Human mucosal associated invariant T (MAIT) CD8+ and Tc17 cells are important tissue-homing cell populations, characterized by high expression of CD161 (++) and type-17 differentiation, but their origins and relationships remain poorly defined. By transcriptional and functional analyses, we demonstrate that a pool of polyclonal, precommitted type-17 CD161++CD8αβ+ T cells exist in cord blood, from which a prominent MAIT cell (TCR Vα7.2+) population emerges post-natally. During this expansion, CD8αα T cells appear exclusively within a CD161++CD8+/MAIT subset, sharing cytokine production, chemokine-receptor expression, TCR-usage, and transcriptional profiles with their CD161++CD8αβ+ counterparts. Our data demonstrate the origin and differentiation pathway of MAIT-cells from a naive type-17 precommitted CD161++CD8+ T-cell pool and the distinct phenotype and function of CD8αα cells in man.
Introduction
Human mucosal associated invariant T cells (MAIT) are defined by an invariant usage of the T-cell receptor chain Vα 7.2, restriction by the major histocompatibility complex (MHC)–related protein MR1, and most recently have been shown to exhibit high expression of the C-type lectin CD161 (CD161++), and IL18R.1 Human MAIT cells have been described to be CD8αβ, CD8αα, or double-negative (DN) although a differential role for these different subsets has not been explored.
Independently, we have described a human tissue-homing CD161++CD8+ T-cell subset to be Tc17 cells, enriched at inflammatory sites including liver and joints.2 Type-17 function has been recently confirmed in the MAIT cell population.3 CD161++CD8+ and MAIT-cells share key differentiation factors with Th17 cells, including cytokine expression (IL-17A and IL22), transcription factors (RORγt and RUNX2), chemokine receptors (CCR6 and CCR2), and cytokine receptors (IL23R and IL18R). There is growing recognition that these data describe the same phenomenon in parallel or overlapping populations, although this has not been fully defined to date, and the relationship between the 2 subsets remains unclear. Given the recent emergence of these cell types in diverse diseases, including multiple sclerosis,4 this remains a significant unanswered question.
CD161 was first identified as a potential lineage identifier for human Th17 cells when it was found to be a highly up-regulated gene on microarray comparison of gene expression between Th1, Th2, and Th17 clones, and circulating Th17 cells were contained within the CCR6+CD161+CD4+ population.5 Cord blood CD161+ CD4+CD8−, CD8+CD4− and CD4−CD8− TCRαβ+, and TCRγδ+ cells already express IL-23R and RORγt mRNA, and produce IL-17, unlike their CD161 counterparts. The transcription factor RORγt has been defined as the driver for the hallmark features of these cells, as CD161, IL-23R, and IL-17 expression could be directly induced by RORC2 transduction of CD161- cord cells.6 In humans, CD161/NKR-P1A encoded by the KLRB1 gene, is expressed by a wide variety of human immune cells; natural killer (NK) cells, NK T cells, CD4+ T cells, CD8+ T cells, and γδ T cells. Lectin-like transcript-1 (LLT1)7,8 and PILAR9 have been identified as ligands for CD161, although the role of such ligation on CD161++CD8+/ MAIT-cells remains to be defined.
NK T cells and MAIT-cells are the only lymphocyte populations to have a restricted TCR repertoire and restricting MHC molecule that is conserved between species. NK T cells are more abundant in mice, whereas MAIT-cells are more numerous in man, representing up to 15% of human CD8+ T cells. Their developmental pathways are distinct. NK T cells are selected, expand and develop their innate-like phenotype, and function before exit from the thymus. They already express the transcription factor ZBTB16, which is crucial for their ready innate/effector functions.10,–12 MAIT-cells are naive and low in number in the thymus,1 and very small amounts of the TCR Vα7.2-J33 transcript are found in cord blood;13 yet MAIT-cells are abundant in adults and have acquired an effector memory phenotype. Mouse studies demonstrate that MAIT-cells require MR1 expression on a nonhematopoietic cell in the thymus for selection. Their subsequent expansion requires peripheral B cells and mucosal commensal flora. Human MAIT-cells also express the transcription factor ZBTB16, but this has only been described in the effector memory population found in adults.1
Most TCRαβ+ CD8+ T cells express CD8 as a heterodimer of CD8α and β chains. However, it is recognized that a subgroup of CD8+ T cells exists, which express CD8α as a homodimer, so-called CD8αα cells. In comparison to the coreceptor function of CD8αβ, the CD8αα homodimer is believed to act as a corepressor and experimental mouse models support a potential regulatory role for CD8αα cells.14,15 CD8αα expressing cells have been most extensively described to be an intraepithelial cell population of the small intestine in mice. Murine models suggest 2 pathways of differentiation for this CD8αα subset. The first model is a subset of single-positive CD8αα cells that undergo agonist selection in the thymus, where they co-express CD8αα, CD8αβ, and CD4. The expression of CD8αα allows survival of cells with self-reactive TCRs that would otherwise be negatively selected under conventional thymic selection. Such cells are observed to migrate preferentially to the gut mucosa as CD8−CD4− cells, and re-express CD8αα because of antigen stimulation and exposure to IL-15. The second pathway describes conventionally-selected activated CD4 and CD8αβ cells that express CD8αα on migration to the gut.16 In addition, in a murine LCMV model, high avidity CD8αβ cells transiently expressed CD8αα during early antigen-induced activation, and these cells were thought to show a preferential ability to differentiate to memory cells on adoptive transfer.17 In humans, CD8αα/CD8α+βlow cells are found exclusively within the memory cell population, with the overall proportion of these cells shown to increase with age.18 Despite extensive study in mice, there is no published data on the differentiation of these cells in humans, other than that of the MAIT cell literature. MAIT-cells include a CD8αα population, but how these are related developmentally and functionally to CD8αβ populations has not been previously examined and indeed the functional and phenotypic profiles of human CD8αα T cells are not clear.
Here we define the differentiation pathway of CD161++ CD8+ T cells and human MAIT-cells and their relationship to one another; in doing so we also define the ontogeny and function of human CD8αα T cells. The naive CD161++CD8+ cell population in cord blood bears the hallmarks of type-17 cells and already expresses the transcription factor ZBTB16, despite few Vα7.2+ MAIT-cells being present, and a wide variety of TCR-Vβ chains being expressed. Vα7.2+ MAIT-cells expand from this population in adults, and with this we observe the emergence of CD8αα cells, which are exclusive to this subset in man. CD161++CD8αα cells share comparable phenotype, type-17 cytokine expression, gene expression, tissue distribution, and MAIT TCR-α and -β usage to their CD161++ CD8αβ counterparts, and we could readily derive CD161++CD8αα cells from CD161++CD8αβ cells in vitro. Together, these data robustly define the differentiation and functional capacity of these striking human T-cell populations.
Methods
Study subjects
Twenty adult healthy donor whole blood/ PBMC samples, 22 umbilical cord blood samples (Stem Cell Services, NHS Blood and Transplant), 12 healthy 10-week-old infant PBMCs (South African TB Vaccine Initiative Field Site). One biopsy sample was taken from healthy terminal ileum at screening colonoscopy for colorectal carcinoma, 5 paired PBMC and explant liver samples from patients with chronic hepatitis C (Transplantation Program), and 8 explant liver samples from patients with chronic liver disease (Transplantation Program) were used in the analysis. One hundred ninety-one random anonymized ethylene diamine tetra-acetic acid blood samples were used for postclinical analysis in the age-correlation study as previously described.19 All subjects were recruited in agreement with local ethics committees of all participating institutions.
T-cell recovery
Mononuclear cells were isolated from EDTA peripheral blood and cord blood samples by Ficoll-Histopaque density gradient centrifugation (Lymphoprep; Axis Sheild).
Liver infiltrating lymphocytes (LILs) were freshly isolated from explanted liver tissue. Explanted human liver tissue was diced and washed in PBS/10% (vol/vol) 2% FCS, and resuspended in RPMI-1640/10% (vol/vol) FCS. This suspension was then homogenized in a Stomacher-400 circulator (Seward), and filtered through a nylon mesh (60 μm). Cells were then washed in RPMI-1640/10% FCS, layered onto Lympholyte (VH Bio), and density gradient centrifugation applied. Separated LILs at the interface were removed, washed, and cell viability assessed by trypan blue exclusion.
Lamina propria mononuclear cells (LPMCs) were isolated from endoscopic biopsy samples. Biopsies were put in a 1mM DDT solution in HBSS (Sigma-Aldrich) put in a shaking incubator at room temperature for 15 minutes, washed 3 times in HBSS, and placed in a gentleMACS tube (Miltenyi Biotech) with collagenase A/DNAse solution, and shaken on a gentle gentleMACS dissociator (Miltenyi Biotech) brain01 setting. Samples were shaken at 37°C for 1 hour, and then placed on an energetic gentleMACS brain01 program and washed in HBSS.
Antibodies
Anti–CD8α-PerCP, anti–CD3-AmCyan, anti–CD3-FITC, anti–CD8β-APC, anti–IFN-γ-FITC, anti–CD45RA-FITC, anti–CD45RO-FITC, and anti–CD62L-APC (BD Biosciences); anti IL-22-APC/PE, anti–CCR7-FITC, anti–CCR6-PE/FITC, anti–CXCR6-PE, anti–CCR2-PE, and anti–CCR5-APC (R&D Systems); anti-CD3–Pacific orange and live/dead violet fixable cell stain (Invitrogen); anti–IL-17A-PE, anti–Ki-67-FITC, and IL18R-PE (eBioscience); anti–CXCR6-Alexa647 (Biolegend); T-cell receptor Vβ antibodies against 24 V β families (Vβ 1, 2, 4, 5.3, 7.2, 8, 9, 11, 14, 16, 17, 18, 23-PE, and Vβ 3, 5.1, 5.2, 7.1, 12, 13.1, 13.6, 14, 16, 17, 20, 21.3, 22-FITC), anti–CB8β-PE and anti–CD161-PE (Beckman Coulter); anti–CD161-PE/FITC (Miltenyi Biotech); and anti-V α7.2 (3C10; Olivier Lantz).
Immunofluorescent staining
Whole peripheral/cord blood or PBMCs/LPMCs/LILs in PBS were incubated with anti-surface antigen antibodies at 4°C for 20 minutes. Whole blood samples were lysed with 10% FACS-lysis buffer (BD Biosciences) in sterile deionized water. All cells were washed in phosphate buffered saline (PBS) and fixed with 1% formaldehyde in PBS.
Intracellular cytokine staining was performed on freshly isolated PBMCs in complete medium (RPMI 1640 containing 10% FCS, 1% streptomycin/penicillin, and L-glutamine), stimulated with leukocyte activation cocktail (BD Biosciences) and incubated (37°C, 5% CO2) for 4 hours. Cells were washed in PBS and stained with antibodies against surface antigens and incubated at 4°C for 20 minutes. After fixation/permeabilization (FoxP3 staining kit, BD Biosciences), cells were stained with antibodies against intracellular antigens, incubated at 4°C for 20 minutes and washed in 10% PermWash buffer (BD Biosciences) in sterile deionized water.
Intracellular staining for Ki-67 was performed on mononuclear cells before and after culture. Cells were fixed/permeabilsed (FoxP3 staining kit; BD Biosciences) and washed ×3 in 10% PermWash buffer (BD Biosciences). Cells were stained with anti–Ki-67 FITC and incubated at 4°C for 20 minutes. Cells were subsequently stained with antibodies against surface antigens.
FACS-analysis was performed on BD FACS-Caliber and LSRII cytometers, and analyzed using FlowJo Version 8.8.6 software (TreeStar).
Cell sorting
Cell sorting of adult PBMCs for culture was performed by easy-sep magnetic-bead CD8 enrichment (StemCell Technologies), followed by staining with a PE-labeled CD8β antibody and magnetic-bead PE-positive selection (StemCell Technologies). Cells were then washed in PBS and placed in RPMI-1640/10% FCS media.
Adult PBMCs/cord blood for micro-array were sorted by magnetic-bead CD8 enrichment (StemCell Technologies) followed by fluorescent antibody staining and use of a MoFlo MLS cell sorter (DAKO). Sorted cell populations were snap frozen and stored at −800C before RNA extraction.
RNA extraction
Total RNA was extracted from snap frozen sorted cell samples using the RNeasy mini kit (QIAGEN), according to the manufacturer's instructions.
Microarray
cDNA was amplified from total RNA using the Ovation Pico WTA System (NuGEN), fragmented and labeled using the Encore Biotin Module kit (NuGEN) before hybridization to GeneChip Human Genome U133 Plus 2.0 arrays (Affymetrix) using the hybridization, wash and stain kit (Affymetrix), and scanned with the GeneChip scanner 3000 7G (Affymetrix).
The output .CEL files were processed and analyzed using the R statistical software (Version 2.11; http://www.R-project.org), and BioConductor packages.20 Signal data were background corrected and normalized using the robust multichip average (RMA) method,21 which is implemented in the affymetrix BioConductor package.
Statistical testing was performed using the Linear Models for Microarray Analysis (limma) package.22 Raw P values were corrected for multiple testing using the false discovery rate controlling procedure of Benjamini and Hochberg,23 adjusted P values below .05 were considered significant. Gene annotation was added to the final probe list from the NetAffx Analysis Center via the Affymetrix website (http://www.affymetrix.com).
Gene set enrichment analysis was performed using the GSEA Version 2.0 software, developed at the Broad Institute of MIT and Harvard24,25 (http://www.broadinstitute.org/gsea/index.jsp). All microarray data are available for viewing at the Gene Expression Omnibus (GEO) under accession number GSE33425.
Real-time PCR
cDNA synthesis was performed with RNA (10 ug/mL) using a mixture of random and oligo (dT) primers (50mM), dNTPs (10mM), and heated to 65°C for 10 minutes, after which Superscript III reverse transcriptase (Invitrogen) was added. The reaction mix was then allowed to amplify at 50°C for 1 hour. Quantitative real-time PCR reactions were carried out using a LightCycler 480 (Roche Diagnostics) using cDNA from the above reaction and primers (IL23R_F-ccatctctacagggcaccttac, IL23R_R-cgatcattcccaataaaagtcc; IL18RAP_F-cagatattctggatcctgtcgag, IL18RAP_R-tgctttgcagctaatagttaaagg; RORC_F-cagcgctccaacatcttct, RORC_R-ccacatctcccacatggact; ZBTB16_F-gcacagttttcgaagga, ZBTB16_R-cagaagacggccatgtca; GAPDG_F-agccacatcgctcagacac, GAPDH_R-gcccaatacgaccaaatcc; Eurofins, mwg Operon). The light-cycler 480 universal probe library (Roche) was used according to the manufacturer's instructions, with cycling conditions as follows; 95°C for 15 minutes and 55 cycles of 94°C for 15 seconds, 54°C for 30 seconds and 72°C for 30 seconds. Fold change in expression was determined by the 2-ΔΔCT method.26
Cell cultures
CD8αβ cell populations were isolated from adult PBMCs as previously described, and placed in culture in RPMI-1640/10% FCS media, with or without anti-CD3/CD28 dynabeads (Invitrogen), and with or without cytokines; IL-2 (25 ng/mL) and IL-15 (10 ng/mL), before splitting, harvesting, and antibody-staining for FACs analysis on day 1, 2, or 3. Media was changed and cytokines added on days 3 and 6. Cultures containing anti-CD3/CD28 beads were placed in an EasySep magnet to remove the beads. Cells were washed in PBS before analysis by flow cytometry.
Statistics
Statistical analysis, other than that described for the microarray experiments, was performed using Prism Version 4 software (GraphPad). A P value of < .05 was considered significant.
Results
Coreceptor and TCR usage of CD161++CD8+/ MAIT cells
MAIT-cells in humans have been previously defined by their invariant use of the TCR Vα 7.2 chain,27 and more recently shown to express high levels of CD161 and type-17 properties.3 There is growing understanding that the cellular subset described bears striking similarity to that also described as CD161++CD8α+ TCRαβ+ T cells.2 However differences in modes of FACS-based description of these subsets have to date made direct comparisons difficult.
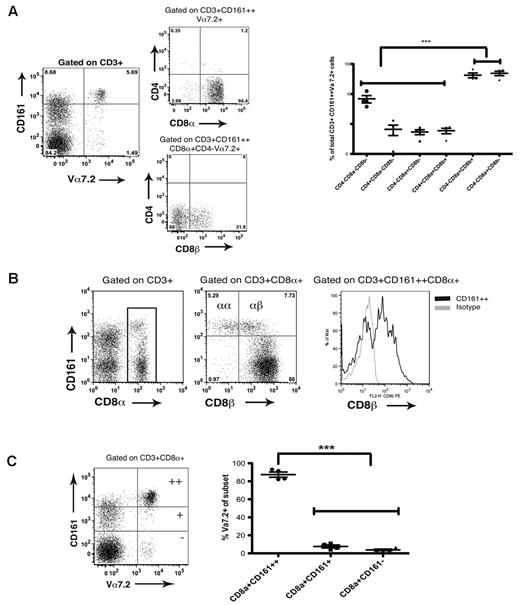
MAIT cells have so far been described to be Vα7.2+ and either CD8β+ or DN, using a system of gating based on CD3, CD4, and CD8β antibodies. In studies of CD161++CD8α+ T cells a significant reduction in the expression of CD8β by CD161++ cells compared with CD161+ and CD161− subsets (CD161++ vs CD161−: −4.43 Log2 fold change; adjusted P = 4.13 × 10−6, CD161++ vs CD161+: −2.08 Log2 fold change; adjusted P = .0053) was previously shown by gene expression profiling.2 Here, by combining these approaches and additional use of a CD8α antibody, we show the CD3+CD161++Vα 7.2+ population to be described > 90% CD8α+CD8β− or CD8α+CD8β+ (49% and 42% of CD3+CD161++Vα 7.2+ subset, respectively). Other cell types, predominantly double-negative cells (6.9%), represented < 10% of the subset (Figure 1A).
MAIT cell and CD161++CD8+ populations are overlapping subsets. (A) CD3+CD161++Vα7.2+ T cells are predominantly CD8αα+ and CD8αβ+ cells. Left hand panel shows representative FACS plots of coreceptor usage by Vα7.2+ T cells. Cumulative data shown in right hand panel. ***P < .001 (1-way ANOVA). (B) Representative FACS plots show a prominent CD8α+CD8β− population exclusive to the CD161++CD8α+ subset in peripheral blood. Data representative of 20 healthy donors. (C) CD161++CD8α+ T cells are predominantly Vα7.2+ (mean 87% Vα7.2+) compared with the CD161+ and CD161− CD8α+ subsets. **P < .001 (1-way ANOVA).
MAIT cell and CD161++CD8+ populations are overlapping subsets. (A) CD3+CD161++Vα7.2+ T cells are predominantly CD8αα+ and CD8αβ+ cells. Left hand panel shows representative FACS plots of coreceptor usage by Vα7.2+ T cells. Cumulative data shown in right hand panel. ***P < .001 (1-way ANOVA). (B) Representative FACS plots show a prominent CD8α+CD8β− population exclusive to the CD161++CD8α+ subset in peripheral blood. Data representative of 20 healthy donors. (C) CD161++CD8α+ T cells are predominantly Vα7.2+ (mean 87% Vα7.2+) compared with the CD161+ and CD161− CD8α+ subsets. **P < .001 (1-way ANOVA).
By further FACS analysis of peripheral blood mononuclear cells (PBMCs) from healthy donors, we demonstrated the CD8αα population in humans to be exclusive to the CD161++CD8α+ subset (Figure 1B). In mice, CD8αα cells are described to be either TCRαβ+ or TCRγδ+, but we have previously demonstrated the CD161++CD8+ T-cell subset to be exclusively TCRαβ+,2 and so are confident that this human subset does not contain a TCRγδ+ population. Murine gut-derived lymphocytes have also been described to co-express CD4 and CD8αα; however, we have not found such a population among the human PBMCs studied (data not shown). We have identified this human CD8αα subset in both liver and small bowel tissue as also exclusive to the CD161++ subset, and demonstrated relative enrichment of CD161++CD8αα T cells in the liver compared with peripheral blood in patients with chronic hepatitis C (HCV; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Depletion of the CD161++CD8α+ subset from peripheral blood in chronic HCV has been previously described.2
We next analyzed to what extent CD161++ CD8α+ T cells express the MAIT TCR Vα7.2. We found the CD161++CD8α+ subset in healthy adults to have predominant but, interestingly, not exclusive usage of the TCR Vα 7.2 chain (mean of 87% usage; Figure 1C). We found, using a panel of Vβ antibodies, that the CD161++CD8+ population consistently used the TCR Vβ chains 2 and 13.2 (17.8% and 9.4%, respectively) as had been previously suggested using a PCR approach (supplemental Figure 2A).13 A higher proportion of MAIT-cells (CD161++Vα7.2+) appear to use Vβ 2 (mean 28.3%) than the total CD161++CD8α+ subset; this is accounted for by leaving out the CD161++Vα 7.2− cells in the analysis of MAIT cells. CD161++Vα 7.2− cells have a significantly reduced usage of Vβ 2 and would have been included in the previous analysis of the overall CD161++CD8α+ population (supplemental Figure 2B). The overall mean Vβ 2 usage for both CD161++Vα7.2+ and CD161++Vα7.2− subsets combined is 17.3%, in keeping with the previous data. We also found that CD161++CD8α+Vα7.2+ T cells did not exclusively use Vβ chains 2 and 13.2, with these chains only accounting for 33% of their Vβ usage (supplemental Figure 2B).
CD161++CD8α+ T cells in cord blood are a preprogrammed type-17 subset
A CD161++CD8α+ subset was clearly seen in cord blood (Figure 2A). In adult peripheral blood CD161++CD8α+ T cells have an effector memory phenotype (largely CD45RA−CD45RO+CCR7lowCD62Llow).2 In cord blood they expressed markers of naive cells (CD45RA+CD45RO−); however, these cells were also CCR7low, CD62Llow (Figure 2B), suggesting that they may be preprogrammed in terms of migration patterns, even before change of CD45 isoform.
A naive CD161++CD8α+ T-cell population can be identified in cord blood. (A) Representative FACS plot shown of cord blood showing the CD161++CD8α+ cell population. Data representative of 22 cord samples. (B) CD161++CD8α+ T cells in cord blood are CD45RA+, CD45RO−, CCR7low, and CD62Llow. Representative FACS plots and cumulative data shown. **P < .001, *P < .05 (1-way ANOVA).
A naive CD161++CD8α+ T-cell population can be identified in cord blood. (A) Representative FACS plot shown of cord blood showing the CD161++CD8α+ cell population. Data representative of 22 cord samples. (B) CD161++CD8α+ T cells in cord blood are CD45RA+, CD45RO−, CCR7low, and CD62Llow. Representative FACS plots and cumulative data shown. **P < .001, *P < .05 (1-way ANOVA).
To examine this in more detail we performed microarray analysis of the gene expression profiles of the CD161++CD8α+ subset compared with the CD161+ and CD161− CD8α+ subsets in both cord and adult blood. This demonstrated that in cord blood the CD161++CD8α+ population already bore the strong hallmark of the type-17 CD161++CD8α+ population, as described in adults2 (Figure 3A). We demonstrated a highly significant correlation on gene set enrichment analysis comparing both up-regulated and down-regulated gene sets between cord and adult CD161++ versus CD161− datasets (Figure 3B). Microarray data were confirmed by both FACS analysis for CCR6 and IL-18R and real-time PCR for RORC, ZBTB16, IL-18RAP, and IL-23R (Figure 3C-D).
CD161++CD8α+ T cells in cord blood are a preprogrammed type-17 subset. (A) Comparison of log fold change in gene expression for selected genes between CD161++CD8α+ versus CD161−CD8α+ T cells in adult and cord blood. Data obtained from 3 cord samples and 3 adult healthy donors. (B) Gene set enrichment summary plots comparing gene expression on microarray analysis between CD161++CD8α+ versus CD161−CD8α+ subsets from adult and cord blood. Left panel shows the plot for up-regulated genes, right hand panel shows plot for down-regulated genes. Normalized enrichment score (NES), up-regulated genes, = 4.21, P < .00001. NES, down regulated genes, = -3.25, P < .0001. (C) Representative FACS plots and cumulative date showing CD161++ CD8α+ cells in cord blood express high levels of IL-18R and CCR6. **P < .001 (1-way ANOVA). (D) Relative fold change in gene expression for RORC, ZBTB16, IL-18RAP, and IL-23R in CD161++CD8α+ compared with the CD161+ and CD161−CD8α+ subsets in cord blood by real time PCR analysis.
CD161++CD8α+ T cells in cord blood are a preprogrammed type-17 subset. (A) Comparison of log fold change in gene expression for selected genes between CD161++CD8α+ versus CD161−CD8α+ T cells in adult and cord blood. Data obtained from 3 cord samples and 3 adult healthy donors. (B) Gene set enrichment summary plots comparing gene expression on microarray analysis between CD161++CD8α+ versus CD161−CD8α+ subsets from adult and cord blood. Left panel shows the plot for up-regulated genes, right hand panel shows plot for down-regulated genes. Normalized enrichment score (NES), up-regulated genes, = 4.21, P < .00001. NES, down regulated genes, = -3.25, P < .0001. (C) Representative FACS plots and cumulative date showing CD161++ CD8α+ cells in cord blood express high levels of IL-18R and CCR6. **P < .001 (1-way ANOVA). (D) Relative fold change in gene expression for RORC, ZBTB16, IL-18RAP, and IL-23R in CD161++CD8α+ compared with the CD161+ and CD161−CD8α+ subsets in cord blood by real time PCR analysis.
CD161++ CD8αα cells are tissue homing, type 17 T cells
We have previously shown CD161++CD8α+ T cells to be a unique functional subset with distinct chemokine receptor expression (CCR6, CXCR6, and CCR2) and cytokine secretion profile (IL-17A, IL-22, and IFN-γ).2 We next analyzed in adult blood whether CD161++CD8αα T cells shared these characteristics with CD161++CD8αβ T cells (ie, largely CD8αα+ vs CD8αβ+ MAIT cells).
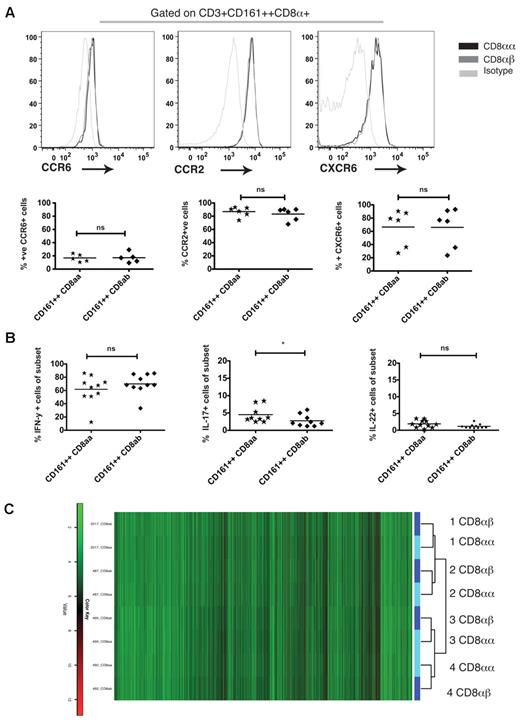
First, we analyzed the phenotype of the CD8αα cells, focusing on chemokine receptors. We demonstrated that in adult blood CD161++CD8αα T cells shared the chemokine-receptor expression profile of CD161++CD8αβ cells, with equivalent levels of CCR6, CCR2, and CXCR6 demonstrated in both subsets (Figure 4A).
Distinct phenotypic and functional features of the CD161++CD8α+ population are shared between the CD161++ CD8αα+ and CD8αβ subsets. (A) Representative histograms showing CD161++ CD8αα+ and CD8αβ cells to express CCR6, CCR2, and CXCR6. Cumulative data shown. ns = non significant (Mann-Whitney test). (B) Cumulative data showing expression of IFN-γ, IL-17 and IL-22 by CD161++ CD8αα and CD8αβ cells. *P = .0467, ns = non significant (Mann- Whitney). (C) Heat-map showing up (red) and down (green) regulated gene expression of CD161++CD8αα (light blue) compared with CD8αβ (dark blue) cells on microarray.
Distinct phenotypic and functional features of the CD161++CD8α+ population are shared between the CD161++ CD8αα+ and CD8αβ subsets. (A) Representative histograms showing CD161++ CD8αα+ and CD8αβ cells to express CCR6, CCR2, and CXCR6. Cumulative data shown. ns = non significant (Mann-Whitney test). (B) Cumulative data showing expression of IFN-γ, IL-17 and IL-22 by CD161++ CD8αα and CD8αβ cells. *P = .0467, ns = non significant (Mann- Whitney). (C) Heat-map showing up (red) and down (green) regulated gene expression of CD161++CD8αα (light blue) compared with CD8αβ (dark blue) cells on microarray.
The CD161++CD8αα subset also shared functional characteristics with the CD8αβ subset defined by cytokine secretion after stimulation with PMA/ionomycin, secreting IL-17A, IL-22, and IFNγ. CD161++CD8αα T cells secreted slightly more IL-17A than CD161++CD8αβ T cells (P = .0467), with no difference found in levels of IFN-γ or IL-22 secretion (Figure 4B).
To assess the differences at a global transcriptional level, we performed a microarray of sorted CD161++CD8αα and CD161++CD8αβ cells. We observed very few differences in gene expression profiles of these 2 cell populations, with none of the up or down-regulated genes achieving a significant adjusted P value (Figure 4C). Clustering of data to individuals rather than the cell subsets further supported the idea that these subsets are very closely related to one another phenotypically and functionally (data not shown).
The CD161++CD8αα subset is derived from the CD161++CD8αβ subset
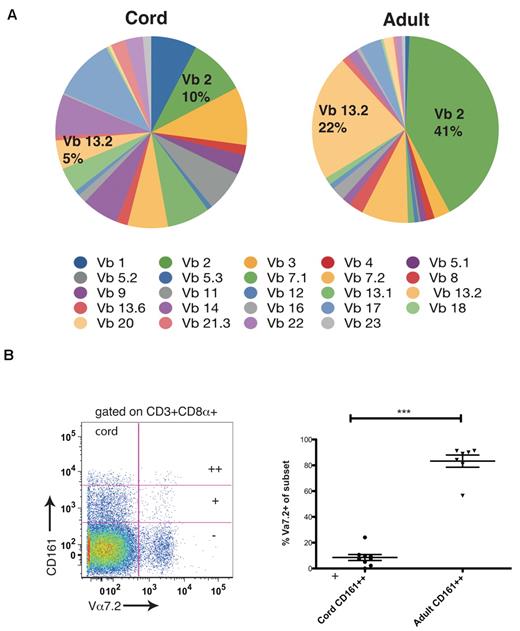
In adult peripheral blood, the CD161++CD8α+ subset had a narrow TCR usage (Figure 1D, supplemental Figure 2), with predominant usage of the MAIT Vα 7.2 chain and Vβ chains 2 and 13.2. We investigated this in cord blood using identical methods. By comparing proportional usage of TCR Vβ chains in cord and adult blood we found that such polarity of usage was not seen in the CD161++ CD8α+ population of cord blood, which consistently showed broad usage of TCR Vβ chains (Figure 5A). In addition, a very small proportion of cells used the Vα 7.2 chain compared with adult blood (mean of 8% vs 83%; Figure 5B).
CD161++CD8α+ T cells in cord blood have broad T-cell receptor usage compared with adults. (A) Comparison of proportional TCR Vβ usage by CD161++CD8α+ T cells between cord and adult blood using TCR Vβ antibody panel. Data obtained from 8 cord blood samples and 10 adult healthy donors. (B) Representative FACS plot of Vα7.2 usage by CD161++/+/− subsets in cord blood (C) Comparison of TCR Vα 7.2 usage between the CD161++CD8α+ subsets in cord and adult blood. ***P = .002 (Mann-Whitney).
CD161++CD8α+ T cells in cord blood have broad T-cell receptor usage compared with adults. (A) Comparison of proportional TCR Vβ usage by CD161++CD8α+ T cells between cord and adult blood using TCR Vβ antibody panel. Data obtained from 8 cord blood samples and 10 adult healthy donors. (B) Representative FACS plot of Vα7.2 usage by CD161++/+/− subsets in cord blood (C) Comparison of TCR Vα 7.2 usage between the CD161++CD8α+ subsets in cord and adult blood. ***P = .002 (Mann-Whitney).
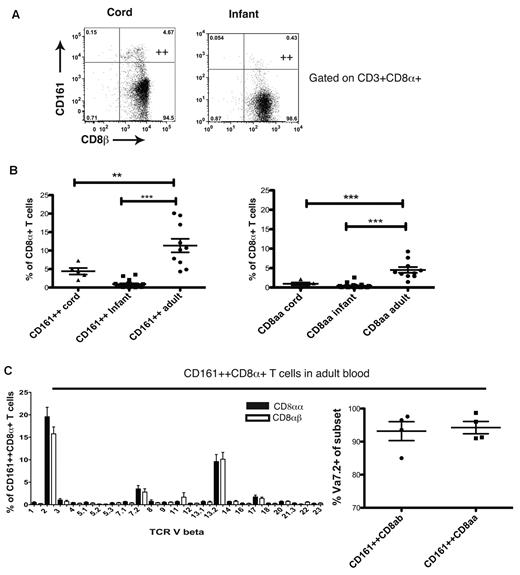
CD161++CD8αα cells were not detectable in cord or infant peripheral blood samples (Figure 6A). We observed a marked expansion of the CD161++CD8+ T-cell subset and the emergence of the CD161++CD8αα subset in adulthood (Figure 6B) and a positive correlation is seen between age and frequency of MAIT/CD161++CD8α+ T cells (r = 0.43, P = .0002, data not shown). To address the clonal relatedness of the populations, we analyzed the Vβ usage of the CD161++CD8αα and CD161++ CD8αβ subsets using flow cytometry. The CD8αα subset shared very closely the narrow MAIT TCR usage of the CD161++CD8αβ subset (Figure 6C). Taken together, these data suggest a common origin of the CD161++CD8αβ and CD161++CD8αα subsets.
The CD161++ CD8αα population emerges on expansion of the CD161++CD8αβ subset with age. (A) CD161++ CD8αα cells are not found in cord or infant blood. (B) Comparison of CD161++CD8α+ and CD161++CD8αα populations in cord, infant, and adult blood. **P < .001, ***P < .0001 (1-way ANOVA). (C) Comparison of TCR Vβ and Vα usage between CD161++ CD8αβ and CD161++CD8αα subsets in adult blood.
The CD161++ CD8αα population emerges on expansion of the CD161++CD8αβ subset with age. (A) CD161++ CD8αα cells are not found in cord or infant blood. (B) Comparison of CD161++CD8α+ and CD161++CD8αα populations in cord, infant, and adult blood. **P < .001, ***P < .0001 (1-way ANOVA). (C) Comparison of TCR Vβ and Vα usage between CD161++ CD8αβ and CD161++CD8αα subsets in adult blood.
To further investigate the origin of the CD161++ CD8αα cells, we isolated CD8αβ+ T cells and cultured them under a variety of conditions (R10 media alone, IL-2 and IL-15, with and without anti-CD3/CD28 beads) as previous reports from mouse studies have identified IL-15 to be of importance in induction/ maturation of CD8αα cells.28,29 No significant variation in the proportions of CD161++, CD161+, and CD161− cell populations was observed after culture (Figure 7A). We found even without the addition of exogenous cytokines, by day 1 a CD8β low population is observed in all CD161 subsets (++, +, and −), which persists out to day 7 of culture (Figure 7B). The emergence of a CD8β- population, corresponding to CD8αα cells, was observed exclusively in the CD161++ population and remains stable beyond day 1 of culture to day 7 (Figure 7C). Having used a CD8β monoclonal antibody to perform positive selection in these experiments, the down-regulation of CD8β could be because of internalization of the CD8αβ/antibody complex. However, because the reduction seen is specific to the CD161++ population and additionally we see no corresponding change in CD8α expression in any CD161 subset, we think this is unlikely to account for our findings (Figure 7D). To ensure that such a population was not the result of proliferation of a residual CD8αα population after sorting, using Ki-67 we showed that the CD161++ cell population had not proliferated significantly over this period, even when cocultured with anti-CD3/CD28 beads (Figure 7E). This is consistent with previous reports that this subset proliferates poorly in culture.3,30,31
CD161++ CD8αα T cells can be derived from CD161++CD8αβ cells in culture. (A) CD161++, CD161+ and CD161− populations of the CD8 enriched/CD8β+ selected PBMCs remain stable over the 7 days of culture in R10 media alone. (B) Representative FACS plots demonstrating change in CD8β expression of CD161++, CD161+ and CD161− populations of the CD8 enriched/CD8β+ selected PBMCs in media alone over 7 days of culture. CD8αα population seen only in CD161++ population. (C) Representative histograms showing change in CD8β expression of the CD161++CD8α+ population of the CD8 enriched/CD8β+ selected PBMCs over 7 days of culture (top panel). Cumulative data shown (bottom panel), *P < .05, **P < .001, and ***P < .0001 (1-way ANOVA). Data representative of 4 experiments. (D) Cumulative data showing no significant change in the CD8α+ T-cell population of cultures over course of experiment (1-way ANOVA). (E) Ki-67 proliferation data for sorted CD8β+ cells left in culture media alone or with CD3/CD28 beads for 48 hours. Baseline Ki-67 staining (gray), after culture Ki-67 staining (black). Data representative of 2 experiments.
CD161++ CD8αα T cells can be derived from CD161++CD8αβ cells in culture. (A) CD161++, CD161+ and CD161− populations of the CD8 enriched/CD8β+ selected PBMCs remain stable over the 7 days of culture in R10 media alone. (B) Representative FACS plots demonstrating change in CD8β expression of CD161++, CD161+ and CD161− populations of the CD8 enriched/CD8β+ selected PBMCs in media alone over 7 days of culture. CD8αα population seen only in CD161++ population. (C) Representative histograms showing change in CD8β expression of the CD161++CD8α+ population of the CD8 enriched/CD8β+ selected PBMCs over 7 days of culture (top panel). Cumulative data shown (bottom panel), *P < .05, **P < .001, and ***P < .0001 (1-way ANOVA). Data representative of 4 experiments. (D) Cumulative data showing no significant change in the CD8α+ T-cell population of cultures over course of experiment (1-way ANOVA). (E) Ki-67 proliferation data for sorted CD8β+ cells left in culture media alone or with CD3/CD28 beads for 48 hours. Baseline Ki-67 staining (gray), after culture Ki-67 staining (black). Data representative of 2 experiments.
Discussion
CD161++CD8α+ T cells are emerging as an important lineage of human CD8+ T cells. Here, we have clarified the relationship of the CD161++CD8α+ T-cell subset to MAIT cells. CD161++CD8α+ T cells (in adults) in addition to being defined by high expression of the C-type lectin CD161 and a pattern of phenotypic markers shared with Th17 cells, predominantly share the invariant TCR usage (Vα 7.2 and Vβ 2/13.2) of MAIT cells.
Human MAIT cells have so far been described to be CD8αα, CD8αβ, or double-negative. By the use of an extended antibody panel, we have been able to demonstrate that CD161++Vα7.2+ cells express CD8αα or CD8αβ, with other coreceptor combinations making up less than 10% of this population, and the CD8αα cell population in humans to be exclusive to this subset. The CD161++CD8α+ T cell pool therefore largely encompasses the MAIT-cell population. The phenotype and function of the remaining 10% of CD161++Vα7.2+ cells (predominantly DN cells) warrants further study. Given their high levels of CD161 expression, they may share phenotypic and functional features of the other populations that are CD161++, with coreceptor expression being a secondary and possibly nonfixed feature of these cells.
CD161++CD8α+ T cells in cord blood appear already a distinct subset in their expression of CCR6, IL-18R, IL-23R, RORγt, and ZBTB16. This suggests that they are a pre committed population, rather than cells that differentiate later from conventional T cells, a finding similar to that in CD4+ T cells.5 Gene expression and phenotyping data from both cord and adults indicates that their genetic signature is strong at both stages, despite there being minimal MAIT Vα7.2+ cells in cord blood. MAIT cells appear to expand from this pool of naive CD161++CD8α+ T cells, suggesting that it is the phenotype/transcriptional signature that defines the subset primarily, and not a specific TCR. We do not know at what stage in development this occurs; that is, pre- or intra-thymically. However, given the identification of IL-18R as a highly up-regulated gene shared between hematopoietic stem cells and CD161++CD8α+ T cells, that also share ABCB1-dependent multidrug efflux ability,30 it could be hypothesized that this differentiation occurs early. The specificity of the diverse pool of CD161++ but Vα7.2− cells found in the cord blood is also of future interest; these could include MR1-restricted but Vα7.2− populations, and other populations restricted by non classic ligands, as well as conventionally class I restricted CD8+ T cells.
The differentiation pathway of MAIT cells has been previously explored in both mice and humans.1 Unlike NK T cells, MAIT cells do not exit the thymus a mature, ready selected and expanded population.32 Small numbers of CD161++Vα7.2+ T cells have been identified in the human thymus and cord blood, and are described to be naive (CD45RA+CD45RO−). The requirement for gut flora and peripheral MR1 expression for development of the homologous MAIT-cells in murine models27 supports the idea that this narrowing of TCR usage with age is because of antigen-driven peripheral expansion. Our data, although encompassing an extended definition of this subset to all CD161++CD8α+ T cells, is consistent with this model. In cord blood this subset has broad TCR usage and chemokine receptor expression (CCR6+, CCR7low, CD62Llow), which would suggest preferential migration to tissue and not lymph nodes as naive cells. Peripheral encounters with antigen/MR1 probably drives the expansion seen with age of cells within the CD161++CD8α+ subset expressing the MAIT TCR. Future investigations correlating these populations more precisely with increasing age of study subjects are warranted to support this hypothesis.
NK T cells express high levels of the transcription factor ZBTB16 compared with other conventional T cells and intrinsic to their ready innate/effector function. From our microarray data, ZBTB16 was found to be one of the most up-regulated genes in both cord and adult CD161++CD8α+ T cells.2 Although previously reported in MAIT cells from adult donors, expression at this earlier stage of development has not been described,33 and probably plays an important role in driving the innate-like functions of this subset. How this expression is initiated is not yet clear, but of direct relevance it has been shown that some human CD4+ T cells within the thymus may acquire ZBTB16 expression and also demonstrate CD161 expression with diverse TCR usage as a naive postthymic population.34
Although we did not exclude NK T cells for the cell sorts performed, we feel confident that the up-regulation of ZBTB16 in the cord CD161++CD8α+ T-cell population is not because of contamination by CD161+iNK T cells. NK T cells make up a very small fraction of CD3+ cells in cord blood (0.05%), are 89.7% CD4+,35 and < 1% of the CD161++CD8α+ cord population used the Vβ11 chain (Figure 5). By sorting on a CD3+CD8α+CD161++ gate the chance of contamination by NK T cells, and these contributing substantially to the expression profile obtained here is very small.
CD8αα cells, like their CD161++CD8αβ counterparts, are differentiated down a type-17 pathway, with similar effector function (IFNγ, IL-17, IL-22) and chemokine receptor expression (CCR2, CCR6 and CXCR6). The overall function of the cells therefore appears to be potentially distinct from the regulatory one suggested for CD8αα T cells found in murine models,14,15 although their in vivo role in man remains to be fully defined. We show human CD8αα cells to be present in the liver and small bowel, and based on previous data,2 we predict infiltrates of CD161++CD8αα cells will also be found in other tissues (ie, tonsil and inflammatory arthritis). Redistribution to the liver as seen in chronic HCV suggests an active role in inflammation or immunity. Overall, their significance in chronic hepatitis is not yet known although previously we found a robust inverse correlation between fibrosis score and the frequency of CD8+ T cells cosecreting IL-17 and IFNγ.2 A specific differential role for CD8αα cells, probably related to the function of the CD8αα molecule itself, requires further study.
The fact that little difference is observed between the phenotype, function, gene expression on microarray or TCR Vα, and Vβ usage between adult CD161++/MAIT CD8αβ+ and CD161++/MAIT CD8αα+ cells suggests that the 2 populations are very closely linked. We propose that the latter population emerges at a later stage from within the same cellular pool as CD161++CD8αβ+ cells, and CD8β expression is regulated within it. First, in infant PBMCs and cord blood, broad TCR-usage of the CD161++ subset is observed—no different to other, for example CD161− populations. Second, CD161++CD8αα cells are not present in cord or infant blood, but are detectable in adulthood, whereas precursor Tc17 lineage populations are present at both stages. Third, we show that CD161++ CD8αα cells can be derived from CD161++ CD8αβ cells in culture. This is seen in the absence of specific stimulation or proliferation after just 24 hours and would suggest that the transition between CD161++CD8αα and CD8αβ cell populations is a rapid and simple process. These data are in contrast with murine studies suggesting that CD8αα cells are a distinct lineage, positively selected in the thymus, which migrate preferentially to the gut as CD4−CD8− T cells, and re-express CD8αα on exposure to IL-15. However, they are consistent with murine data that suggest CD8αα can be co-expressed on conventionally selected CD4, CD8αβ and DN T cells.16,28,29,36,37 Culture experiments here used CD8 enriched PBMCs and so we are unable to comment on the possibility of induction of CD8αα on CD4 and DN T cells in humans under similar experimental conditions. Given the existing mouse data, it will be important to look for these among gut-derived lymphocytes in man.
In keeping with the idea that CD8αα acts as a corepressor, rather than a coreceptor like CD8αβ, a regulatory role for CD8αα cells in mice has been proposed. Murine CD8αα cells have been shown to protect against experimental autoimmune encephalitis15 and colitis38 in adoptive transfer models. In fact, mice that lack the CD8αα ligand TL, which is constitutively expressed in the murine gut, develop a more severe phenotype of colitis in experimental models.39 In humans CD8αα is likely to play an analogous role, potentially imposing an important threshold to activation of these innate-like T cells, dampening activation on encounter with MR1 expressing cells. They may also play a role in determining the overall functional phenotype of the cells (eg, predominant IL-17 vs IL-22 production) depending of the type of activation or homeostatic signals received.
In conclusion, we have defined the origins and relationships of human CD161++ CD8+, MAIT TCR+ T cells, and CD8αα T cells. We propose a model whereby there is primary expression of CD161 and its associated phenotype on a precommitted naive T-cell population with diverse TCRs. This is followed by postnatal expansion of MAIT TCR+ populations within this pool after encounter with antigen, and ready transition to a CD8αα status, including at tissue sites. Once expanded, the potent cytokine secretary activity and homing pattern of the CD8+ T-cell populations studied here suggests a central role in host defense and tissue inflammation in human health and disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. Worth (Jenner Institute, University of Oxford) for technical expertise with cell sorting, and N. Haining (Dana-Farber Cancer Institute, Harvard Medical School) for advice and support with the microarray experiments.
This research was supported by the Medical Research Council, the Wellcome Trust (WT091663MA), the NIHR Biomedical Research Program (Oxford), the James Martin School for the 21st Century (Oxford), and the NIAID U19 Bio-defense Program (NIH NIAID 1U19AI082630-01).
National Institutes of Health
Authorship
Contribution: L.J.W. and P.K. wrote the paper and designed the experiments; L.J.W., Y.-H.K., M.O.S., and H.T. performed the experiments; L.J.W. analyzed the data; N.R., V.F., A.L., and N.S. provided technical support; Y.O., A.G., T.J.S., W.A.H., G.M.L., D.H.A., O.L., and F.P. provided critical material; and E.B., D.H.A., T.J.S., and O.L. read the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul Klenerman, Peter Medawar Building for Pathogen Research, University of Oxford, Oxford, OX1 3SY, United Kingdom; email: paul.klenerman@ndm.ox.ac.uk




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal