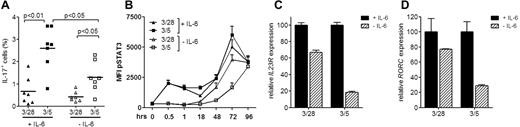
With interest we read the letter by Ayyoub and coworkers who compare the efficacy of Th17 differentiation from naive FOXP3+CD25+CD127− NTregs and conventional naive T cells.1 We previously reported that compared with CD28, alternative costimulation via CD5 enhanced Th17 differentiation from CD4+CD45RA+CD4RO− naive T cells.2 CD5 promotes Th17 development through elevation of IL-23R expression, resulting in prolonged STAT3 activation and enhanced levels of ROR-γt. Ayyoub and coworkers also observed increased Th17 induction in naive CD4+ T cells by CD5. Costimulation of NTregs via CD28 or CD5 both induce more Th17, from which the authors conclude that Th17 differentiation from conventional naive CD4+ T cells is less efficient. We are pleased that our data on naive CD4+ T cells are confirmed, but observed a relatively weak efficiency of Th17 differentiation using naive CD4+ T cells. Moreover, no enhanced CD5-mediated expression of IL-23R and ROR-γt was observed. We noticed that the experiments were performed in absence of IL-6, which has been reported to be required for optimal Th17 development.3 Although not shown in our publication, we extensively investigated the contribution of IL-6 in the development of Th17 cells from naive CD4+ T cells (Figure 1). This data underlines the importance of IL-6 in Th17 development, especially on CD5 costimulation. Without IL-6, CD5 costimulation was still superior for Th17 induction, in agreement with Ayyoub et al's findings, but the efficiency of Th17 differentiation was strongly reduced (Figure 1A). In mice, Nishihara and coworkers showed that IL-6 requirement of conventional naive CD4+ T cells for Th17 development is mediated by STAT3.4 Indeed, omission of IL-6 abrogated STAT3 activation by CD28 or CD5 costimulation within the first 18 hours (Figure 1B). Especially for CD5 costimulation, this was followed by less pSTAT3 induction at later time points (Figure 1B). In line with this, IL-23R levels were also decreased in the absence of IL-6 (Figure 1C). Again, the strongest impact was observed on CD5-stimulated cells (± 80% reduction vs ± 35% with CD28, P < .05, paired t test, n = 3). As expected,2 this also correlated with strongly decreased levels of ROR-γt (P < .05, paired t test, n = 3; Figure 1D). Thus, IL-6 plays an important role in Th17 differentiation from naive CD4+ T cells, not only on classic CD28 stimulation,3 but even more strongly in CD5 stimulation. The lack of IL-6 may explain why Ayyoub and coworkers did not observe increased IL-23R or ROR-γt in CD5-stimulated naive CD4+ T cells. Interestingly, their data also show that NTregs still efficiently form Th17 without IL-6. This may reflect earlier observations showing reduced IL-6R signaling in natural Tregs compared with CD4+CD25− T cells and only weak IL-6–induced STAT3 activation in Tregs compared with strong pSTAT3 induction in CD4+CD25− T cells.5 It is tempting to speculate that the latter also explains why CD5 is not superior to CD28 in NTreg costimulation. Taken together, the data of Ayyoub and coworkers show interesting new insights. To ascertain, however, whether Th17 development from NTregs is superior to that from conventional naive CD4+ T cells, the concept that in different CD4+ subsets Th17 differentiation may be controlled by distinct regulatory factors should be integrated in the comparison. We look forward to further studies that will address this issue.
CD5 costimulation requires IL-6 for optimal Th17 differentiation. (A) FACS-sorted naive CD4+CD45RA+CD45RO− T cells were stimulated via plate-coated CD3/CD28 or CD3/CD5 antibodies under Th17 polarizing conditions (30 ng/mL IL-23, 10 ng/mL IL-1β, 10 ng/mL IL-6, 10 ng/mL TGF-β and 10μg/mL anti-IFN-γ) in the presence of 10 U/mL of IL-2. Intracellular levels of IL-17A were measured at day 11 after 5 hours of restimulation with PMA, ionomycin, and BFA. Data are from 7 independent experiments of individual donors. Statistical analyses were performed with paired t test. (B) Naive T cells were stimulated by plate-bound CD3/CD28 or CD3/CD5 antibodies and cultured in Th17-inducing conditions. Phospho-STAT3 levels were measured by FACS. Data shown are from 6 individual experiments with different donors, with mean + SD. (C-D) Real-time semi-quantitative PCR (ABI PRISM 7000, SYBR green method) of IL23R (C) and RORC (D) mRNA expression of naive T cells stimulated for 3 days via coated CD3/CD28 or CD3/CD5 antibodies under Th17-polarizing conditions. Results were normalized to 18S rRNA. Data shown are mean ± SD of triplicate measurement from 1 representative experiment of 3 independent experiments using different donors.
CD5 costimulation requires IL-6 for optimal Th17 differentiation. (A) FACS-sorted naive CD4+CD45RA+CD45RO− T cells were stimulated via plate-coated CD3/CD28 or CD3/CD5 antibodies under Th17 polarizing conditions (30 ng/mL IL-23, 10 ng/mL IL-1β, 10 ng/mL IL-6, 10 ng/mL TGF-β and 10μg/mL anti-IFN-γ) in the presence of 10 U/mL of IL-2. Intracellular levels of IL-17A were measured at day 11 after 5 hours of restimulation with PMA, ionomycin, and BFA. Data are from 7 independent experiments of individual donors. Statistical analyses were performed with paired t test. (B) Naive T cells were stimulated by plate-bound CD3/CD28 or CD3/CD5 antibodies and cultured in Th17-inducing conditions. Phospho-STAT3 levels were measured by FACS. Data shown are from 6 individual experiments with different donors, with mean + SD. (C-D) Real-time semi-quantitative PCR (ABI PRISM 7000, SYBR green method) of IL23R (C) and RORC (D) mRNA expression of naive T cells stimulated for 3 days via coated CD3/CD28 or CD3/CD5 antibodies under Th17-polarizing conditions. Results were normalized to 18S rRNA. Data shown are mean ± SD of triplicate measurement from 1 representative experiment of 3 independent experiments using different donors.
Authorship
Acknowledgments: This work was supported by grants from the Landsteiner Foundation for Blood Research (grants 0533 and 0816) and Sanquin Blood Supply Foundation (PPOC 09-032).
Contribution: Y.S., J.d.W., H.K.B., F.J.M.M., and T.J. performed experiments; and Y.S., J.d.W., E.C.d.J., S.M.v.H. and M.L.K. designed experiments, analyzed the results and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: S. Marieke van Ham, Sanquin Research, Department of Immunopathology, Plesmanlaan 125, 1066 CX, Amsterdam, The Netherlands; e-mail: m.vanham@sanquin.nl.
References
Author notes
Y.S. and J.d.W. contributed equally to this work, sharing first authorship.
M.L.K., E.C.d.J., and S.M.v.H contributed equally to this work, sharing last authorship.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal