-5, either 5000 U/d (●) or 500 U/d (gray circle) from 9 to 18 days after immunization, delayed onset of EAN (n = 6 per group); 5000 U/d fully suppressed EAN, with P < .001 on days 14-21, P < .03 on days 13 and 21. This dose was used in all subsequent experiments. With 500 U of IL-5, clinical severity was less at days 13-16 and day 18 (P < .05). Clinical disease was scored as 1+ for limp tail, 2+ for hind leg weakness, 3+ for paraplegia, and 4+ for quadriplegia.18,19 Bottom panel shows weight loss was also reduced in IL-5–treated animals. There is early weight loss after immunization, which continues in controls. Weight loss is arrested with treatment with 5000 U of IL-5 and partially reduced with treatment with 500 U of IL-5.
To confirm whether such changes may be relevant to human disease, we cultured human peripheral blood CD4+CD25+ Tregs with alloantigen and IL-4 or IL-2. There was marked proliferation induced by either IL-2 or IL-4 (data not shown). Human CD4+CD25+ T cells also expressed Il-5rα when activated by IL-4, but not by IL-2 (Figure 1B). This showed that in the presence of IL-4 and Ag, human Tregs also can express Il-5rα. This suggests that any effect of rIL-5 on Treg function could be relevant to immunoregulation in humans.
Effect of rIL-5 therapy on induction of EAN
After immunization with autoantigen in CFA, there is induction of Th1, Th2,25,29,30 and Th1719 cytokines in the lymph node draining the site of immunization. We reasoned that the induction of Th2 cytokines such as IL-4 could with the immunizing autoantigen activate nTregs in the regional node and induce Il-5ra in the Ag-specific Tregs. Thus, we examined whether treatment with rIL-5 could promote in vivo expansion of the Ag-specific Tregs that express Il-5rα to inhibit the effectors of immune-mediated injury.
Lewis rats were immunized with PNM/CFA to induce EAN, and one group was treated with rIL-5 (5000 U/d for 10 days)20,21 from day 9 after immunization, a time in which activated Tregs could be induced by the PNM and IL-4 could be produced by activated CD4+CD25− T cells. This treatment with rIL-5 markedly delayed onset of clinical paralysis compared with controls with EAN, and a mild disease only developed after cessation of rIL-5 at day 19 (Figure 1C). rIL-5 treatment at 500 U/d was less effective (Figure 1C). Weight loss was arrested in the high-dose group and partial reduced in the low-dose rIL-5 group (Figure 1C), indicating that rIL-5 therapy had a beneficial effect on the animals and was well tolerated.
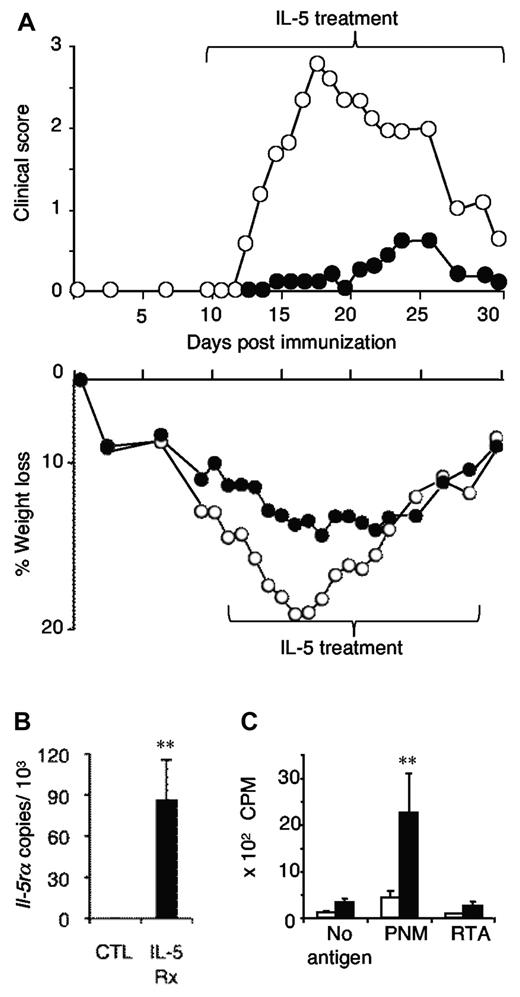
Longer treatment with 5000 U/d of rIL-5 from day 9 until day 30 after immunization markedly suppressed EAN over that period, with a maximal clinical score achieved being 0.5 (Figure 2A). By contrast, controls developed severe disease from day 12 to day 25, with a peak score of 3.5 (P < .001; days 15-27). Again, weight loss was markedly reduced with IL-5 treatment. Thus, rIL-5 treatment given before the onset of symptoms markedly suppressed EAN.
Demonstration that long-term IL-5 treatment suppresses EAN and induces Ag-specific CD4+CD25+ Tregs that express Il-5rα. (A) Extended treatment with rIL-5 (5000 U/d; (●) from day 9 to day 30 after immunization prevented onset of significant clinical disease (top panel; P < .001 on days 15-27, and P < .02 on days 14 and 29; n = 6/group) compared with controls with EAN (○). Weight loss (bottom panel) was significantly reduced at days 10-22, P < .05. (B) CD4+CD25+ T cells were prepared from the lymph nodes and spleens of rIL-5–treated animals and from untreated controls in panel A, at the end of the treatment, which was day 30 after immunization. CD4+CD25+ T cells from rIL-5–treated animals (IL-5Rx) expressed Il-5rα, whereas cells from controls (CTL) had no Il-5rα (**P = .02). (C) The same CD4+CD25+ T cells from rIL-5–treated animals (■) proliferated to specific immunizing Ag PNM but not autologous stimulator cells with no Ag or RTA (**P = .02). CD4+CD25+ T cells from untreated EAN controls (□) did not respond to any stimulus. These data show that IL-5 promoted induction of Ag-specific Tregs that expressed Il-5rα mRNA.
Demonstration that long-term IL-5 treatment suppresses EAN and induces Ag-specific CD4+CD25+ Tregs that express Il-5rα. (A) Extended treatment with rIL-5 (5000 U/d; (●) from day 9 to day 30 after immunization prevented onset of significant clinical disease (top panel; P < .001 on days 15-27, and P < .02 on days 14 and 29; n = 6/group) compared with controls with EAN (○). Weight loss (bottom panel) was significantly reduced at days 10-22, P < .05. (B) CD4+CD25+ T cells were prepared from the lymph nodes and spleens of rIL-5–treated animals and from untreated controls in panel A, at the end of the treatment, which was day 30 after immunization. CD4+CD25+ T cells from rIL-5–treated animals (IL-5Rx) expressed Il-5rα, whereas cells from controls (CTL) had no Il-5rα (**P = .02). (C) The same CD4+CD25+ T cells from rIL-5–treated animals (■) proliferated to specific immunizing Ag PNM but not autologous stimulator cells with no Ag or RTA (**P = .02). CD4+CD25+ T cells from untreated EAN controls (□) did not respond to any stimulus. These data show that IL-5 promoted induction of Ag-specific Tregs that expressed Il-5rα mRNA.
CD4+CD25+ Tregs from these rIL-5–treated but not control rats expressed mRNA for Il-5rα (Figure 2B) and proliferated in vitro when stimulated with syngeneic APCs primed with the immunizing Ag PNM, but not those primed with another third-party autoantigen (RTA) or unprimed cells (Figure 2C). Thus, IL-5 promoted Ag-specific Tregs that expressed Il-5rα and responded to the immunizing but not other Ags.
Effect of rIL-5 therapy after onset of clinical disease in EAN
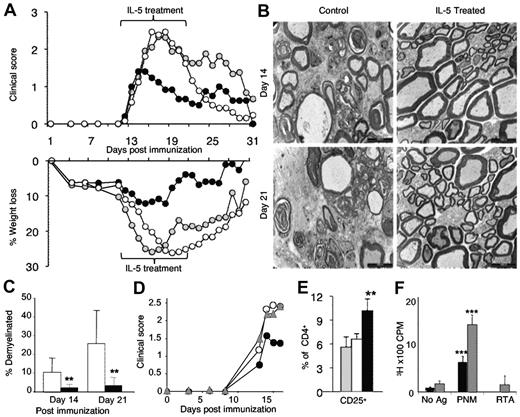
Because clinical autoimmunity presents after tissue injury and symptoms, we examined the effect of treatment with 5000 U/d rIL-5, commenced on the day of onset of clinical EAN. In our experiments, clinical paralysis was first evident on day 12 or day 13. Clinical disease severity and weight loss were significantly reduced 2 days after commencing rIL-5 therapy, and there was more rapid recovery (Figure 3A) compared with untreated controls or sham-treated controls. Again, weight loss was arrested within days of commencement of rIL-5 treatment. rIL-5 treatment had a similar effect on EAN in 4 separate experiments.
The effects of treatment with IL-5 after the onset of clinical EAN. (A) rIL-5 treatment (5000 U/d for 10 days; ●; n = 15) commenced on the day after onset of clinical EAN, usually day 12, reduced severity of EAN within 2 days, compared with sham-treated (gray circle; n = 10) or untreated (○; n = 13) controls. In contrast to both sham and untreated controls, rIL-5–treated animals had significantly lower clinical scores between day 15 and day 21 (P < .008) and greater weight loss between day 14 and day 26 (P < .03). At days 14 and 21 after immunization, nerves were examined. (B) Electromicrographs (bar represents 1 μm) showed major disruption with numerous demyelinated nerves and edema with mononuclear cell infiltrates in controls, whereas rIL-5–treated animals had well-preserved myelin sheaths and only occasional demyelinated nerves. (C) The area of demyelinated nerves was significantly less at both day 14 (**P < .001) and day 21 (**P < .001) in rIL-5–treated animals (■) compared with untreated controls (□; n = 10/group). (D) The specificity of the rIL-5 effect was shown by coadministration of rIL-5 and mAb that blocks IL-5 function from day 23 after immunization (gray triangle). This anti–IL-5-blocking mAb abolished the effect of rIL-5 treatment on EAN because the course of EAN in these animals was similar to untreated EAN (○; n = 5/group). rIL-5 treatment from day 13 diminished severity of EAN (●) with significant differences to the rIL-5/anti-IL-5 monoclonal treatment group at day 15 (**P = .004) and day 16 (**P = .003). The rIL-5 CD4+CD25+ T cells were prepared from these rIL-5–treated and control groups and examined at day 17 after immunization. (E) An increased number of CD4+CD25+ T cells were observed in peripheral lymphoid tissues of rIL-5–treated animals (■) compared with control EAN (□) and normal Lewis rats (▩; **P < .01). CD4+CD25+ T cells were taken from animals 4 days after rIL-5 treatment commenced. (F) Enriched CD4+CD25+ T cells from these rIL-5–treated rats only proliferated to the immunizing Ag PNM and not to unprimed autologous stimulator cells with no Ag or with RTA (***P < .001). Addition of rIL-5 (▩) to culture significantly enhanced proliferation to PNM (***P < .001) compared with cells without rIL-5 that responded to PNM (■). IL-5 had little effect on proliferation of these cells to unprimed autologous stimulators or RTA-primed stimulators. This shows that Tregs were Ag-specific and responded to IL-5.
The effects of treatment with IL-5 after the onset of clinical EAN. (A) rIL-5 treatment (5000 U/d for 10 days; ●; n = 15) commenced on the day after onset of clinical EAN, usually day 12, reduced severity of EAN within 2 days, compared with sham-treated (gray circle; n = 10) or untreated (○; n = 13) controls. In contrast to both sham and untreated controls, rIL-5–treated animals had significantly lower clinical scores between day 15 and day 21 (P < .008) and greater weight loss between day 14 and day 26 (P < .03). At days 14 and 21 after immunization, nerves were examined. (B) Electromicrographs (bar represents 1 μm) showed major disruption with numerous demyelinated nerves and edema with mononuclear cell infiltrates in controls, whereas rIL-5–treated animals had well-preserved myelin sheaths and only occasional demyelinated nerves. (C) The area of demyelinated nerves was significantly less at both day 14 (**P < .001) and day 21 (**P < .001) in rIL-5–treated animals (■) compared with untreated controls (□; n = 10/group). (D) The specificity of the rIL-5 effect was shown by coadministration of rIL-5 and mAb that blocks IL-5 function from day 23 after immunization (gray triangle). This anti–IL-5-blocking mAb abolished the effect of rIL-5 treatment on EAN because the course of EAN in these animals was similar to untreated EAN (○; n = 5/group). rIL-5 treatment from day 13 diminished severity of EAN (●) with significant differences to the rIL-5/anti-IL-5 monoclonal treatment group at day 15 (**P = .004) and day 16 (**P = .003). The rIL-5 CD4+CD25+ T cells were prepared from these rIL-5–treated and control groups and examined at day 17 after immunization. (E) An increased number of CD4+CD25+ T cells were observed in peripheral lymphoid tissues of rIL-5–treated animals (■) compared with control EAN (□) and normal Lewis rats (▩; **P < .01). CD4+CD25+ T cells were taken from animals 4 days after rIL-5 treatment commenced. (F) Enriched CD4+CD25+ T cells from these rIL-5–treated rats only proliferated to the immunizing Ag PNM and not to unprimed autologous stimulator cells with no Ag or with RTA (***P < .001). Addition of rIL-5 (▩) to culture significantly enhanced proliferation to PNM (***P < .001) compared with cells without rIL-5 that responded to PNM (■). IL-5 had little effect on proliferation of these cells to unprimed autologous stimulators or RTA-primed stimulators. This shows that Tregs were Ag-specific and responded to IL-5.
After 2 days of rIL-5 treatment, which was day 14 after immunization, the extent and severity of demyelination of nerves was less in rIL-5–treated animals than in controls (Figure 3B). The architecture of nerves from rIL-5–treated animals was well preserved with only occasional nerve demyelinated, whereas there were many demyelinated nerves and a greater cellular infiltration in controls, especially in the perivenular area. Quantitative studies of demyelination found that at day 14 the area of demyelination of nerves was 1.8% in rIL-5–treated rats compared with 10.6% of controls (P < .001; Figure 3C). At day 21, after 9 days of treatment with rIL-5, the area of demyelination was 3.1% compared with 27.1% in controls (P < .0001). Thus, rIL-5 treatment commenced after onset of clinical disease reduced development of clinical paralysis, weight loss, and demyelination. In fact, there was minimal demyelination of nerves, suggesting the effector T-cell and macrophage responses that mediate demyelination in EAN were reduced at a tissue level.
Administration of a mAb that blocked IL-5 inhibited the effects of treatment with rIL-5 on clinical EAN (Figure 3D). This shows that the amelioration of EAN was because of the IL-5 in the preparation used which was from CHO-K1 cells transfected with the gene for rat IL-5.20
rIL-5 treatment had no adverse effects on general well-being of the rats. Serial monitoring of blood counts in animals in which rIL-5 treatment commenced after the onset of clinical EAN at day 12 after immunization with PNM/CFA is shown in Table 1. No change in the whole blood count was observed through the period of observation, compared with normal Lewis rats before immunization. rIL-5–treated rats had more eosinophils at day 14 (P = .004) and day 21 (P < .001) than controls, but not at day 28. At day 21, monocytes (P = .04) and lymphocytes (P = .02) were less in rIL-5–treated animals than in controls. The induction of EAN with PNM/CFA induced significant changes in leukocyte subsets, including an increase in neutrophils in both controls and rIL-5–treated animals at days 14, 21, and 28 (P < .05). A complementary decrease was observed in lymphocytes (P < .02) which was greater with rIL-5 treatment at day 21. In controls, eosinophils were reduced at days 14, 21, and 28 (P < .05), and this occurred in rIL-5–treated animals after cessation of treatment, whereas rIL-5–treated animals at day 14 had no drop in eosinophils and by day 21 had more than did normal Lewis rats before immunization (P = .03). Thus, rIL-5 did induce an eosinophilia, as would be expected. rIL-5 first prevented the reduction in eosinophils induced by the onset of EAN, probably because of increased production of corticosteroids that occurs after immunization with CFA.
Comparison of peripheral blood white cell counts between rIL-5 treated and control EAN rats
| . | Day 0 . | Day 14 . | Day 21 . | Day 28 . | |||
|---|---|---|---|---|---|---|---|
| Normal . | rIL-5 Rx . | Control . | rIL-5 Rx . | Control . | rIL-5 Rx . | Control . | |
| WBC count | 12.8 ± 4 | 14.1 ± 4.1 | 13.6 ± 4.2 | 12.3 ± 3.9 | 14.6 ± 4.6 | 15.3 ± 6.5 | 14.7 ± 3.5 |
| Neutrophil | 2.4 ± 0.7* | 4.4 ± 3.6 | 4.9 ± 2.9 | 4.1 ± 2.4 | 4.1 ± 3.1 | 5.0 ± 2.8 | 4.3 ± 2.2 |
| Eosinophil | 0.1 ± 0.08* | 0.07 ± 0.04† | 0.04 ± 0.03 | 0.2 ± 0.09‡ | 0.05 ± 0.03 | 0.05 ± 0.04 | 0.04 ± 0.04 |
| Basophil | 0.23 ± 0.1 | 0.2 ± 0.1 | 0.28 ± 0.7 | 0.8 ± 0.7 | 0.5 ± 0.4 | 0.4 ± 0.4 | 0.3 ± 0.3 |
| Monocytes | 1.1 ± 0.4 | 1.2 ± 0.5 | 1.2 ± 0.5 | 0.9 ± 0.3§ | 1.4 ± 0.6 | 1.5 ± 0.6 | 1.28 ± 1.0 |
| Lymphocytes | 12 ± 3.7* | 7.8 ± 3.9 | 7.9 ± 4.4 | 6.7 ± 2.3‖ | 8.4 ± 3.7 | 8.5 ± 4.2 | 9.0 ± 3.0 |
| . | Day 0 . | Day 14 . | Day 21 . | Day 28 . | |||
|---|---|---|---|---|---|---|---|
| Normal . | rIL-5 Rx . | Control . | rIL-5 Rx . | Control . | rIL-5 Rx . | Control . | |
| WBC count | 12.8 ± 4 | 14.1 ± 4.1 | 13.6 ± 4.2 | 12.3 ± 3.9 | 14.6 ± 4.6 | 15.3 ± 6.5 | 14.7 ± 3.5 |
| Neutrophil | 2.4 ± 0.7* | 4.4 ± 3.6 | 4.9 ± 2.9 | 4.1 ± 2.4 | 4.1 ± 3.1 | 5.0 ± 2.8 | 4.3 ± 2.2 |
| Eosinophil | 0.1 ± 0.08* | 0.07 ± 0.04† | 0.04 ± 0.03 | 0.2 ± 0.09‡ | 0.05 ± 0.03 | 0.05 ± 0.04 | 0.04 ± 0.04 |
| Basophil | 0.23 ± 0.1 | 0.2 ± 0.1 | 0.28 ± 0.7 | 0.8 ± 0.7 | 0.5 ± 0.4 | 0.4 ± 0.4 | 0.3 ± 0.3 |
| Monocytes | 1.1 ± 0.4 | 1.2 ± 0.5 | 1.2 ± 0.5 | 0.9 ± 0.3§ | 1.4 ± 0.6 | 1.5 ± 0.6 | 1.28 ± 1.0 |
| Lymphocytes | 12 ± 3.7* | 7.8 ± 3.9 | 7.9 ± 4.4 | 6.7 ± 2.3‖ | 8.4 ± 3.7 | 8.5 ± 4.2 | 9.0 ± 3.0 |
Data are expressed as 106 cells/mL (n = 5/group).
WBC indicates white blood cell.
Blood counts before immunization were significantly different (P < .05) from all controls at days 14, 21, and 28 after induction of EAN with PNM/CFA.
IL-5–treated animals significantly different from controls, P = .004.
IL-5–treated animals significantly different from controls, P < .001.
IL-5–treated animals significantly different from controls, P = .04.
IL-5–treated animals significantly different from controls, P = .02.
Examination of the regional lymph node draining the site of immunization showed that after 9 days of rIL-5 therapy there was no difference in weight (32.2 ± 12.8 mg) compared with untreated controls (28.6 ± 12.0 mg; n = 10 per group). rIL-5 treatment reduced the percentage of CD4+ T cells to 37.7% ± 2.5% compared with 57.6% ± 3.2% in controls (P < .001). The fall in CD4+ T-cell numbers was because of increased numbers of B cells, because rodent B cells (unlike human B cells) express Il-5rα, and rIL-5 therapy expanded these cells.
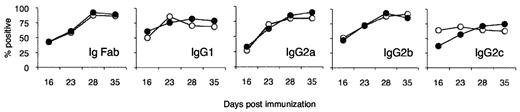
rIL-5 treatment had no effect on anti-PNM Ab titers nor did it induce an IgG isotype shift to a Th2 type with more IgG1 and less complement-fixing IgG2 isotypes (Figure 4). Anti-PNM Abs have been implicated as mediators of demyelination in EAN31 ; however, activation of the membrane attack complex of complement is not essential for demyelination in EAN.19 Because there was no alteration in anti-PNM Ab titers or Ab isotype, the effect of rIL-5 on B cells in the lymph node at the site of immunization was not the explanation for its effects on EAN.
Effect of IL-5 treatment on anti-PNM Ab isotypes. Comparison of titers taken at day 12 after immunization of anti-PNM Abs in groups treated with IL-5 (●) from day of onset of clinical disease to untreated controls (○; n = 5/group). No significant differences were observed in total Ig (IgFab), IgG1, IgG2a, IgG2b, and IgG2c titers at days 14, 21, 28, and 35 after immunization. Data are expressed as a percentage relative to a control sera from a Lewis rat hyperimmunized with PNM.
Effect of IL-5 treatment on anti-PNM Ab isotypes. Comparison of titers taken at day 12 after immunization of anti-PNM Abs in groups treated with IL-5 (●) from day of onset of clinical disease to untreated controls (○; n = 5/group). No significant differences were observed in total Ig (IgFab), IgG1, IgG2a, IgG2b, and IgG2c titers at days 14, 21, 28, and 35 after immunization. Data are expressed as a percentage relative to a control sera from a Lewis rat hyperimmunized with PNM.
Demonstration that the rIL-5 activates Ag-specific Tregs
rIL-5 treatment increased CD4+CD25+ T cells within peripheral lymphoid tissues from < 6% of CD4+ T cells in normal and control EAN rats to > 9% (Figure 3E). Further, CD4+CD25+ Tregs, taken 4 days after rIL-5 treatment was commenced, proliferated to PNM, the specific Ag, but not to third-party autoantigen (RTA) or syngeneic stimulator cells with no Ag (Figure 3F). The addition of rIL-5 to cultures only enhanced proliferation to PNM, not other Ags, confirming that only Ag-specific Tregs responded to rIL-5 (Figure 3F). This response is in contrast to the response of Tregs to IL-2 or IL-4, whereby proliferation is induced to all Ags.3 The increased proliferation of Tregs to specific Ags induced by rIL-5 confirmed that the induction of il5ra results in expression of functional IL-5Rα on autoantigen-specific Tregs. Further, this IL-5Rα when activated by IL-5 promoted proliferation of these Tregs.
The requirement for CD4+CD25+ T cells was tested by depletion of CD25+ cells with a mAb to CD25 (NDS61).23 This abrogated the benefits of rIL-5 treatment on clinical EAN (Figure 5A). Anti-CD25 mAb treatment did not itself affect the severity of EAN, albeit it delayed the onset of paralysis by 2-3 days.
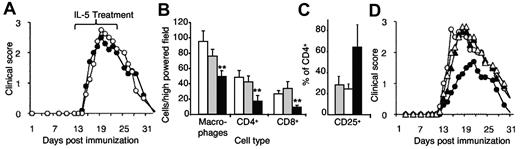
Characterization of the central role of CD4+CD25+ T cells and IL-4 in mediating the IL-5 effect. (A) Pretreatment with NDS61, a mAb that depletes CD25+ cells,23 eliminated any effect of rIL-5 treatment (●) because the clinical course was similar to controls treated with NDS61, but no IL-5 (○; n = 6/group). In contemporary controls not treated with NDS61 (n = 5/group), IL-5 inhibited EAN (data not shown). (B) The cauda equina nerves of animals treated with IL-5 (■) had reduced macrophage (**P = .002), CD4+ (**P = .002), and CD8+ (**P = .008) T-cell infiltrates than untreated (□) controls and IL-5/NDS61 (CD25+ depleted; ▩). Samples were taken from animals in panel A at day 31 (n = 5/group). (C) IL-5–treated animals (■) had a greater proportion of CD25+CD4+ T cells in nerves than untreated controls and NDS61/IL-5–treated animals (▩). Data are expressed as mean ± SD and number of cells counted as per high-power field (n = 5). (D) Blocking IL-4 prevented rIL-5 inhibition of EAN. Animals immunized to develop EAN were treated with MRCOX81, a monoclonal that blocks IL-4.22 The disease course was similar in animals treated with anti–IL-4, with (▴) or without (▵) rIL-5, and untreated controls (○). Controls only treated with rIL-5 had a reduction in severity of EAN (●; n = 5/group).
Characterization of the central role of CD4+CD25+ T cells and IL-4 in mediating the IL-5 effect. (A) Pretreatment with NDS61, a mAb that depletes CD25+ cells,23 eliminated any effect of rIL-5 treatment (●) because the clinical course was similar to controls treated with NDS61, but no IL-5 (○; n = 6/group). In contemporary controls not treated with NDS61 (n = 5/group), IL-5 inhibited EAN (data not shown). (B) The cauda equina nerves of animals treated with IL-5 (■) had reduced macrophage (**P = .002), CD4+ (**P = .002), and CD8+ (**P = .008) T-cell infiltrates than untreated (□) controls and IL-5/NDS61 (CD25+ depleted; ▩). Samples were taken from animals in panel A at day 31 (n = 5/group). (C) IL-5–treated animals (■) had a greater proportion of CD25+CD4+ T cells in nerves than untreated controls and NDS61/IL-5–treated animals (▩). Data are expressed as mean ± SD and number of cells counted as per high-power field (n = 5). (D) Blocking IL-4 prevented rIL-5 inhibition of EAN. Animals immunized to develop EAN were treated with MRCOX81, a monoclonal that blocks IL-4.22 The disease course was similar in animals treated with anti–IL-4, with (▴) or without (▵) rIL-5, and untreated controls (○). Controls only treated with rIL-5 had a reduction in severity of EAN (●; n = 5/group).
The effect of rIL-5 on the cellular infiltrate in nerves was compared with controls and animals depleted of CD25+ T cells but treated with rIL-5. rIL-5 treatment in hosts with normal CD25+ T-cell population had a significantly reduced infiltration of macrophages (P = .002), CD4+ T cells (P = .002), and CD8+ T cells (P = .008) into nerves compared with nontreated controls and those treated with anti-CD25 mAb and rIL-5 (Figure 5B). rIL-5–treated rats, not depleted of CD25+ cells, had an increased proportion of CD4+CD25+ T cells in their nerves (57% of CD4+ T cells) compared with untreated controls (20% of CD4+ T cells; P < .001; Figure 3C).
Taken together, these data show that CD25+ cells were essential for IL-5 to reduce immune inflammation and, that at the site of inflammation, there was an increased proportion of CD4+CD25+ T cells.
Our hypothesis is that the Th2 response induced by immunization with PNM/CFA secretes IL-4, which with PNM, induces Ag-specific Tregs to the Ts2 phenotype expressing Il-5rα. To test this, we treated Lewis rats immunized with PNM/CFA with an IL-4–blocking mAb from 5 days after immunization, then commenced rIL-5 therapy 9 days after immunization. Pretreatment with anti–IL-4 mAb totally prevented any effect of rIL-5 therapy on the clinical course of EAN. This experiment confirmed that host-derived IL-4 was required to induce Il-5rα–expressing Tregs that could respond to the IL-5 (Figure 5C).
Effect of rIL-5 on induction of T effector cell subsets
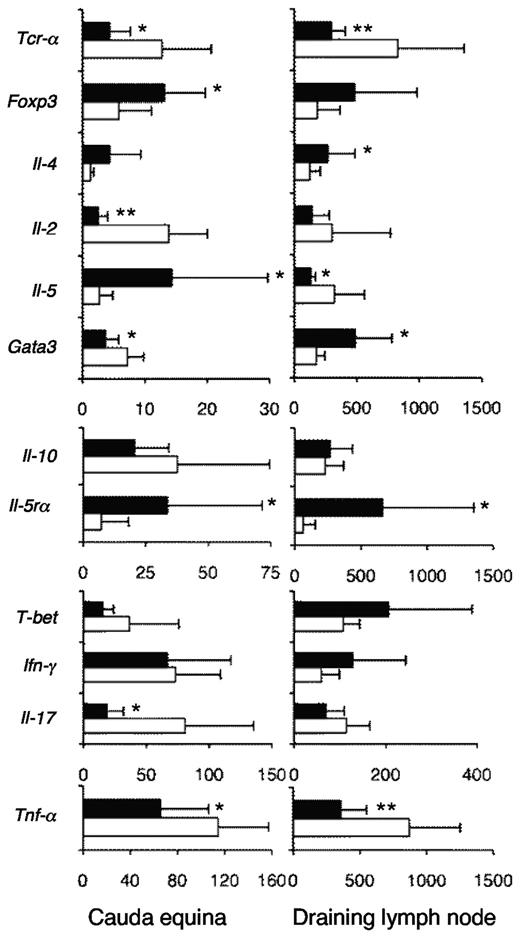
The principal mediators of autoimmunity in EAN are Th17 cells, which produce IL-17A, Th1 cells that produce IL-2, and macrophages that produce TNF-α.32 Nerves from rIL-5–treated animals had reduced levels of mRNA for Il-2, Il-17A, and Tnf-α, compared with controls, showing reduced infiltration of all 3 effector cells types (Figure 6A). Foxp3 and Il-5rα mRNA were increased in nerves from rIL-5 compared with nerves from controls, consistent with increased infiltration of IL-5–responsive Tregs. After rIL-5 therapy, mRNA of Th2 cytokines Il-4, Il-5, and Il-10 mRNA levels were similar or relatively increased compared with controls.
Effect of rIL-5 treatment on induction of inflammatory T-cell subtypes in EAN. RT-PCR of cauda equina nerves (n = 10/group; *P ≤ .05, **P ≤ .01) showed that rIL-5 treatment for 9 days after onset of EAN (■) reduced the mRNA for TCR-α (Tcr-α), indicating there were less T cells than in EAN controls (□; P < .05; n = 10/group), but enhanced foxp3 (P = .05) and Il-5rα (P < .015), consistent with infiltration of Tregs (n = 10 rat samples/group). There was reduced mRNA for Il-2 (**P < .001), a Th1 cytokine, and Il-17A (**P = .008), a Th17 cytokine, and of Tnf-α a macrophage cytokine (*P < .05), indicating reduced infiltration of these autoimmune effector cells. mRNA of Th2 cytokines Il-5 (P = .03) and Il-4 mRNA (P = .08) were similar or increased in rIL-5–treated animals, indicating sparing of Th2 compared with Th1 and Th17 cells. T-beta and Gata3 were not increased. In summary, there was enhanced Ts2-associated mRNA (Foxp3, Il-5rα, with similar Ifn-γ), whereas there was suppressed Th1 (Il-2, T-bet) and Th17 (Il-17A), and sparing of Th2 (Il-4, Il-5, Il-10) cytokines. RT-PCR of the popliteal lymph nodes draining the site of immunization showed treatment with IL-5 for 9 days after onset of EAN had less mRNA for Tcr-α (**P < .006) and Tcr-β (P < .001; data not shown) than controls (n = 10 rat samples/group). The reduced Tcr-α was consistent with the increased B-cell numbers because of the effects of rIL-5. There was more mRNA for Il-5rα (*P = .04), Foxp3 (P = .05), and Gata3 (P < .01) in the IL-5–treated animals compared with controls. There was no differences in mRNA levels for Il-2, Il-17A, Il-10, or T-bet, but mRNA for Tnf-α (**P < .002) and Il-5 (*P < .02) was reduced in the IL-5–treated animals. The increased Foxp3 and Il-5rα and unchanged Ifn-γ were consistent with the presence of Ts2 cells. There was no suppression of Th1 or Th17 responses and sparing of some but not all Th2 cytokines.
Effect of rIL-5 treatment on induction of inflammatory T-cell subtypes in EAN. RT-PCR of cauda equina nerves (n = 10/group; *P ≤ .05, **P ≤ .01) showed that rIL-5 treatment for 9 days after onset of EAN (■) reduced the mRNA for TCR-α (Tcr-α), indicating there were less T cells than in EAN controls (□; P < .05; n = 10/group), but enhanced foxp3 (P = .05) and Il-5rα (P < .015), consistent with infiltration of Tregs (n = 10 rat samples/group). There was reduced mRNA for Il-2 (**P < .001), a Th1 cytokine, and Il-17A (**P = .008), a Th17 cytokine, and of Tnf-α a macrophage cytokine (*P < .05), indicating reduced infiltration of these autoimmune effector cells. mRNA of Th2 cytokines Il-5 (P = .03) and Il-4 mRNA (P = .08) were similar or increased in rIL-5–treated animals, indicating sparing of Th2 compared with Th1 and Th17 cells. T-beta and Gata3 were not increased. In summary, there was enhanced Ts2-associated mRNA (Foxp3, Il-5rα, with similar Ifn-γ), whereas there was suppressed Th1 (Il-2, T-bet) and Th17 (Il-17A), and sparing of Th2 (Il-4, Il-5, Il-10) cytokines. RT-PCR of the popliteal lymph nodes draining the site of immunization showed treatment with IL-5 for 9 days after onset of EAN had less mRNA for Tcr-α (**P < .006) and Tcr-β (P < .001; data not shown) than controls (n = 10 rat samples/group). The reduced Tcr-α was consistent with the increased B-cell numbers because of the effects of rIL-5. There was more mRNA for Il-5rα (*P = .04), Foxp3 (P = .05), and Gata3 (P < .01) in the IL-5–treated animals compared with controls. There was no differences in mRNA levels for Il-2, Il-17A, Il-10, or T-bet, but mRNA for Tnf-α (**P < .002) and Il-5 (*P < .02) was reduced in the IL-5–treated animals. The increased Foxp3 and Il-5rα and unchanged Ifn-γ were consistent with the presence of Ts2 cells. There was no suppression of Th1 or Th17 responses and sparing of some but not all Th2 cytokines.
In the lymph node draining the site of immunization, RT-PCR identified less mRNA for Tcr-α (P < .002) and Tcr-β (P < .001) than for controls (Figure 6B). This reduction in TCR is because of the expansion in the proportion of B cells, because of IL-5–induced proliferation. No difference in mRNA levels was observed for Il-2, Il-17, Il-10, T-bet, or Gata3. There was more mRNA for Il-5rα (P < .05), Il-5 (P < .03), Il-4 (P < .02), and Foxp3 (P = .05) in the rIL-5–treated animals compared with controls. mRNA for Tnf-α was reduced in the rIL-5–treated animals (P < .002). Paradoxically, mRNA for Ifn-γ was not reduced in the nerves or lymph nodes in rIL-5–treated animals. Ts2 cells express Ifn-γ,3 which could explain the presence of Ifn-γ in rIL-5–treated rats.
These findings confirmed that there is expression of Il-4 during induction of EAN and that this cytokine could have induced the Il-5rα–expressing Ts2 cells. These data suggested that, although rIL-5 increases Treg numbers in the regional node, it did not suppress the generation of Th17 and Th1 lineages of effector cells. Taken together, these results suggest that induction of Th1, Th2, and Th17 responses in the immunizing lymph node is not impaired and that there is an increase in the number of Ts2-like Tregs in the lymph node draining the site of immunization. The marked reduction in Th1 and Th17 cytokines in the nerves suggests the activated Ts2-like Tregs are inhibiting the entry or survival of these effector lineage Th1 and Th17 cells and macrophages, in the site of autoimmune inflammation, which in EAN is the peripheral nerves.
Discussion
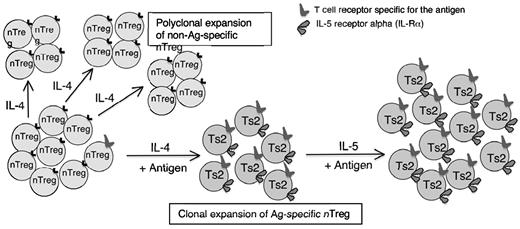
This study found that IL-5 promotes induction of Ag-specific Tregs that have been activated by IL-4 and autoantigen. It shows that our findings in allograft tolerance3 also apply to tolerance in autoantigens. The separate Th1- and Th2-dependent pathways of activation of naive natural CD4+CD25+ Tregs into Ag-specific CD4+CD25+ T cells3 provided potential mechanisms by which Ag-specific Tregs can be activated and expanded. Early in the immune response, IL-4 released by Ag-activated CD4+CD25− T cells will promote polyclonal expansion of Tregs. Within this population, those Tregs with receptors for specific Ag are activated and express Il-5rα (Figure 7). These Ag-specific Tregs can be further expanded by rIL-5 therapy or IL-5 produced by Th2 cells. This activation of nTregs by IL-4 and Ag is a potential therapeutic pathway for induction of Ag-specific Tregs to control autoimmune and other immune-mediated inflammatory responses.
Proposed mechanism of action of IL-5 on IL-4–induced Ag-specific Tregs to expand Th2 cytokine–dependent Tregs of the Ts2 phenotype. Within the naive CD4+CD25+ T-cell population there are a small number of Tregs with TCR specific for Ag that can be induced to Ts2,3 by stimulation with Ag in the presence of IL-4. These Ag-specific Tregs express Il-5rα and are further expanded by IL-5, produced by maturing Th2 cells or exogenous rIL-5. Most nTregs have TCR that do not recognize the Ag in question, which are activated by IL-4 and induced to proliferate but do not differentiate to express Il-5rα. They remain as nTregs and do not mediate Ag-specific tolerance. This study showed that rIL-5 therapy enhanced expansion of Ts2 cells that had been activated by immunizing Ag and production of IL-4 by the host T cells. The Il-5rα–expressing Ag-specific Tregs were selectively expanded by rIL-5 therapy to suppress autoimmunity, including Th1 cells, Th17 cells, and macrophages.
Proposed mechanism of action of IL-5 on IL-4–induced Ag-specific Tregs to expand Th2 cytokine–dependent Tregs of the Ts2 phenotype. Within the naive CD4+CD25+ T-cell population there are a small number of Tregs with TCR specific for Ag that can be induced to Ts2,3 by stimulation with Ag in the presence of IL-4. These Ag-specific Tregs express Il-5rα and are further expanded by IL-5, produced by maturing Th2 cells or exogenous rIL-5. Most nTregs have TCR that do not recognize the Ag in question, which are activated by IL-4 and induced to proliferate but do not differentiate to express Il-5rα. They remain as nTregs and do not mediate Ag-specific tolerance. This study showed that rIL-5 therapy enhanced expansion of Ts2 cells that had been activated by immunizing Ag and production of IL-4 by the host T cells. The Il-5rα–expressing Ag-specific Tregs were selectively expanded by rIL-5 therapy to suppress autoimmunity, including Th1 cells, Th17 cells, and macrophages.
In this report, we have shown that naive CD4+CD25+FOXP3+ Tregs cultured with PNM, the specific Ag for EAN, and IL-4 were induced to proliferate and express Il-5rα. Culture of nTregs with PNM and IL-2 did not induce Il-5rα, but induced ifngr (data not shown). Thus, an autoantigen was shown to induce the same changes reported with alloactivation of nTregs in culture with IL-4 or IL-2.3 We also observed that human CD4+CD25+ T cells activated with IL-4, but not IL-2, were induced to express Il-5rα. Thus, the effects of rIL-5 we show in our rodent studies may also be applicable to human disease.
Further, rIL-5 administered in high doses commencing before onset of clinical EAN blocked induction of clinical symptoms. This was associated with induction of specific PNM-responsive CD4+CD25+ T cells that express Il-5rα. The commencement of rIL-5 on day 9 after immunization but before onset of symptoms suppressed onset of clinical EAN, albeit there was a relapse immediately after rIL-5 was stopped. This suggested that ongoing rIL-5 was required to maintain the Ag-specific Tregs.
Treatment with rIL-5 after the onset of clinical symptoms also markedly reduced the severity of EAN, with effects evident within 2 days. rIL-5 treatment prevented demyelination; reduced infiltration of macrophages, CD4+, and CD8+ T cells into nerves; and was associated with less Th1 and Th17 cytokines, suggesting these effector lineages had been suppressed at the site of inflammation. After rIL-5 treatment there were more CD4+CD25+ T cells, as well as more foxp3, Il-5rα, and Ifn-γ in nerves consistent with an infiltrate of Ts2-like Tregs. Further, the CD4+CD25+ T cells in peripheral lymphoid tissue of these animals responded to PNM but not self-or third-party autoantigen, and this proliferative response was enhanced in the presence of rIL-5. This shows that the rIL-5 therapy within 4 days enhanced the Ag-specific IL-5–responsive Tregs. Proof that the effects of IL-5 depended on IL-4–activated CD25+ Tregs was shown by depletion of CD25+ T cells with an anti-CD25 mAb and the blocking of IL-4 with an anti–IL-4 mAb. These results support our hypothesis that the Th2 component of response to PNM/CFA released IL-4 together with PNM activated naive CD4+CD25+ Treg with TCR that recognized PNM to express Il-5rα. These Ag-specific Tregs then can respond and proliferate to IL-5 and PNM in vitro and in vivo. These expanded Ag-specific Ts2-like cells in turn inhibited immune inflammation and nerve demyelination, thereby reducing the severity of clinical EAN and weight loss.
Dominance of Th2 responses33 and Th2 cytokines IL-4,22,25 IL-10,34 and IL-1335 can protect against autoimmunity, but their effects are variable. Th2 cells do not directly mediate tolerance. Immune regulation between Th1 and Th2 effector types was the first example of cross talk between CD4+ T cells.36,37 Polarization of a CD4+ T-cell response to Th2 is associated with suppression of Th1 by IL-4 and IL-10.36,37 IFN-γ produced by Th1 inhibits Th2.37 In the 1990s, popular dogma was that Th2 responses prevent damaging effector responses in allograft rejection and autoimmunity. Therapy with Th2 cytokines, especially with IL-4,38 IL-5,21 and IL-1339 delay allograft rejection and reduce autoimmunity.22,40 Th2 dominance was considered an explanation for acquired immune tolerance, until it was shown that Th2 induction and IL-4 are not essential for induction of tolerance.41 rIL-5 can delay allograft rejection by inhibiting Th1,21 but there are no other reports of IL-5 inhibiting immunity. In this study, rIL-5 reduced the Th1 and Th17 response in the nerves but not in the lymph node draining the site of immunization. mRNA of Th2 cytokines was abundant in both the node of immunization and the nerves. This suggests that rIL-5 induced Ag-specific Tregs that migrated to the site of inflammation where they suppressed the damaging Th1 and Th17 immune response, rather than inhibiting the immune response in lymphoid tissues.
The main known function of IL-5 in humans is to induce eosinophilia, which occurs when Th2 cells are activated in allergic and antiparasitic immune responses. There was no explanation for how rIL-5 may mediate immune-protective effects, such as a delay in allograft rejection with inhibition of Th1 responses.21 This led us to examine the cytokines that activate nTregs and Ag-specific Tregs. A systematic examination of the cytokine responsiveness of nTregs identified that they could be induced to proliferate by IL-4 as well as IL-2. We found that nTregs activated with a specific alloantigen and IL-4 had changes in their cytokine and cytokine receptor expression.3 Particularly, we observed expression of mRNA for the Th2 cytokine receptors Il-4rα and Il-5rα, but not for the Th1 cytokine receptors Ifnγr and Il-12rβ2. These cells continued to express CD25 and FOXP3, but they were not induced to express Gata3 or T-bet.3 They had enhanced potency over nTregs in suppression of responses of naive CD4+ T cells to the original stimulating alloantigen in vitro and in vivo; at least a 10-fold increase in potency was identified.3 We called these IL-4 Ag-activated Tregs Ts2 cells because they were activated by the Th2 cytokine IL-4 and induced to express Il-5rα, a receptor for a Th2 cytokine. Because IL-4 is only produced early in a Th2 response, we reasoned that the activated Treg may need late Th2 cytokines, such as IL-5, to promote their survival. Our early studies on Ag-specific CD4+ T cells showed that Ag-specific Tregs died without specific Ag and cytokines derived from activated T cells.17 This led to our hypothesis that IL-5 may promote Ag-specific Tregs activated by IL-4 in Th2 responses.
In support of the concept that different Th responses induce different activated CD4+CD25+ Tregs, human Tregs have recently been shown to express different chemokine receptors consistent with them being targeted to tissues with Th1, Th2, or Th17 responses.42 A significant proportion of Tregs express CCR8, the chemokine receptor expressed by Th2, and these cells migrate to sites of Th2 inflammation which release its ligand CCL1. Twenty percent% of CD4+CCR8+ T cells are FOXP3+, and specific Ag-induced CCR8+CD4+CD25+ Tregs regulate Th2 granuloma.43 These findings support our finding that Th1 and Th2 responses induced separate pathways of Treg activation that migrate to the sites with the relevant Th subclass effector of inflammation.
In multiple sclerosis, parasitic infestation–induced eosinophilia reduces relapses and increases CD4+CD25+ Tregs.44 Parasitic infestation also prevents autoimmunity in rodents.45 Trials of therapeutic parasitic infestation in inflammatory bowel disease46 and multiple sclerosis47 are being conducted. During parasitic infestations, CD4+CD25+ T cells develop in parallel with Th2 polarization48 and inhibit Th1 responses.49 It has been suggested that the immune system evolved with persistent parasitic infestations inducing Th2 dominance to inhibit innate and Th1/Th17 immunity.50 Communities with low parasite infestation rates have an increased incidence of autoimmunity.50 The parasite effect may in part also explain the lower incidence of autoimmunity in communities in latitudes closer to the equator and with poorer hygiene.50 Our results suggest that one of the beneficial effects of parasites may be high levels of IL-5 expanding IL-5Rα–expressing Ag-specific Tregs to control autoimmunity. This hypothesis requires further investigation.
These findings provide new avenues for therapies to promote induction of specific immune tolerance to autoimmunity by promoting the Th2 pathway for activation of Ag-specific CD4+CD25+ Tregs. These Ts2-like Tregs inhibited both Th1 and Th17 effector responses while sparing Th2 cells and thus may contribute to Th2 polarization.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mr Botrous and Hematology at Liverpool Hospital for expert technical assistance and Professor A. Basten for helpful critique of the manuscript.
This work was supported by grants from Bob and Jack Ingham, Liverpool, Australia; Multiple Sclerosis Research Australia; National Health & Medical Research Council Australia; Juvenile Diabetes Research Foundation; Novartis; and University of New South Wales, particularly Vice Chancellor Rory Hume, as well as anonymous donations.
The use of animals was in accordance with approval of the University of New South Wales Animal Ethics Committee.
Authorship
Contribution: G.T.T., S.J.H., and B.M.H. designed research; G.T.T., N.M.C., N.D.V., K.M.P., R.B., C.M.R., and M.N. performed the research; M.K. performed electron microscopy and nerve demyelination studies; G.T.T., S.J.H., N.M.C, N.D.V., C.M.R., and B.M.H. analyzed the data; and G.T.T., S.J.H., and B.M.H. wrote the paper.
Conflict-of-interest disclosure: B.M.H. and S.J.H. hold patents or pending patents related to IL-5 and antigen-activated Treg. The remaining authors declare no competing financial interests.
Correspondence: Suzanne J. Hodgkinson, Department of Neurology, UNSW and Liverpool Health Service, Locked Bag 7103, Liverpool BC 1871, Australia; e-mail: s.hodgkinson@unsw.edu.au.
References
Author notes
G.T.T. and S.J.H. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal