The exact role of Langerhans cells (LCs) in the induction and regulation of T-cell immunity is an important focus of research with possible implications for the immunotherapy of cancer.1-3 Recently we reported on the phenotypic and functional characterization of dendritic cell (DC) subsets in skin-draining sentinel lymph nodes (SLNs) of early-stage melanoma patients.4 Our data showed that skin-migrated LCs in metastasis-free SLNs that were excised between 12 and 94 days after removal of the primary tumor (mean Breslow thickness 1.5 mm, range 0.5-3.6 mm) displayed a semi-mature phenotype, with on average 50% of the LCs expressing the maturation marker CD83.4 Despite their generally mature phenotype, LCs displayed poor T-cell stimulatory capacities and induced low levels of IFN-γ.
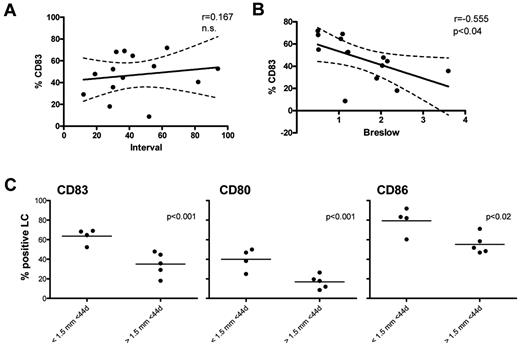
In response to our publication Gerlini et al show that in their hands LCs in tumor-negative melanoma SLNs display a more immature phenotype, with an average of 17% of the LCs expressing CD83.5 The authors reason that this difference in CD83 expression likely lies in the used experimental methods; whereas in our study SLN samples were digested with low-dose DNAse and collagenase-A, in theirs mechanical dissociation in PBS was used. We deem this unlikely as expression levels of other activation markers (ie, CD80 and CD86) were entirely equivalent between both studies.4,5 More likely, and as already pointed out by Gerlini and colleagues, the difference lies in the studied patient groups. We specifically selected our patient group to reflect a steady state based on tumor negative nodes and relatively long intervals since the removal of the primary tumor. In their dataset, the mean interval between tumor resection and removal of the SLNs was 16 days compared with 44 days in ours. In a correlative analysis we found a slight nonsignificant increase in percentage of CD83+ LCs in relation to longer intervals between tumor and SLN dissection (Figure 1A). In addition, the SLN negative patients studied by Gerlini et al had melanomas with a mean Breslow thickness of 2.43 mm, whereas in our published dataset only 2/14 patients had a Breslow thickness that exceeded 2.43 mm. Interestingly, we found a significant inverse correlation between CD83 expression on LCs and Breslow thickness in our dataset (Figure 1B). Indeed, significantly lower percentages of LCs in the SLNs expressed CD83, CD8, and CD86 when the SLNs had been removed from patients with a relatively large primary tumor (> 1.5 mm) within a shorter-than-mean excision interval (< 44 days, see Figure 1C). These additional analyses provide a possible explanation for the observed difference in LC activation state in negative SLNs between our study and that of Gerlini et al as the latter comprised patients with larger Breslow thickness and shorter intervals between tumor resection and SLN excision. In addition, it raises the question whether the lower percentage of CD83+ LCs in their SLN+ patient group might not be related to larger Breslow thickness (mean 3.21 mm) rather than SLN status. This would be in keeping with the growing recognition that immunologic conditioning of the SLNs by the primary melanoma precedes and enables the metastatic process.6,7
Langerhans cell (LC) activation in relation to the interval between primary melanoma removal and sentinel lymph node (SLN) excision and Breslow thickness. Human SLN single-cell suspensions from early-stage melanoma patients (SLN−, n = 14) were obtained after informed consent in accordance with the Declaration of Helsinki and phenotypically analyzed by flow cytometry using (combinations of) the following monoclonal antibodies diluted in PBS supplemented with 0.1% BSA and 0.02% NaN3 (FACS buffer) and incubated for 30 minutes at 4°C: CD11c-APC, CD14-PerCP_Cy5, CD1a-PE, CD1a-FITC, CD80-FITC, CD86-FITC (BD Biosciences), Langerin-PE (intracellular staining by use of the BD Fix-Perm kit), and CD83-FITC (Beckman Coulter Immunotech). After incubation, cells were washed in FACS buffer to remove excess antibodies. Cells (0.25-0.5 × 106) were analyzed on a FACSCalibur flow cytometer (BD Biosciences) equipped with CellQuest Pro 6.0 analysis software.4 Correlations between percentage of CD83+ LCs in SLNs and (A) excision interval (in months) and (B) Breslow thickness (in mm) were determined using the Pearson r test. Differences were considered significant when P < .05; 95% confidence intervals are depicted. (C) CD83, CD80, and CD86 expression levels among LCs (by percentage positive cells), compared among patients with relatively small (Breslow < 1.5 mm) and large (Breslow > 1.5 mm) primary tumors and excision intervals < 44 days. Differences were considered significant when P < .05 in an unpaired 2-sided Student t test.
Langerhans cell (LC) activation in relation to the interval between primary melanoma removal and sentinel lymph node (SLN) excision and Breslow thickness. Human SLN single-cell suspensions from early-stage melanoma patients (SLN−, n = 14) were obtained after informed consent in accordance with the Declaration of Helsinki and phenotypically analyzed by flow cytometry using (combinations of) the following monoclonal antibodies diluted in PBS supplemented with 0.1% BSA and 0.02% NaN3 (FACS buffer) and incubated for 30 minutes at 4°C: CD11c-APC, CD14-PerCP_Cy5, CD1a-PE, CD1a-FITC, CD80-FITC, CD86-FITC (BD Biosciences), Langerin-PE (intracellular staining by use of the BD Fix-Perm kit), and CD83-FITC (Beckman Coulter Immunotech). After incubation, cells were washed in FACS buffer to remove excess antibodies. Cells (0.25-0.5 × 106) were analyzed on a FACSCalibur flow cytometer (BD Biosciences) equipped with CellQuest Pro 6.0 analysis software.4 Correlations between percentage of CD83+ LCs in SLNs and (A) excision interval (in months) and (B) Breslow thickness (in mm) were determined using the Pearson r test. Differences were considered significant when P < .05; 95% confidence intervals are depicted. (C) CD83, CD80, and CD86 expression levels among LCs (by percentage positive cells), compared among patients with relatively small (Breslow < 1.5 mm) and large (Breslow > 1.5 mm) primary tumors and excision intervals < 44 days. Differences were considered significant when P < .05 in an unpaired 2-sided Student t test.
Authorship
Acknowledgments: This work was supported by The Netherlands Organization for Scientific Research, NWO VIDI grant 917-56-321 to T.D.d.G., Stichting Cancer Center Amsterdam (CCA), and a Dutch Cancer Society (KWF BUIT-4346) fellowship to R.v.d.V.
Contribution: M.F.C.M.v.d.H. performed research, analyzed and interpreted data, and cowrote the manuscript; B.D.K. analyzed data and drafted the manuscript; B.J.R.S. performed research; P.A.M.v.L, S.M., and M.P.v.d.T. treated patients and supplied the clinical research material; A.J.M.v.d.E. and R.J.S. designed the research; R.v.d.V. performed research, analyzed and interpreted data, and drafted and cowrote the manuscript; and T.D.d.G. designed the research, interpreted data, and drafted and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Tanja D. de Gruijl, Department of Medical Oncology, VU University Medical Center, De Boelelaan 1117-CCA 2.44, 1081 HV Amsterdam, The Netherlands; e-mail: td.degruijl@vumc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal