Abstract
Tissue-type plasminogen activator (t-PA) can modulate permeability of the neurovascular unit and exacerbate injury in ischemic stroke. We examined the effects of t-PA using in vitro models of the blood-brain barrier. t-PA caused a concentration-dependent increase in permeability. This effect was dependent on plasmin formation and potentiated in the presence of plasminogen. An inactive t-PA variant inhibited the t-PA–mediated increase in permeability, whereas blockade of low-density lipoprotein receptors or exposed lysine residues resulted in similar inhibition, implying a role for both a t-PA receptor, most likely a low-density lipoprotein receptor, and a plasminogen receptor. This effect was selective to t-PA and its close derivative tenecteplase. The truncated t-PA variant reteplase had a minor effect on permeability, whereas urokinase and desmoteplase were ineffective. t-PA also induced marked shape changes in both brain endothelial cells and astrocytes. Changes in astrocyte morphology coincided with increased F-actin staining intensity, larger focal adhesion size, and elevated levels of phosphorylated myosin. Inhibition of Rho kinase blocked these changes and reduced t-PA/plasminogen–mediated increase in permeability. Hence plasmin, generated on the cell surface selectively by t-PA, modulates the astrocytic cytoskeleton, leading to an increase in blood-brain barrier permeability. Blockade of the Rho/Rho kinase pathway may have beneficial consequences during thrombolytic therapy.
Introduction
The plasminogen-activating enzyme system is widely appreciated for its role in fibrinolysis and thrombolysis1 and in other areas related to remodeling of the extracellular matrix.2 However, this enzyme system also has a major impact in the CNS under both physiologic and pathologic circumstances.3,4 The literature has amassed much data implicating tissue-type plasminogen activator (t-PA), plasmin, or both in cognitive function, memory, and anxiety3 and in addictive behavior.5,6 Under pathologic conditions, including ischemic stroke and traumatic brain injury, t-PA has been shown to facilitate neurotoxic events via potentiation of glutamate receptor signaling.3,7 Although direct neurotoxicity of t-PA has been demonstrated by some studies7,8 but not others,9 t-PA also has been shown to modulate permeability of the neurovascular unit.10-12 Hence, under pathologic conditions, the deleterious consequences of t-PA may be because of direct neurotoxicity, increased permeability of the blood-brain barrier (BBB), or a combination.
Much effort has been devoted to understanding the mechanism by which t-PA modulates BBB permeability. t-PA delivered intravenously has been shown to cross brain endothelial cells via transcytosis without compromising BBB integrity,10,13 whereas others have shown that t-PA can enter the parenchyma during pathologic conditions where it further enhances BBB breakdown.12 In some paradigms of BBB breakdown,12,14 the damaging effect of t-PA has been reported as plasmin-dependent15,16 or -independent11,12,17 contingent on the cellular context and time-frame of the experiments performed. Hence, t-PA–mediated modulation of BBB permeability may invoke more than 1 pathway involving plasminogen, other substrates, or a combination.
Here, we have established mouse and human primary cell models of the BBB and investigated the ability of t-PA to increase permeability in vitro. We also explored the effect of a variety of other plasminogen activators on BBB permeability, the role of plasmin, and the signaling pathways essential for this effect. Our results demonstrate that the ability of t-PA to increase permeability is unique to t-PA and its close variant tenecteplase. This property of t-PA was completely dependent on plasmin generation, required the presence of cell surface receptors for both t-PA and plasminogen, and involved marked shape changes in brain endothelial cells and astrocytes. Furthermore, t-PA/plasmin-induced modulation of the actin cytoskeleton and downstream signaling in astrocytes occurs via the Rho/Rho kinase (ROCK) pathway.
Methods
Human plasminogen (Plgn) and (−)-blebbistatin were from Calbiochem. TNK-tPA (tenecteplase; metalyse) and human t-PA (rt-PA; actilyse) were from Boehringer Ingelheim. Urokinase (u-PA) was from Medac. Both u-PA and rt-PA were dialyzed to remove the original vehicle components.18 DSPAα1 (desmoteplase), catalytically inactive t-PA (“ct-PA”) and reteplase were supplied by PAION Deutschland. Reteplase was dialyzed against phosphate-buffered saline (PBS) to remove the original vehicle components. Recombinant human α2–anti-plasmin was provided by Bernadine Lu (Monash University). HA1077 and Y27632 were from Cayman Chemicals. The PI3 kinase inhibitor LY294002 was provided by Dr Simone Schoenwaelder (Monash University). U0126, fluorescein isothiocyanate (FITC)–conjugated BSA, trypsin, trypsin inhibitor, poly-d-lysine, and aprotinin were from Sigma-Aldrich. Tranexamic acid (TXA) was from Pharmion.
Cell culture
SVG human fetal astroglial cells19 were cultured in minimum essential medium/Earle balanced salt solution (HyClone Laboratories) with 20% FCS, 2mM l-glutamine, and 50 U/mL penicillin/streptomycin. Primary human brain microvascular endothelial cells (hBECs; ACBRI 376) were from Cell Systems (CSC) and were cultured in gelatin-coated flasks in CSC complete medium (based on DMEM/F-12 with 15mM HEPES) supplemented with 10% CSC JetFuel. For experimentation hBECs were cultured in CSC serum-free medium (DMEM/F-12 with 15mM HEPES, human serum albumin, and CSC RocketFuel). SVG cells and hBECs were maintained for up to 20 and 15 passages, respectively.
Preparation of mouse glial cells
Animal procedures were approved by the Alfred Medical Research and Education Precinct Animal Ethics Committee or the University of Melbourne Animal Ethics Committee. Glial cultures were prepared from a whole mouse brain,20,21 with modifications. In brief, 1-day-old C57Bl/6 mice were killed by decapitation, their brains removed and placed in ice-cold Hanks-balanced salt solution (HBSS) with 10mM HEPES-NaOH, pH 7.3, 1mM sodium pyruvate, 1.15mM MgSO4, and 3 g/L bovine serum albumin (BSA; HBSS+). Brains were stripped from meninges, dissected into small pieces, and then digested (10 minutes) with HBSS+ containing 0.2 g/L trypsin and 80 U/mL DNAse-I. Digestion was stopped with HBSS+ containing 2.63mM MgSO4, 0.52 g/L trypsin inhibitor, and 80 U/mL DNAse-I (HBSS+TI). The tissue was centrifuged, resuspended in HBSS+TI (3 mL), and triturated through a fire-polished Pasteur pipette. After centrifugation at 610g for 7 minutes, cells were resuspended in DMEM/F-12 (HyClone Laboratories) with 10% FCS, 2mM l-glutamine, and 50 U/mL penicillin/streptomycin and then seeded in flasks (100 000 cells/cm2) and maintained under standard conditions. Medium was changed after 24 hours (days in vitro 1) and every 3 days for 2 weeks. These cultures contained primarily astrocytes (identified by immunostaining for glial fibrillary acidic protein [GFAP]) and ∼ 15% microglia (detected by CD11b immunostaining). Cultures were used within the first 3 passages because astrocytes were shown to maintain GFAP expression during this time frame.
Preparation of mouse endothelial cells and assembly of the mouse in vitro BBB model
See supplemental Figure 4 for preparation of mouse endothelial cells and assembly of the mouse in vitro BBB model (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Coculture of SVG and hBECs: assembly of the human in vitro BBB model
The luminal surface of Transwell polyester inserts (6.5 mm in diameter; pore size 3 μm; Corning Life Sciences) was coated with bovine collagen I (20 μg/cm2; Trevigen). SVG cells (4 × 104) were seeded on the underside (abluminal) surface (inserts were turned upside down) and left to adhere for 4 hours at 37°C. Inserts were returned to the normal orientation into 800 μL of medium/well (24-well plates). hBECs (2 × 104) in 200 μL were seeded onto the collagen-coated luminal surface of the membrane. The SVG/hBEC-seeded inserts were cocultured for 3 days in hBEC medium. A schematic representation of this is shown in supplemental Figure 1. Supplemental Figure 1 also presents scanning electron micrographs of the luminal surface showing that abluminally cultured astrocytes can extend projections through the pores enabling contact with cells on the luminal side. The properties of this system were characterized by FITC-BSA permeability assays (see “BBB permeability assessment” and supplemental Figure 1). Astrocytes were found to modulate permeability not only by providing a physical barrier (supplemental Figure 1C) but also by providing essential support to hBECs that resulted in tightening of the endothelial monolayer (supplemental Figure 1D-E).
Stimulation of the in vitro BBB
The abluminal and luminal media were replaced with 0.6 and 0.1 mL of serum-free media, respectively. The luminal medium was aspirated again and replaced with 0.1 mL of serum-free media with selected proteases. Aprotinin, α2–anti-plasmin, ct-PA, HA1077, RAP, and TXA were added simultaneously with the proteases. Each experimental group was tested in triplicate, and at least 3 independent experiments undertaken.
BBB permeability assessment
At the end of the stimulation period, 10 μL of 3.85 mg/mL FITC-conjugated BSA was added to the luminal chamber (final concentration, 0.35 mg/mL) of the human in vitro BBB model. For the mouse in vitro BBB model, inserts were transferred into new wells containing 0.6 mL of fresh serum-free medium before addition of FITC-BSA. After 1 hour, the abluminal media was sampled (50 μL), and fluorescence was measured with a VICTOR-II microplate reader (PerkinElmer). Changes in permeability were calculated relative to coated inserts without cells, which served as a reference for maximal permeability. The following formula was used: permeability (% of max) = (FITC read of experimental insert − average FITC read of the vehicle group)/(FITC read of the blank insert − average FITC read of the vehicle group) × 100.
Stimulation and imaging of astrocytes and hBECs
SVG cells, mouse astrocytes, or hBECs were washed with serum-free medium. Proteases were added in fresh serum-free medium at the specified concentrations. α2–anti-plasmin, aprotinin, and TXA were added simultaneously with the proteases. HA1077, Y27632 and blebbistatin were added 15 to 30 minutes before protease addition. Cells were fixed after 12 to 24 hours with 4% paraformaldehyde. Phase-contrast micrographs (see Figures 4, 5C, and 6B and supplemental Figures 2C and 3) were obtained using a DM-IRB microscope (Leica) equipped with an ORCA-AG digital camera (Hamamatsu) with objectives NPLAN ×10 magnification air, 0.22 numeric aperture (NA); NPLAN ×40 magnification air, 0.55 NA. Acquisition software for all images was MetaMorph 7.5 from MDS Analytical Technologies (except for Figure 4G, where DVTool was used). Image processing was performed with ImageJ Version 1.42q (National Institutes of Health).
Immunocytochemistry
Cells cultured on μ-slides (8-well; Ibidi) were fixed for 20 minutes with ice-cold 4% paraformaldehyde, washed with Tris-buffered saline ([TBS]: 154mM NaCl, 50mM Tris-HCl, pH 7.6) and blocked for 1 hour at 4°C with TBS containing 10% heat-inactivated horse serum (HS) and 0.1% Triton X-100 (TX-100). Rabbit anti–cow GFAP (Dako), mouse anti-vinculin (Sigma-Aldrich), or rabbit anti-phosphomyosin light chain (pMLC; Cell Signaling Technology), were applied overnight at 4°C, diluted 1:100 in TBS + 4% HS + 0.1% TX-100. Cells were washed 3 times with TBS and then incubated for 3 hours at 4°C with the appropriate fluorescent secondary antibody (AlexaFluor 568–conjugated anti–rabbit IgG or AlexaFlour 488–conjugated anti–mouse IgG antibodies; Invitrogen) diluted 1:1000 in TBS + 4% HS + 0.1% TX-100. After 3 additional TBS washes, Hoechst (5 μg/mL) was added for 0.5 hours at 4°C in TBS + 0.1% TX-100 as a nuclear counterstain. For F-actin staining, cells were incubated for 2 hours with 13.2nM AlexaFluor 568–conjugated phalloidin (Invitrogen) in TBS + 4% HS + 0.1% TX-100 and then washed 3 times with TBS. Micrographs (see Figures 5 and 6A and supplemental Figure 4E) were taken on a Cell Observer system microscope (Carl Zeiss Axio Observer Z1, with objectives EC Plan-Neofluar ×10 magnification air, 0.3 NA; EC Plan-Neofluar ×40 magnification air, 0.75 NA; excitation and emission filters for 4′,6-diamidino-2-phenylindole, fluorescein, and Texas Red fluorophores; camera AxioCamMR3; and acquisition software AxioVision 4.8). Image processing was performed with ImageJ Version 1.42q.
Mouse astrocyte lysate preparation for signaling analysis
Mouse glial cultures (passages 0-2) were seeded in poly-d-lysine–coated 12-well plates at 150 000 cells/well. Subconfluent wells (days in vitro 2-7) were washed and equilibrated at 37°C for 4 hours in fresh serum-free medium. Proteases were directly added to the medium for 2 hours. HA1077, Y27632, and C3 exoenzyme were added 15 to 30 minutes before protease addition. Whole cell lysates were prepared using ice-cold radioimmunoprecipitation assay buffer (50mM Tris-HCl, pH 7.4; 150mM NaCl, 1mM EDTA, 1% TX-100, 1% Na-deoxycholate, 0.1% SDS, and protease and phosphatase inhibitor cocktails [Roche] and 10μM aprotinin) and frozen in liquid nitrogen.
Western blotting
Lysates were applied to 10% to 14% SDS-PAGE gels under reducing conditions. Phosphorylated MLC (pMLC), -p44/42 MAPK (pERK), or -Akt (pAkt) were detected by rabbit anti-pMLC 2 (Ser19), mouse anti-pERK, and rabbit anti-pAkt (Ser473) antibodies, respectively (Cell Signaling Technology). Antibodies against total proteins (ERK and Akt from Cell Signaling Technology and MLC from Santa Cruz Biotechnology (mouse anti-MRCL3/MRLC2/MYL9 [E-4]), glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Millipore), and β-tubulin served as loading controls. Western blotting was performed as described previously.17
Statistical analysis
Bar graphs represent mean ± SEM. Comparison of experimental groups was performed by 1-way ANOVA with Newman-Keuls posthoc correction or by Student t test. Probability values of < .05 are considered significant.
Results
t-PA–mediated increase in BBB permeability is plasmin-dependent and unique to t-PA
t-PA (10nM-1μM) was added to the luminal side of the human BBB model, and permeability was assessed after 24 hours. As shown in Figure 1A, t-PA caused a concentration-dependent increase in transfer of FITC-BSA over a 24-hour period, with a significant increase seen in the presence of 100nM t-PA. t-PA required its protease activity in this process because ct-PA17 had no effect (Figure 1B). This effect was plasmin-dependent because addition of α2–anti-plasmin (Figure 1C) or the plasmin inhibitor aprotinin (data not shown) blocked the ability of t-PA to increase permeability. We then tested whether plasmin alone could substitute for t-PA. Surprisingly, addition of 500nM plasmin to the cultures failed to increase permeability (Figure 1D). The activity of plasmin was confirmed using amidolytic assays (data not shown). Hence, although plasmin formation is essential, additional factor(s) are required for t-PA to modulate permeability.
t-PA–mediated increase in BBB permeability is plasmin-dependent and unique to t-PA and its highly related variant tenecteplase. (A) Increasing concentrations of human t-PA were added to the luminal chamber of the in vitro BBB and the transfer of FITC-BSA to the abluminal chamber assessed after 24 hours (n = 5; ***P < .001, **P < .01 compared with “no cells”). (B) t-PA–mediated increase in permeability requires catalytically active t-PA. t-PA (1μM) and ct-PA (1μM) were added to the BBB as described in panel A, and permeability was assessed after 24 hours. As shown, inactive t-PA does not increase permeability of the BBB (n = 3). (C) t-PA–mediated increase in permeability is plasmin-dependent. t-PA (1μM) was added to the BBB as described in panel A in the absence or presence of α2–anti-plasmin (α2AP; 1μM), and permeability was assessed after 24 hours. α2AP blocked the ability of t-PA to increase permeability (n = 4). (D) Plasmin cannot substitute for t-PA to increase permeability. t-PA (1μM) or human plasmin (500nM) was added to the luminal chamber of the BBB, and permeability was assessed after 24 hours. As shown, plasmin alone fails to promote an increase in permeability (n = 3). (E-H) t-PA (1μM) was added to the luminal chamber of the BBB, and the extent of permeability compared at 24 hours with tenecteplase (TNK, 1μM, n = 4; E), reteplase (1μM), and its vehicle (n = 3; F), urokinase (u-PA, 1μM, n = 6; G), or desmoteplase (DSPA, 1μM, n = 6; H). Only t-PA and its structurally related variant TNK-tPA can augment BBB permeability. In all panels, bars represent mean ± SEM (in B-H, ***P < .001, *P < .05 compared with all other groups).
t-PA–mediated increase in BBB permeability is plasmin-dependent and unique to t-PA and its highly related variant tenecteplase. (A) Increasing concentrations of human t-PA were added to the luminal chamber of the in vitro BBB and the transfer of FITC-BSA to the abluminal chamber assessed after 24 hours (n = 5; ***P < .001, **P < .01 compared with “no cells”). (B) t-PA–mediated increase in permeability requires catalytically active t-PA. t-PA (1μM) and ct-PA (1μM) were added to the BBB as described in panel A, and permeability was assessed after 24 hours. As shown, inactive t-PA does not increase permeability of the BBB (n = 3). (C) t-PA–mediated increase in permeability is plasmin-dependent. t-PA (1μM) was added to the BBB as described in panel A in the absence or presence of α2–anti-plasmin (α2AP; 1μM), and permeability was assessed after 24 hours. α2AP blocked the ability of t-PA to increase permeability (n = 4). (D) Plasmin cannot substitute for t-PA to increase permeability. t-PA (1μM) or human plasmin (500nM) was added to the luminal chamber of the BBB, and permeability was assessed after 24 hours. As shown, plasmin alone fails to promote an increase in permeability (n = 3). (E-H) t-PA (1μM) was added to the luminal chamber of the BBB, and the extent of permeability compared at 24 hours with tenecteplase (TNK, 1μM, n = 4; E), reteplase (1μM), and its vehicle (n = 3; F), urokinase (u-PA, 1μM, n = 6; G), or desmoteplase (DSPA, 1μM, n = 6; H). Only t-PA and its structurally related variant TNK-tPA can augment BBB permeability. In all panels, bars represent mean ± SEM (in B-H, ***P < .001, *P < .05 compared with all other groups).
We next compared the ability of a panel of well-described plasminogen activators (tenecteplase [TNK-tPA],22 reteplase,23 u-PA, and desmoteplase24 ) to alter BBB permeability. Of the plasminogen activators tested, only TNK-tPA retained any significant capacity to increase permeability and was ∼ 60% as effective as t-PA on an equimolar basis (Figure 1E). Reteplase displayed only minor increases in permeability (∼ 15%; Figure 1F), whereas urokinase and desmoteplase were without effect (Figure 1G-H). Hence only t-PA and its close derivative TNK-tPA can modulate this in vitro human model of the BBB.
t-PA–mediated plasmin generation in situ potently modulates human BBB permeability
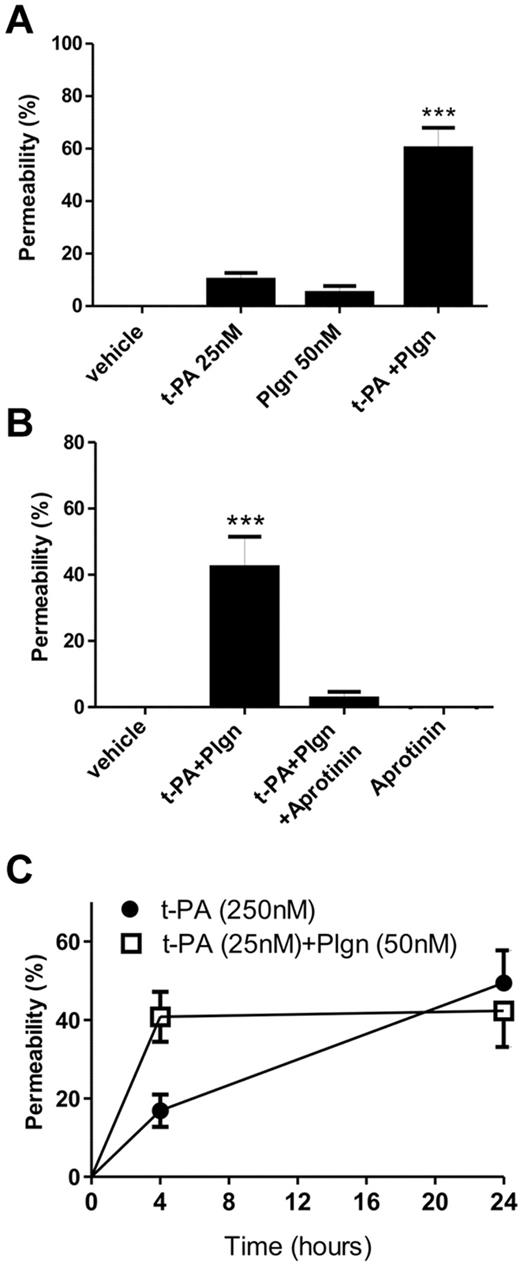
Our finding that plasmin generation was required indicates that trace levels of plasminogen are present (eg, in the culture media). We repeated the permeability experiments with lower concentrations of t-PA (25nM) in the presence of 50nM plasminogen. Although addition of 25nM t-PA or 50nM plasminogen alone failed to cause any increase in permeability, profound increases in permeability were seen when cells were coincubated with t-PA + plasminogen (Figure 2A), comparable with that produced using 250nM t-PA alone (Figure 1A). This effect of t-PA + plasminogen also relied on active plasmin because coaddition of aprotinin blocked this increase in permeability (Figure 2B). Time course studies revealed that the t-PA + plasminogen–mediated effect on permeability occurred faster than seen with t-PA alone, reaching maximum increase at 4 hours (Figure 2C). Hence, t-PA–induced in situ plasmin generation is a more potent and physiologically relevant BBB-modulating event.
The human in vitro BBB is highly sensitive to t-PA–mediated in situ plasmin generation. (A) t-PA (25nM) and plasminogen (Plgn; 50nM) were added to the luminal chamber of the BBB either alone or in combination and permeability assessed after 24 hours (n = 4; ***P < .001 compared with all other groups). (B) Inhibition of t-PA–mediated in situ plasmin generation fully protects the BBB. t-PA (25nM) and Plgn (50nM) were added to the luminal chamber of the BBB with or without aprotinin (2μM), and permeability was assessed 24 hours later (n = 5; ***P < .001 compared with all other groups). (C) Time course analysis of BBB disruption at 4 and 24 hours after addition of t-PA (25nM) and Plgn (50nM; n = 5, open squares) or t-PA (250nM) alone (n = 4, full circles). Compared with 250nM t-PA alone, t-PA + Plgn mediate a faster progression of BBB opening that is maximal already at 4 hours. In all panels, bars/data points represent mean ± SEM.
The human in vitro BBB is highly sensitive to t-PA–mediated in situ plasmin generation. (A) t-PA (25nM) and plasminogen (Plgn; 50nM) were added to the luminal chamber of the BBB either alone or in combination and permeability assessed after 24 hours (n = 4; ***P < .001 compared with all other groups). (B) Inhibition of t-PA–mediated in situ plasmin generation fully protects the BBB. t-PA (25nM) and Plgn (50nM) were added to the luminal chamber of the BBB with or without aprotinin (2μM), and permeability was assessed 24 hours later (n = 5; ***P < .001 compared with all other groups). (C) Time course analysis of BBB disruption at 4 and 24 hours after addition of t-PA (25nM) and Plgn (50nM; n = 5, open squares) or t-PA (250nM) alone (n = 4, full circles). Compared with 250nM t-PA alone, t-PA + Plgn mediate a faster progression of BBB opening that is maximal already at 4 hours. In all panels, bars/data points represent mean ± SEM.
t-PA–mediated opening of the BBB requires a cell surface receptor
We considered the possibility that t-PA may require the binding to a cell surface receptor to allow plasmin to be generated in a spatially optimal manner. We first compared the ability of t-PA to increase permeability in the presence of a 10-fold molar excess of the catalytically inactive t-PA variant ct-PA. ct-PA fully inhibited the ability of active t-PA to increase permeability over 4 hours (Figure 3A), consistent with the notion that t-PA requires a cell surface receptor(s) to promote BBB opening.
t-PA requires a cell surface receptor to modulate BBB permeability. (A) t-PA (250nM) was added to the luminal chamber of the BBB either alone or in combination with 2.5μM of ct-PA, and permeability was assessed after 4 hours. As shown, ct-PA inhibits the ability of active t-PA to increase BBB permeability (n = 4; ***P < .001). (B) t-PA (250nM) or the LDLR blocker RAP (500nM) was added either alone or in combination to the luminal chamber of the BBB, and permeability was assessed after 24 hours. The presence of RAP significantly inhibits t-PA–mediated BBB injury (n = 4-6; **P < .01). (C-D) t-PA (250nM; C) or t-PA (25nM) and Plgn (50nM; D) were added to the luminal chamber of the BBB either alone or in combination with the lysine analog TXA (10mM, added to the luminal and abluminal chambers), and permeability was assessed after 24 hours. As shown, the presence of TXA effectively blocks the ability of t-PA alone or on combination with Plgn to enhance BBB permeability. In panels C-D, n = 3 (***P < .001). In all panels, bars represent mean ± SEM.
t-PA requires a cell surface receptor to modulate BBB permeability. (A) t-PA (250nM) was added to the luminal chamber of the BBB either alone or in combination with 2.5μM of ct-PA, and permeability was assessed after 4 hours. As shown, ct-PA inhibits the ability of active t-PA to increase BBB permeability (n = 4; ***P < .001). (B) t-PA (250nM) or the LDLR blocker RAP (500nM) was added either alone or in combination to the luminal chamber of the BBB, and permeability was assessed after 24 hours. The presence of RAP significantly inhibits t-PA–mediated BBB injury (n = 4-6; **P < .01). (C-D) t-PA (250nM; C) or t-PA (25nM) and Plgn (50nM; D) were added to the luminal chamber of the BBB either alone or in combination with the lysine analog TXA (10mM, added to the luminal and abluminal chambers), and permeability was assessed after 24 hours. As shown, the presence of TXA effectively blocks the ability of t-PA alone or on combination with Plgn to enhance BBB permeability. In panels C-D, n = 3 (***P < .001). In all panels, bars represent mean ± SEM.
t-PA and plasminogen are known to bind to several cell surface binding proteins, including annexin II, histone H2, and PLG-RTK (for review, see Medcalf25 ). This binding requires exposed lysine residues and is inhibited by lysine analogs (TXA).26 Other well-characterized receptors for t-PA are members of the low-density lipoprotein receptor (LDLR) family.27 To explore the contribution of LDLRs and lysine containing t-PA/plasminogen receptors in the modulation of BBB permeability by t-PA, experiments were performed in the presence of the pan-LDLR blocking agent, receptor-associated protein (RAP),28 or with TXA. Inclusion of 500nM RAP with t-PA alone (Figure 3B) or 10mM TXA with either t-PA alone or t-PA + plasminogen (Figure 3C-D) significantly reduced the ability of t-PA to increase permeability. The inability of RAP to fully block the t-PA effect suggests that other t-PA receptors may be playing a role. Collectively, both LDLRs and lysine-dependent receptors are involved in the mechanism by which t-PA opens the BBB.
t-PA alone or in combination with plasminogen alters endothelial cell and astrocyte morphology
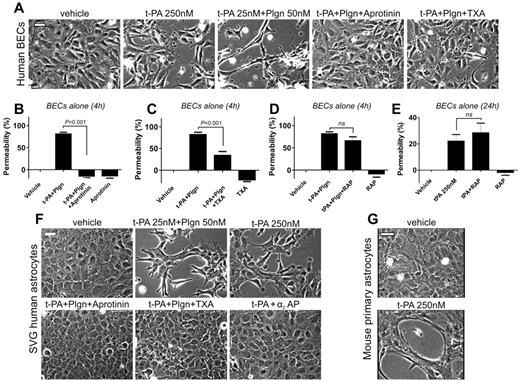
For t-PA/plasminogen to cause an increase in albumin passage across at least 2 cell layers implies that they have the capacity to alter the integrity of the BBB structure. We therefore treated isolated cultures of brain endothelial cells and astrocytes with t-PA or with t-PA + plasminogen and looked for changes in cell morphology. Endothelial morphology was only mildly altered in the presence of t-PA (250nM) but was strongly influenced by treatment with t-PA (25nM) + plasminogen (50nM; Figure 4A). Marked increases in permeability were observed after short (4-hour) stimulation of endothelial cells with t-PA + plasminogen (Figure 4B-D). Both permeability and morphologic changes were blocked by aprotinin (Figure 4A-B) or TXA (Figure 4A,C), confirming a requirement for cell surface–bound plasmin. Treatment with RAP, however, failed to reduce t-PA + plasminogen– (Figure 4D) or t-PA–induced permeability changes (Figure 4E). This suggests that LDLRs may not play the same role in brain endothelial cells and that the LDLR component underlying the ability of t-PA/plasminogen to modulate the human BBB model is restricted to astrocytes. Pronounced morphologic changes also were seen in human SVG and primary mouse astrocytes: t-PA added alone (250nM) or in combination with plasminogen (25nM t-PA + 50nM plasminogen) caused substantial changes in human astrocyte morphology after 16 hours that were blocked by α2–anti-plasmin, aprotinin or TXA (Figure 4F). Addition of t-PA (250nM) to mouse astrocytes also produced similar changes as seen in human astrocytes (Figure 4G) and were inhibited by α2–anti-plasmin or by TXA (data not shown), consistent with the findings in the human BBB model.
t-PA causes marked morphologic and functional changes in both brain endothelial cells (BECs) and astrocytes via plasmin. (A) Representative phase contrast images of human BECs 12 hours after stimulation with t-PA (250nM) alone or t-PA (25nM) + Plgn (50nM) in the absence or presence of aprotinin (2μM) or TXA (10mM). Aprotinin and TXA fully block t-PA/Plgn–mediated changes in endothelial cell morphology, suggesting that cell surface–associated active plasmin is responsible for this effect. Scale bars represent 40 μm. (B-E) Permeability analysis of BECs alone on porous membranes 4 hours after stimulation by luminal addition of t-PA (25nM) and Plgn (50nM) with or without aprotinin (2μM; n = 3-7; B), TXA (10mM added to the luminal and abluminal chambers; n = 5; C), or RAP (0.5μM; n = 6; D). The effect of t-PA (250nM) alone or in combination with RAP (0.5μM) after 24 hours is shown in panel E (n = 4). Hence, endothelial cell permeability is also strongly influenced by cell surface–bound, active plasmin. (F) Phase-contrast images of SVG human astrocytes 16 hours after treatment with vehicle, t-PA (25nM) + Plgn (50nM) with or without aprotinin (2μM) or TXA (10mM) or with t-PA (250nM) alone, or in combination with 250nM α2AP. Scale bars represent 40 μm. (G) Phase-contrast images of primary mouse astrocytes 16 hours after treatment with vehicle or t-PA (250nM) alone. Both human SVG and mouse astrocytes undergo marked morphologic changes after treatment with t-PA/Plgn. Scale bars represent 40 μm.
t-PA causes marked morphologic and functional changes in both brain endothelial cells (BECs) and astrocytes via plasmin. (A) Representative phase contrast images of human BECs 12 hours after stimulation with t-PA (250nM) alone or t-PA (25nM) + Plgn (50nM) in the absence or presence of aprotinin (2μM) or TXA (10mM). Aprotinin and TXA fully block t-PA/Plgn–mediated changes in endothelial cell morphology, suggesting that cell surface–associated active plasmin is responsible for this effect. Scale bars represent 40 μm. (B-E) Permeability analysis of BECs alone on porous membranes 4 hours after stimulation by luminal addition of t-PA (25nM) and Plgn (50nM) with or without aprotinin (2μM; n = 3-7; B), TXA (10mM added to the luminal and abluminal chambers; n = 5; C), or RAP (0.5μM; n = 6; D). The effect of t-PA (250nM) alone or in combination with RAP (0.5μM) after 24 hours is shown in panel E (n = 4). Hence, endothelial cell permeability is also strongly influenced by cell surface–bound, active plasmin. (F) Phase-contrast images of SVG human astrocytes 16 hours after treatment with vehicle, t-PA (25nM) + Plgn (50nM) with or without aprotinin (2μM) or TXA (10mM) or with t-PA (250nM) alone, or in combination with 250nM α2AP. Scale bars represent 40 μm. (G) Phase-contrast images of primary mouse astrocytes 16 hours after treatment with vehicle or t-PA (250nM) alone. Both human SVG and mouse astrocytes undergo marked morphologic changes after treatment with t-PA/Plgn. Scale bars represent 40 μm.
t-PA–mediated morphologic changes in astrocytes are associated with alteration of the actin cytoskeleton and focal adhesion
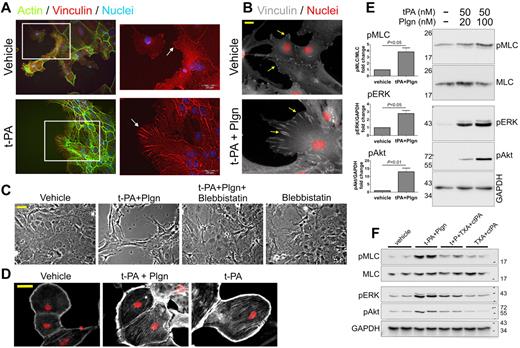
The profound changes in astrocyte morphology by t-PA suggest that t-PA was affecting adhesion, contractility of astrocytes, or both, processes that are usually driven by changes in the actin cytoskeleton. Vinculin is a major constituent of the focal adhesion complex that is involved in anchoring filamentous-actin (F-actin) to the membrane and represents a key element in the transmembrane linkage of the extracellular matrix to the actin cytoskeleton. Indeed, t-PA caused an increase in F-actin staining intensity and in focal adhesion as represented by an increase in vinculin size (Figure 5A-B); focal adhesion length was notably extended in both human (data not shown) and primary mouse astrocytes on treatment with 1μM t-PA (Figure 5A) and with low concentrations of t-PA (50nM) in the presence of 20nM plasminogen (Figure 5B).
Treatment with t-PA/Plgn causes marked changes in the actin cytoskeleton, an increase in focal adhesion size, and generation of contractile forces in primary mouse astrocytes. (A) Representative immunofluorescence images of primary mouse astrocytes showing changes in F-actin and focal adhesion after treatment with t-PA (1μM) or its vehicle. Marked increase in stress fiber formation (left images, green) and vinculin size (right magnified images, red, indicated by arrows) are demonstrated after treatment with t-PA. Nuclei appear in blue. Scale bars represent 20 μm. (B) Representative immunofluorescence images of primary mouse astrocytes demonstrating changes in focal adhesion 24 hours after treatment with t-PA (50nM) + Plgn (20nM). Marked increase in vinculin (white, arrows) dimension is observed. Nuclei appear in red. Scale bars represent 20 μm. (C) Phase-contrast images of primary mouse astrocytes treated with vehicle, t-PA (50nM) + Plgn (20nM), or blebbistatin (a selective myosin ATPase inhibitor; 10μM) either alone or in combination for 24 hours. The full blockade of t-PA/Plgn–mediated morphology changes by blebbistatin indicates that contractile forces are generated in astrocytes by these proteases. Scale bar represents 40 μm. (D) Representative immunofluorescence images of serum-starved primary mouse astrocytes (48 hours) subjected to treatment with vehicle, t-PA (50nM) + Plgn (20nM) or t-PA alone (500nM) for 4.5 hours. Immunocytochemical analysis shows an increase in pMLC staining intensity and distribution (white) in astrocytes after stimulation with t-PA and Plgn or with high levels of t-PA alone. Cell nuclei are counterstained red. Scale bar represents 40 μm. (E) Western blot analysis confirming a concentration-dependent increase in pMLC as well as in pERK and pAkt levels in primary mouse astrocytes after t-PA/Plgn treatment. Astrocytes were serum-starved for 4 hours and then treated for 2 hours with t-PA (50nM) in the presence of either 20 or 100nM Plgn. A representative blot is shown on the right, and quantitation of fold changes in phospho-proteins after treatment with t-PA 50nM + Plgn (100nM) is presented on the left (n = 4-5). (F) Representative Western blot analysis demonstrating a strong attenuation of t-PA (10nM) + Plgn (50nM)–mediated increase in pMLC, pERK, and pAkt levels by coaddition of ct-PA (200nM) and TXA (20mM). Stimulation was performed as described in panel E.
Treatment with t-PA/Plgn causes marked changes in the actin cytoskeleton, an increase in focal adhesion size, and generation of contractile forces in primary mouse astrocytes. (A) Representative immunofluorescence images of primary mouse astrocytes showing changes in F-actin and focal adhesion after treatment with t-PA (1μM) or its vehicle. Marked increase in stress fiber formation (left images, green) and vinculin size (right magnified images, red, indicated by arrows) are demonstrated after treatment with t-PA. Nuclei appear in blue. Scale bars represent 20 μm. (B) Representative immunofluorescence images of primary mouse astrocytes demonstrating changes in focal adhesion 24 hours after treatment with t-PA (50nM) + Plgn (20nM). Marked increase in vinculin (white, arrows) dimension is observed. Nuclei appear in red. Scale bars represent 20 μm. (C) Phase-contrast images of primary mouse astrocytes treated with vehicle, t-PA (50nM) + Plgn (20nM), or blebbistatin (a selective myosin ATPase inhibitor; 10μM) either alone or in combination for 24 hours. The full blockade of t-PA/Plgn–mediated morphology changes by blebbistatin indicates that contractile forces are generated in astrocytes by these proteases. Scale bar represents 40 μm. (D) Representative immunofluorescence images of serum-starved primary mouse astrocytes (48 hours) subjected to treatment with vehicle, t-PA (50nM) + Plgn (20nM) or t-PA alone (500nM) for 4.5 hours. Immunocytochemical analysis shows an increase in pMLC staining intensity and distribution (white) in astrocytes after stimulation with t-PA and Plgn or with high levels of t-PA alone. Cell nuclei are counterstained red. Scale bar represents 40 μm. (E) Western blot analysis confirming a concentration-dependent increase in pMLC as well as in pERK and pAkt levels in primary mouse astrocytes after t-PA/Plgn treatment. Astrocytes were serum-starved for 4 hours and then treated for 2 hours with t-PA (50nM) in the presence of either 20 or 100nM Plgn. A representative blot is shown on the right, and quantitation of fold changes in phospho-proteins after treatment with t-PA 50nM + Plgn (100nM) is presented on the left (n = 4-5). (F) Representative Western blot analysis demonstrating a strong attenuation of t-PA (10nM) + Plgn (50nM)–mediated increase in pMLC, pERK, and pAkt levels by coaddition of ct-PA (200nM) and TXA (20mM). Stimulation was performed as described in panel E.
These data suggest that t-PA/plasminogen activates the cell adhesion machinery. We then assessed the contractile state of astrocytes after t-PA/plasminogen treatment. Addition of the myosin ATPase inhibitor blebbistatin, which inhibits the movement of myosin on actin microfilaments, fully blocked the action of t-PA and plasminogen on astrocyte morphology (Figure 5C), confirming that t-PA/plasminogen activates contractile forces in astrocytes.
Contractile forces in cells are known to be associated with changes in the phosphorylation of myosin.29 Accordingly, we evaluated changes in pMLC levels in mouse astrocytes after treatment with t-PA + plasminogen. Our immunohistochemical analysis revealed significant increases in the distribution and levels of pMLC in astrocytes 4.5 hours after treatment with t-PA ± plasminogen (Figure 5D). To confirm that pMLC levels were altered in these cells, Western blot analysis for pMLC was performed. A 3.8 ± 0.6-fold (mean ± SEM, n = 4) increase in pMLC was seen in Western blots of whole astrocyte lysates after 2-hour treatment with t-PA and plasminogen (Figure 5E). We also observed a pronounced increase in the phosphorylation status of ERK (2.78 ± 0.38-fold, n = 4) and Akt (13 ± 2.3-fold, n = 5; Figure 5E), demonstrating that t-PA/plasminogen treatment of astrocytes also activates the MAP kinase and PI3 kinase signaling pathways, respectively, an observation that has been seen in other cell systems.30,31
We then tested whether the signaling observed in astrocytes after treatment with t-PA/plasminogen was transmitted through cell surface receptors. To achieve this, lower concentrations of t-PA (10nM) and plasminogen (50nM) were used with a combination of inactive t-PA (ct-PA) and TXA to improve assay sensitivity. As shown (Figure 5F), coaddition of ct-PA and TXA had a strong attenuating effect on all signaling pathways tested. However, inclusion of RAP and TXA was less effective at blocking signaling. Because RAP was only partially effective at blocking t-PA–induced permeability changes (Figure 3B), its inability to block signaling may be a reflection of limitations in sensitivity of the Western blot approach. Nonetheless, t-PA/plasminogen treatment of astrocytes triggers profound changes in the actin cytoskeleton that include receptor-dependent signaling, generation of contractile forces, and activation of the cell adhesion process.
ROCK inhibitors block t-PA–induced effects on astrocytes and on the in vitro BBB
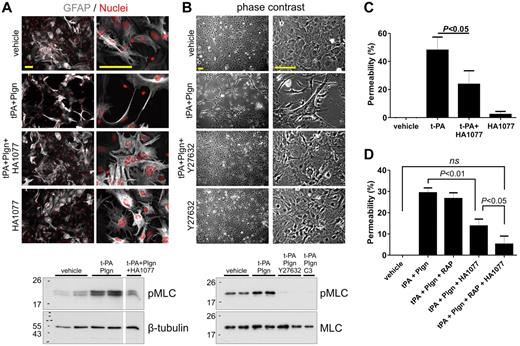
Phosphorylation of MLC is classically linked to the Rho/Rho kinase pathway.32 We speculated that disruption of this pathway with selective inhibitors would block t-PA–mediated changes in astrocyte morphology and antagonize t-PA–induced increases in pMLC levels and permeability. Downstream of Rho, the Rho-associated coiled-coil–containing protein kinase (ROCK) mediates a large proportion of Rho signaling.33,34 Addition of the ROCK inhibitor HA1077 (Figure 6A) or Y27632 (Figure 6B) completely blocked the ability of t-PA/plasminogen to alter astrocyte morphology. Consistent with this, addition of either HA1077 (Figure 6A) or Y27632 (Figure 6B) inhibited t-PA/plasminogen–mediated increase in astrocyte pMLC levels. Interestingly, t-PA/plasminogen–induced elevation of pMLC was fully inhibited when Rho itself (rather than its downstream targets) was inactivated by the Rho inhibitor C3 exo-enzyme (Figure 6B bottom). This indicates that the effect of t-PA/plasminogen on astrocytes involves direct Rho activation.
ROCK inhibitors block t-PA/Plgn-induced changes in myosin phosphorylation and morphology in mouse astrocytes and reduce t-PA/Plgn–mediated permeability increase in a human in vitro BBB model. (A) Immunofluorescence images of primary mouse astrocytes treated for 24 hours with vehicle or t-PA (50nM) + Plgn (20nM) in the presence or absence of the Rho kinase inhibitor HA1077 (20μM). GFAP is represented in gray and nuclei in red. Addition of HA1077 fully blocked t-PA/Plgn–induced morphologic changes. Scale bars represent 100 μm. Bottom: representative Western blot demonstrating blockade of t-PA (50nM) + Plgn (100nM)–mediated increase in pMLC in primary mouse astrocytes by HA1077 assessed 2 hours after stimulation of primary mouse astrocytes. (B) Representative phase contrast images showing the blocking effect of Y27632 (20μM) on t-PA (50nM) + Plgn (20nM)–induced morphologic changes in primary mouse astrocytes 24 hours after stimulation. Scale bars represent 100 μm. Bottom: representative Western blot analysis showing a complete inhibition of t-PA (50nM) + Plgn (100nM)–mediated increase in pMLC by Y27632 and by the Rho inhibitor C3 exoenzyme assessed 2 hours after stimulation of primary mouse astrocytes. (C) t-PA (250nM) was added to the luminal chamber of the in vitro human BBB model alone or in combination with HA1077 (20μM, added to both luminal and abluminal chambers). Permeability was assessed after 24 hours. As shown, HA1077 significantly attenuated the ability of t-PA to increase permeability (n = 5). (D) t-PA (25nM) and Plgn (50nM) were added to the luminal chamber of the in vitro human BBB model alone or in combination with HA1077 (20μM, added to both luminal and abluminal chambers) and RAP (0.5μM). Permeability was assessed after 24 hours. As shown, HA1077 significantly reduced the ability of t-PA/Plgn to increase permeability, whereas combination of HA1077 and RAP fully blocked the t-PA/Plgn effect (n = 5).
ROCK inhibitors block t-PA/Plgn-induced changes in myosin phosphorylation and morphology in mouse astrocytes and reduce t-PA/Plgn–mediated permeability increase in a human in vitro BBB model. (A) Immunofluorescence images of primary mouse astrocytes treated for 24 hours with vehicle or t-PA (50nM) + Plgn (20nM) in the presence or absence of the Rho kinase inhibitor HA1077 (20μM). GFAP is represented in gray and nuclei in red. Addition of HA1077 fully blocked t-PA/Plgn–induced morphologic changes. Scale bars represent 100 μm. Bottom: representative Western blot demonstrating blockade of t-PA (50nM) + Plgn (100nM)–mediated increase in pMLC in primary mouse astrocytes by HA1077 assessed 2 hours after stimulation of primary mouse astrocytes. (B) Representative phase contrast images showing the blocking effect of Y27632 (20μM) on t-PA (50nM) + Plgn (20nM)–induced morphologic changes in primary mouse astrocytes 24 hours after stimulation. Scale bars represent 100 μm. Bottom: representative Western blot analysis showing a complete inhibition of t-PA (50nM) + Plgn (100nM)–mediated increase in pMLC by Y27632 and by the Rho inhibitor C3 exoenzyme assessed 2 hours after stimulation of primary mouse astrocytes. (C) t-PA (250nM) was added to the luminal chamber of the in vitro human BBB model alone or in combination with HA1077 (20μM, added to both luminal and abluminal chambers). Permeability was assessed after 24 hours. As shown, HA1077 significantly attenuated the ability of t-PA to increase permeability (n = 5). (D) t-PA (25nM) and Plgn (50nM) were added to the luminal chamber of the in vitro human BBB model alone or in combination with HA1077 (20μM, added to both luminal and abluminal chambers) and RAP (0.5μM). Permeability was assessed after 24 hours. As shown, HA1077 significantly reduced the ability of t-PA/Plgn to increase permeability, whereas combination of HA1077 and RAP fully blocked the t-PA/Plgn effect (n = 5).
We next determined whether inhibition of the Rho/ROCK pathway also blocked the capability of t-PA or t-PA + plasminogen to modulate permeability using the in vitro human BBB model. t-PA–induced increase in permeability was significantly inhibited by HA1077 (Figure 6C). HA1077 also blocked t-PA + plasminogen–mediated increase in BBB permeability (Figure 6D). Importantly, inclusion of both HA1077 (against plasmin-mediated signals) and RAP (against a t-PA receptor) fully blocked the augmenting effect of t-PA + plasminogen on permeability (Figure 6D). Interestingly, HA1077 was ineffective against the effect of t-PA alone or in combination with plasminogen on endothelial cell permeability (ie, in the absence of astrocytes; supplemental Figure 2). This suggests that there are differences in the mechanisms underlying the actions of t-PA/plasminogen on endothelial cells and astrocytes. Collectively, our data reveal that the increase in BBB permeability by t-PA requires activation of the Rho kinase pathway in astrocytes that in turn alters the astrocyte cytoskeleton and that inactivation of this pathway can inhibit these effects of t-PA.
Because t-PA treatment of astrocytes also led to marked increases in pERK and pAkt levels (Figure 5E), we assessed the ability of selective blockers of these pathways to inhibit t-PA–mediated changes in astrocyte morphology. Cells were treated with t-PA in the presence of the MEK inhibitor U0126 and the PI3 kinase/Akt pathway inhibitor LY294002. Although both of these inhibitors fully blocked the ability of t-PA to increase pERK and pAkt levels, respectively, they did not disrupt the ability of t-PA to alter astrocyte morphology (supplemental Figure 3). Hence, of the pathways assessed after t-PA/plasminogen treatment, only the Rho/ROCK pathway is critical in the modulation of astrocyte morphology by t-PA.
t-PA modulates permeability of an in vitro mouse BBB model
Because our work on the human BBB model made use of immortalized human astrocytes, we confirmed our findings in a mouse BBB model using primary mouse brain microvascular endothelial cells and mouse astrocytes. We observed that the effects attributed to t-PA in the human BBB model were fully reproduced in the mouse: mouse and human t-PA initiated identical disrupting effects when applied to the mouse BBB model (supplemental Figure 4A,E), validating the suitability of this mouse model to study human t-PA activity on the BBB. These effects were also plasmin-dependent because α2–anti-plasmin blocked the ability of t-PA to increase permeability (supplemental Figure 4B). Again, BBB disruption was specific to t-PA because u-PA was ineffective at modulating mouse BBB permeability (supplemental Figure 4C). Finally, inhibition of ROCK by HA1077 reduced the augmenting effect of t-PA on mouse BBB permeability by ∼ 33% (supplemental Figure 4D). Taken together, these experiments on mouse primary brain cells reinforce our findings seen using the human model and indicate that the effects of t-PA on the BBB are conserved between human and mouse, and potentially other species.
Discussion
In recent years, nonfibrinolytic roles for t-PA have become prominent in the CNS and mostly in relation to neurotoxicity and modulation of the BBB.10,12 Although the literature is very consistent with regard to the effects of t-PA on the BBB, the mechanism underlying this capacity of t-PA remains controversial. Some reports have suggested a plasmin-independent role for t-PA via members of the LDLR superfamily such as LDLR-related protein (LRP-1)10 or PDGF-CC signaling,12 whereas others have reported t-PA–mediated plasmin generation as crucial for t-PA activity on the BBB.15,16 Moreover, supraphysiologic (micromolar) concentrations of t-PA were often used that can lead to nonphysiologic observations with t-PA alone.7,11,12
We developed human and mouse models of the BBB to evaluate the means by which t-PA influences permeability. Our results revealed that t-PA modulates permeability in a process intrinsically linked with plasmin formation. Furthermore, although t-PA alone increased permeability and promoted endothelial and astrocyte shape changes at concentrations greater than or equal to 100nM, the sensitivity of all test systems was markedly enhanced in the presence of 50nM plasminogen, even with low concentrations of t-PA (10-25nM).
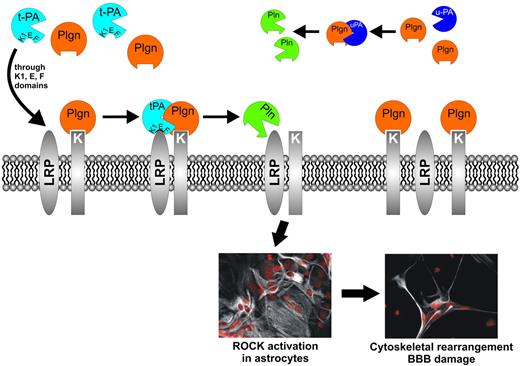
An interesting observation was that plasmin, although essential for t-PA to promote an increase in permeability, was completely incapable of substituting for t-PA, suggesting a requirement for an additional component. Indeed, our competition experiments using a catalytically inactive t-PA gave credence to the idea that a cell surface receptor for t-PA also was required. The identity of this receptor was not revealed but was probably a member of the LDLR family because t-PA–mediated increase in permeability was inhibited by the pan-LDLR blocking agent RAP. A similar blocking effect was seen when t-PA was added to our BBB model in the presence of the lysine analog TXA. Together with the lack of effect of the truncated t-PA variant reteplase that retains its lysine-binding kringle 2 domain, suggesting that t-PA binding is lysine-independent, these observations point to a requirement for a second receptor, probably a plasminogen receptor. We propose a model (Figure 7) where at least 2 cell surface receptors, one receptor for t-PA (an LDLR) and another receptor for plasminogen, are required for this effect.
Proposed model for t-PA/Plgn–mediated, receptor-dependent BBB modulation via the Rho/ROCK pathway. t-PA binds an astrocytic cell surface receptor, possibly LRP-1, via its kringle 1, EGF, or Finger domains (or their combination). The t-PA receptor colocalizes on the cell surface with a Plgn binding receptor (or protein) that uses exposed lysines to bind the Plgn kringles. This receptor-mediated colocalization of t-PA and Plgn provides the “cofactor” requires for effective t-PA–induced plasmin (Pln) generation at specific locations on the astrocyte surface. Plasmin in turn activates a yet-to-be identified target protein, triggering key signaling pathways in the astrocyte (ie, the MAPK, PI3 kinase, and Rho/ ROCK pathways). ROCK activation leads to substantial cytoskeletal changes in the astrocyte, resulting in astrocyte retraction that compromises the BBB structure and increases its permeability. Other plasminogen activators (PAs), such as u-PA, cannot use the same t-PA receptors. Pln activation by these PAs occurs in the extracellular fluid or in other areas on the cell surface that cannot participate in similar signal-transducing events, rendering these PAs ineffective as BBB modulators.
Proposed model for t-PA/Plgn–mediated, receptor-dependent BBB modulation via the Rho/ROCK pathway. t-PA binds an astrocytic cell surface receptor, possibly LRP-1, via its kringle 1, EGF, or Finger domains (or their combination). The t-PA receptor colocalizes on the cell surface with a Plgn binding receptor (or protein) that uses exposed lysines to bind the Plgn kringles. This receptor-mediated colocalization of t-PA and Plgn provides the “cofactor” requires for effective t-PA–induced plasmin (Pln) generation at specific locations on the astrocyte surface. Plasmin in turn activates a yet-to-be identified target protein, triggering key signaling pathways in the astrocyte (ie, the MAPK, PI3 kinase, and Rho/ ROCK pathways). ROCK activation leads to substantial cytoskeletal changes in the astrocyte, resulting in astrocyte retraction that compromises the BBB structure and increases its permeability. Other plasminogen activators (PAs), such as u-PA, cannot use the same t-PA receptors. Pln activation by these PAs occurs in the extracellular fluid or in other areas on the cell surface that cannot participate in similar signal-transducing events, rendering these PAs ineffective as BBB modulators.
Of the plasminogen activators tested in our human BBB model, only TNK-tPA retained the capability to increase permeability, albeit not to the same magnitude. TNK-tPA is a third generation thrombolytic agent that is identical to t-PA except for the substitution of 6 amino acids that empowered TNK-tPA with a longer plasma half-life and higher fibrin-selectivity.22 Reteplase, which displayed only a weak modulating effect, is a truncated t-PA variant that contains only the second kringle domain and the protease domain.23 By inference, 1 or more of the N-terminal domains missing in reteplase (Finger-, EGF-, and kringle 1 domains) are required for t-PA to optimally influence permeability. Both u-PA and desmoteplase were incapable of modulating permeability, suggesting that they both lack the cell surface binding and targeted plasmin generating capacity of t-PA. These findings suggest that plasmin generation needs to be targeted to the cell surface in a t-PA–specific manner via t-PA–specific cell surface receptor(s) to promote permeability.
Some variation was noted in the sensitivity to t-PA between different experimental cohorts in our human BBB model. This could stem from natural variability between primary hBECs isolated from different human donors in expression level of relevant t-PA receptors and tight junction proteins, from changes in primary cell behavior that may occur during cell passaging, or both. Although all experiments were internally controlled, these variations were noticeably minimized when exogenous plasminogen was added together with t-PA, confirming that in situ t-PA–generated plasmin is a uniform effector in our BBB system.
Our study revealed that t-PA, together with plasminogen, was producing effects on both brain endothelial cells and astrocyte morphology. Because our experiments relied on the luminal application of proteases, 1 question concerns the means by which t-PA/plasminogen can access the abluminal compartment. Although t-PA/plasminogen can enter the brain parenchyma from the circulation via physical disruption of the BBB (ie, after ischemic stroke) t-PA also can be actively transported across the intact endothelium via specific LDLR-mediated transcytosis to reach the brain parenchyma.10 Moreover, during ischemia the transport rate increases and becomes LDLR-independent.35 In addition, the strong effect of t-PA/plasminogen on brain endothelial cell morphology (Figure 4A-E) is also likely to facilitate subsequent access of these proteases to the underlying astrocytes. Both t-PA and plasminogen are synthesized in neurons, and these brain-derived levels have been shown to increase after neuronal injury.9,36-38 Hence, both proteins can be presented to the astrocytic layer either from the circulation or from within the parenchyma.
We devoted effort to understand the means by which t-PA/plasminogen were increasing permeability of the BBB. This phenomenon in astrocytes involved an elevation in actin stress fiber formation, an increase in focal adhesion size together with an increase in the phosphorylation of MLC. Because the effect of t-PA on astrocyte morphology also was inhibited by the myosin ATPase inhibitor blebbistatin, this suggested that t-PA/plasminogen were influencing cell contractility and adhesion. A pathway that is well established in modulation of cell adhesion and spreading is the Rho/ROCK pathway.32 Rho, a member of a larger GTPase family, regulates bundling of actin filaments into stress fibers and the formation of focal adhesion complexes. A key component in the Rho-induced cascade is an elevation in levels of phosphorylated myosin.39-42 The dephosphorylation of pMLC is controlled by the MLC phosphatase that in turn is inhibited by the Rho target protein ROCK. Hence, when ROCK is activated, pMLC levels increase because of the inactivation of MLC phosphatase.43
Because our findings suggested ROCK involvement in BBB modulation, we treated astrocytes with t-PA/plasminogen in the presence of 2 different ROCK inhibitors: HA1077 and Y27632. HA1077, also known as fasudil, has the highest affinity to block ROCK-II, whereas Y27632 works as a selective, ATP-competitive inhibitor of 2 Rho-associated kinases, p160ROCK (ROCK-1) and ROCK-II.44,45 Inclusion of either inhibitor not only reduced t-PA/plasminogen–mediated increase in pMLC levels but also blocked the dramatic effects of t-PA on astrocyte morphology. Importantly, inhibition of ROCK using HA1077 also significantly decreased the ability of t-PA and plasminogen to alter permeability using our in vitro human and mouse BBB models. Our study has therefore uncovered a critical pathway through which t-PA modulates the BBB in vitro and by inference in vivo.
Effects of t-PA/plasminogen were also evident on endothelial cells, both at the morphologic and functional levels. hBECs were in fact very sensitive to t-PA–induced cell surface plasmin generation, but the mechanism underlying this effect is less clear because neither the LDLR antagonist RAP nor the ROCK inhibitor HA1077 blocked t-PA/plasminogen–induced permeability or morphology changes. The LDLR and ROCK dependency observed using the intact human BBB model is likely to originate from the astrocytic layer, as supported by our experiments using isolated astrocytes. We postulate that the ROCK pathway is the relevant effector pathway activated by t-PA/plasminogen in astrocytes leading to changes in cell morphology and BBB disruption. It remains to be determined whether any of the other pathways known to be active in brain endothelial cells15,16,46 contribute to this process.
Taken together, the actions of t-PA at modulating permeability are unique to t-PA and its close variant TNK-tPA. The differential effect of plasminogen activators on BBB permeability may be relevant in the context of ischemic stroke. Two clinical studies have shown that administration of t-PA to patients with ischemic stroke can indeed increase permeability of the neurovascular unit.47,48 It would be interesting to determine whether administration of the plasminogen activators that had only mild or no effect on BBB permeability in vitro have similar effects on the neurovascular unit in vivo. A desirable outcome would be to have a thrombolytic agent that maintains effective fibrin-dependent thrombolysis without having untoward influence on the BBB.
Because we have identified the Rho/ROCK pathway as being a target for t-PA/plasminogen in both human and mouse astrocytes, promoting an increase in permeability, we also propose that the use of ROCK inhibitors together with t-PA could be considered as a means to reduce the incidence of intracerebral hemorrhage during thrombolytic therapy in stroke. Fasudil (HA1077) is already being used clinically as a vasodilator in the treatment of cerebral vasospasm and pulmonary hypertension and has been evaluated in ischemic stroke.49 Our studies would suggest a previously unsuspected potential for this drug or related inhibitors during thrombolytic treatment of patients with ischemic stroke.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank PAION Deutschland GmbH for providing desmoteplase, reteplase, and ct-PA; Dr Simone Schoenwaelder (Monash University) for providing Rho kinase inhibitors and for advice; and Dr Warwick Nesbitt (Monash University) for advice.
This study was funded by National Health and Medical Research Council of Australia grant 606658 (R.L.M.).
Authorship
Contribution: B.N. provided intellectual contribution, performed experiments, analyzed data, created the figures, and participated in writing the manuscript; R.F. performed experiments and analyzed data; T.B.P. provided intellectual contribution and data and helped analyze data; A.M.T. provided intellectual contribution and reagents and helped analyze data; and R.L.M provided intellectual contribution, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for T.B.P. is Laboratory of Astrocyte Biology and CNS Regeneration, Center for Brain Repair and Rehabilitation, Institute of Neuroscience and Physiology, Sahlgrenska Academy at the University of Gothenburg, Gothenburg, Sweden.
Correspondence: Robert L. Medcalf, Australian Centre for Blood Diseases, Monash University, 89 Commercial Rd, Melbourne, Victoria 3004, Australia; e-mail: robert.medcalf@monash.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal