Abstract
During embryonic development, multilineage HSCs/progenitor cells are derived from specialized endothelial cells, termed hemogenic endothelium, within the yolk sac, placenta, and aorta. Whether hemogenic endothelial cells contribute to blood cell development at other sites of definitive hematopoiesis, such as in the fetal liver and fetal bone marrow, is not known. Also unknown is whether such cells exist within the vasculature of adult bone marrow and generate hematopoietic stem cells after birth. These issues and their clinical relevance are discussed herein.
Introduction
Blood vessels and blood cells must develop in parallel during mammalian embryogenesis to form a functional circulatory system that provides nutrients and oxygen to all tissues, removes metabolic waste products, enables growth, and prevents toxicity. The origin(s) of vascular and blood cell types during development is not entirely clear and may be different depending on the stage of hematopoiesis (primitive vs definitive), as well as the site of blood cell development.
Primitive hematopoiesis
In the mouse at around embryonic day (E) 7.0 to 7.25, as well as in humans, the first primitive blood cells, composed predominantly of nucleated erythroid progenitors,1,2 and endothelial cells emerge in parallel, temporally and spatially, from extraembryonic yolk sac mesoderm. Although the first blood and endothelial cells appear in clusters referred to as blood islands, endothelial cells and vascular channels form in other regions of the yolk sac and embryo proper as well. As the developing heart forms and begins to contract, oscillatory plasma flow occurs within the vascular channels, and the immature erythroblasts enter the plasma within the vascular plexus.3
Definitive hematopoiesis
This initial burst of “primitive” hematopoietic activity is soon followed and supplanted by the second wave of multilineage (definitive) hematopoiesis, coincident with the onset of synchronous heart beating and pulsatile systemic circulation.3 Interestingly, the site of multilineage HSC/progenitor cell production and/or maintenance changes throughout mammalian gestation. During mouse embryogenesis, definitive hematopoiesis begins in the extraembryonic yolk sac at approximately E8.252,4 and placenta approximately E9.5,5,6 and then within the aorta-gonad-mesonephros (AGM) region of the embryo proper at approximately E10.4,5,7,8 As development progresses, the fetal liver becomes the major site of definitive hematopoiesis at approximately E11 to E129 ; and shortly before birth, this process is established within fetal bone marrow, which remains the predominant site of hematopoiesis postnatally. Although hematopoiesis is known to occur within these distinct tissues, the origin(s) of the multilineage stem/progenitor cells that contribute to this process at each of these sites is not entirely clear.
Developmental origin of HSCs/progenitor cells
At the earliest stages of blood development (primitive hematopoiesis), the primitive hematopoietic and endothelial cells that make up a rudimentary circulatory system emerge simultaneously; thus, their origin(s) has long been vigorously debated. One theory suggests that these lineages are generated from a common bipotent progenitor (hemangioblast),10,11 whereas the other suggests that they are independently fated among mesodermal progenitors during gastrulation.12 To date, this debate has not been resolved at this stage of development.
In contrast, at later stages of blood development (definitive hematopoiesis), it has become increasingly clear over the past few years that multilineage HSCs/progenitor cells arise from specialized vascular endothelial cells that acquire blood-forming potential (hemogenic endothelium), at least within the yolk sac, placenta, and AGM. This is not surprising, given that the possibility had been noted almost 100 years ago. Indeed, the earliest use of the term “hemangioblast” refers to the precursor cells that give rise to blood-forming (hemogenic) endothelial cells, all of which is further discussed in the next section.
Hemangioblasts
In the early 1900s, Florence Sabin noted the physical association of blood cells with the endothelial-lining of blood vessels in the developing chick.13 She observed: “endothelial cells divide so that one daughter cell projects into the lumen and then becomes filled with hemoglobin.” Once a cluster of red blood cells (“red blood corpuscles”) is formed, it breaks free from the vessel wall and floats away in the blood plasma. She coined the term “angioblasts” to describe the cells that give rise to the blood-forming or hemogenic endothelial cells.
More than a decade later, Murray referred to the same precursor cells, derived from the mesenchyme, as “hemangioblasts,” suggesting that this is a more accurate term because both endothelial and blood cells develop there from.14 Thus, Murray was proposing that hemangioblasts are the immediate precursors to hemogenic endothelial cells that generate blood. However, by the 1980s, the term “hemangioblast” had come to be used to describe a bipotent cell, which was presumed to be generated in the primitive streak and exist transiently for establishment of the blood and vascular systems.
The existence of “hemangioblasts” as such in vivo and their contribution to primitive and/or definitive hematopoiesis remain controversial. Although few people would probably argue against the idea that vascular cells and blood cells have, at some point in their ontogeny, a common progenitor of mesodermal origin, there is no definitive evidence in mouse or other established models of blood and vessel development (ie, zebrafish, chick), to date, that specific mesodermal progenitors generate just 2 distinct lineages, vascular endothelial and blood cells, in vivo.
Hemangioblasts proposed in the mouse model are identified by the coexpression of mesoderm-specific marker Brachyury, as well as vascular endothelial growth factor receptor 2 (VEGFR2 or Flk-1). However, indirect tracking of this cell type reveals that it gives rise to endothelial cells and blood cells, as well as vascular smooth muscle cells in the yolk sac.15 These are the only 3 distinct mesodermal lineages in the yolk sac at the stage of development studied. Furthermore, cells expressing Flk-1 were shown in lineage-tracing studies to give rise to multiple mesodermally derived cell types within the embryo proper, including cardiac and skeletal muscle cells.16 Therefore, collective evidence supports the function of murine Bry+Flk-1+ cells as multipotent progenitors that give rise to several mesodermal lineages, including endothelial and blood cells. Whether there is a specific subset that is exclusively bipotent and gives rise to only endothelial and blood cells has yet to be definitively shown in this model.
Indeed, other lineage-tracing studies in the mouse embryo suggest that endothelial and blood cells are independently fated during gastrulation. Kinder et al transplanted small clusters (5-10 cells) of genetically marked (LacZ+) mesodermal progenitor cells into wild-type embryos at the time point in development when endothelial and blood cells are emerging.12 They found that the transplanted cells gave rise to either endothelial or blood cells, and erythrocytes were generated slightly earlier in vivo than endothelial cells. These results do not support the presence/function of hemangioblasts and also do not provide support for the existence of hemogenic endothelial cells during primitive hematopoiesis, which has also not been definitively shown. Thus, the precise cellular origin of the first endothelial and blood cells in the mouse, as well as other species, continues to be debated.
The origin of endothelial and blood cells in the zebrafish was investigated by labeling gastrula-stage embryos via laser activation of caged fluorescein dextran.17 Single cells were UV-activated shortly after gastrulation, at a stage when cells are thought to be “restricted to a single lineage.” The investigators found that most labeled cells gave rise to either Gata-1–expressing (blood) or Flk-1–expressing (endothelial) cells, supporting the idea that most blood and endothelial cells are independently fated, as was observed in the mouse.12 However, they also found that approximately 12% of the labeled mesodermal cells in the zebrafish gave rise to cells that expressed either Flk-1 or Gata-1. The labeled cells were presumed to be hemangioblasts; however, the temporal and/or sequential expression of these genes therein was not shown, so it is possible that the Gata-1–expressing (blood) cells were derived from the Flk-1–expressing (endothelial) cells during the time course of these studies.
Lineage tracing was also performed in the chick embryo at later stages of development, during definitive hematopoiesis, to determine the origin of hematopoietic cells within the periaortic region that do not have an apparent physical association with endothelium. A LacZ-expressing retroviral vector was delivered via cardiac injection to specifically label the aortic endothelium before hematopoietic development in this tissue. The hematopoietic progenitors within the aortic lumen and subsequently within the periaortic region were found to be “born from the aortic endothelium.”18 Thus, although the title of this article suggests that there are “hemangioblasts” in the developing avian embryo, the authors state, “these hemangioblasts may be better described as hemogenic endothelium.”
Hemogenic endothelial cells
Although the hemangioblast debate will probably continue, this issue appears to be relevant to only primitive hematopoiesis, at the onset of hematovascular development. It is now more generally accepted that multilineage HSCs/progenitor cells responsible for the generation of all blood cell types during definitive hematopoiesis arise from hemogenic endothelium, as originally observed by Sabin.13
The evidence that has mounted over the past few years to support the emergence of multilineage HSCs/progenitor cells from the endothelium during definitely hematopoiesis comes from lineage-tracing studies, in vivo imaging, and in vitro clonal analysis, all of which are more thoroughly reviewed elsewhere.19,20 For example, Zovein et al used an inducible Cre-lox system to track the fate of cells expressing VE-cadherin,21 which is thought to be endothelial-specific. They found that VE-cadherin lineage+ cells, labeled within the AGM region before the onset of definitive hematopoiesis, gave rise to blood cells of all lineages in vivo. Dynamic imaging captured such events in real time in the mouse and zebrafish,22,23 and in vitro clonal analysis demonstrated the generation of multilinage hematopoietic colonies from single hemogenic endothelial cells freshly isolated from E9.5 mouse yolk sac.24
Collectively, such studies provide direct evidence that blood cells are generated from the endothelium during definitive hematopoiesis. Even blood cells referred to as “hemangioblast-derived” have recently been proposed to be produced via an endothelial-intermediate,25 as originally suggested by Murray.14 Therefore, definitive hematopoiesis cannot occur in the absence of endothelial cell development.26,27 Furthermore, even if endothelial cells form, defects in their proliferative control can also result in impaired definitive hematopoiesis.24,28,29 Conversely, proper endothelial cell development and arteriovenous specification do not ensure normal definitive hematopoiesis.30 Thus, the signaling and/or transcription factors that regulate the specialization of blood-forming endothelial cells, as well as arterial and venous endothelial cells, are probably distinct from those that promote their specialized functions.
Whereas hemogenic endothelial cells are known to generate multilineage HSCs/progenitor cells in the yolk sac, AGM, and placenta (further reviewed by Sills and Hirschi19 ), there is little evidence that a similar process occurs within fetal liver and bone marrow. The current concept of the progression of developmental hematopoiesis is that HSCs are “born” within the yolk sac (although still controversial) and AGM, and that they then migrate to subsequent sites of definitive hematopoiesis (ie, to fetal liver and fetal bone marrow; extensively reviewed elsewhere, eg, Medvinsky et al20 ). Although early embryonic lineage-tracing studies demonstrate that AGM-derived HSC transition to the fetal liver, and even to adult bone marrow,21 they do not account for all hematopoietic cells within the fetus and adult. Thus, the possibility that HSCs also arise de novo within later sites of definitive hematopoiesis, perhaps via hemogenic endothelium, has not been ruled out. However, a blood-forming function of the vasculature within these tissues, such as the fetal liver and bone marrow, will be difficult to discern from their currently proposed function as a passageway for the influx of circulating blood stem/progenitors generated elsewhere. Nonetheless, endothelial cell differentiation and blood vessel formation are thought to occur within these tissues before the onset of their hematopoietic function (reviewed by Crivellato31 ). Therefore, it is plausible that multilineage stem/progenitor cells are generated de novo from the vasculature in these tissues, although not yet proven.
To convincingly demonstrate the existence and functional importance of hemogenic endothelial cells within various tissues, the precise phenotype of the blood-forming endothelial cells must be defined, relative to endothelial cells that do not have this capability, and distinct from their HSC/progenitor cell progeny. In addition to defining phenotype, one must also demonstrate their localization and fate in vivo and their specialized function on a clonal level during the relevant developmental time frame. Adding to the challenge is the fact that, although endothelium constitutes the luminal layer of all blood vessels within the body, only a small subset has the capacity to generate multilineage HSCs/progenitor cells within distinct sites at different stages of development.
Hemogenic endothelium constitutes less than 2% of total endothelial cells in the mouse yolk sac at approximately E8.524,32 and within the AGM at approximately E10.5 (K.K.H., unpublished data, 2010-2011); thus, the cell populations are relatively rare. Nonetheless, we have defined the phenotype of, and devised an isolation strategy for, hemogenic endothelial cells within the murine yolk sac,24,32 and demonstrated their functional properties on a clonal level.24 The hemogenic endothelial cells within the yolk sac, as well as embryo proper, exhibit dye-efflux properties (so-called “side population,” SP cells),32 are Flk1+cKit+CD45− and give rise to Flk1−cKit+CD45+ SP cells that exhibit multilineage hematopoietic colony-forming activity24,32 (Figure 1). Whether similar cells exist, and serve similar functions, at all sites of definitive hematopoiesis is not known.
However, there is already evidence that the progeny of hemogenic endothelial cells are functionally different depending on their site of origin, and perhaps their developmental stage. For example, although hemogenic endothelial cells within the yolk sac give rise to multilineage progenitors in vitro, these endothelial-derived cells have limited ability to repopulate all blood cell lineages on transplantation in vivo.4 This is in contrast to the multilineage precursors (thought to be “true” HSCs) generated in the embryonic AGM, which can repopulate neonatal and adult recipients.8 Interestingly, although yolk sac– and AGM-derived progenitors perform differently in transplant studies, both are thought to contribute to the adult HSC pool.21,33 These observations suggest that hemogenic endothelial cells arising within different anatomic sites throughout development may be phenotypically and functionally distinct and/or give rise to distinct types of multilineage HSCs/progenitor cells. Thus, it will be important to carefully define and compare the phenotype and function of blood-forming endothelial cells within different hematopoietic tissues at distinct stages of development. Insights gained from such studies will enable their prospective isolation for further phenotypic and genomic analyses, as well as dissection of their molecular regulation.
Postnatal hemogenic endothelium?
Whether hemogenic endothelial cells exist in adult bone marrow, and generate HSCs in situ, has not been proven, and is not a well-accepted concept. The favored concept is that we are born with all of the HSCs that we are going to have, and they are replenished via self-renewal. Support for this idea comes from cell transplantation studies in animal models, in which serial transplantation of HSCs/bone marrow eventually leads to exhaustion of the HSC pool.34,35 However, this does not necessarily disprove the existence and function of hemogenic endothelial cells in adult marrow. Indeed, embryonic lineage-tracing studies, which label hemogenic endothelial cells before definitive hematopoiesis and follow their progeny HSCs through adulthood, demonstrate that, although embryonic HSCs remain functional in the adult, they do not account for all hematopoietic cells generated postnatally.21 It is also important to consider that, in adult bone marrow, HSCs are known to reside in close physical association with sinusoidal endothelial cells,36 and the vasculature provides an important niche for HSC survival and function.37 In procedures in which HSCs are transplanted into irradiated animals and human subjects, the sinusoidal endothelium within the irradiated host marrow is also damaged.38 Perhaps hemogenic endothelial cells reside therein and, like resident HSCs, are eliminated by irradiation. If this were the case, then they would no longer be able to generate new HSCs and contribute to the rescue of the irradiated animals. Thus, successive transplantation of the same HSC pool, in the absence of the generation of new HSCs from the irradiated host, would yield the same outcome, exhaustion of the HSC pool over time.
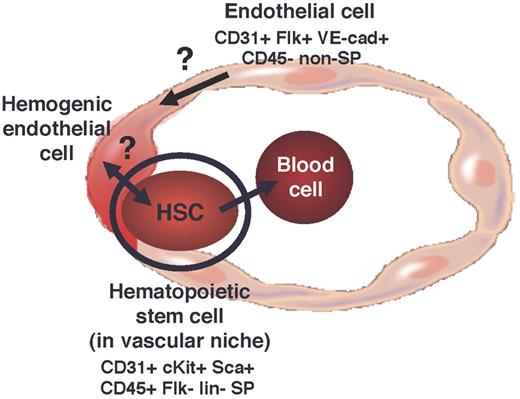
Human clinical studies also support the idea that HSCs are derived via self-renewal. For example, cell-autonomous defects in HSCs lead to hematopoietic failure and death.39 However, it may be interesting to consider yet another possibility that HSCs (endogenous and transplanted) contribute to the formation of their own vascular niche within marrow, and perhaps a subset of HSC-derived endothelium is specialized to become hemogenic, ultimately contributing to HSC generation but not via self-renewal (Figure 2). Thus, HSC transplantation may rescue more than the hematopoietic compartment of bone marrow, and the rescue of the vascular component may be equally important in the maintenance and/or generation of long-term HSCs. Although this is not consistent with the idea that successive transplantation of HSCs, per se, ultimately leads to exhaustion of the blood-forming pool, perhaps hemogenic endothelial cells, generated there from, also exhibit a limited life span via similar mechanisms (ie, telomere shortening40 ).
In support of this idea, previous studies in various laboratories have demonstrated that HSCs can give rise to cells within the marrow (presumably CD34+Flk-1+) that function as endothelial precursor cells (further reviewed by Hirschi et al41 ), and give rise to endothelial cells, as well as other cell types, in response to injury (for example, Jackson et al42 and Majka et al43 ). Perhaps the injury induced by marrow ablation promotes the generation of endothelial cells from the transplanted HSCs that home to this microenvironment and they thereby contribute to the formation/maintenance of their own vascular niche, which, in turn, enables continuous self-renewal and/or de novo generation of HSCs.
Although the occurrence of such a phenomenon has yet to be demonstrated and the existence of hemogenic endothelial cells in adult marrow is highly speculative, it is interesting to note that the phenotype of the adult marrow-derived HSCs is similar to that of embryonic multilineage stem/progenitor cells24,32 (Figures 1 and 2). Both exhibit an SP phenotype,32,44 express CD31, CD45, and cKit, and do not express Flk-1.24,32,42 In the yolk sac, such cells are derived from hemogenic endothelial cells, which are CD31+Flk-1+cKit+CD45− SP cells.24 Whether adult HSCs are similarly derived from hemogenic endothelial cells remains to be determined. However, if this is the case, the phenotype of adult blood-forming endothelial cells is probably somewhat different from their putative embryonic counterparts, given that there are no Flk-1–expressing cells in the SP fraction of adult marrow.42
It is also interesting to note that some of the same signaling pathways and transcription factors that regulate the development and function of embryonic hemogenic endothelium also play an important role in adult hematopoiesis. Although a thorough review of the molecular regulation of hematopoiesis is beyond the scope of this Perspectives (and is extensively reviewed elsewhere; eg, Medvinsky et al20 ), one example is that both embryonic and adult blood formation is dependent on retinoic acid signaling. The absence of active retinoic acid in the embryo leads to hyperproliferation of the endothelium, suppressed development of hemogenic endothelium, and impaired definitive hematopoiesis.24,28,29 Similarly, dysregulation of retinoic acid receptors in adult HSCs impairs their self-renewal and differentiation.45 Runx1 is another molecular regulator that is expressed by embryonic46 and adult47 HSCs, and is critical for proper generation of all blood lineages during development and postnatally. Its disrupted expression/function in the embryo leads to mid-gestation death and lack of definitive hematopoiesis,48 and genetic mutations in humans yield significant hematopoietic disorders.49 Although similarities in phenotype and regulation exist, much work is needed to determine whether embryonic hematopoietic processes are recapitulated postnatally and whether this includes the formation and function of hemogenic endothelial cells.
Clinical significance
Delineating the developmental hierarchy among vascular and blood cells predominantly advances our understanding of embryonic development; however, insights gained can be applied toward understanding adult HSCs and hematopoiesis, as well as in vitro generation of HSCs for clinical therapies. Thorough and careful characterization of the cell types involved in blood cell generation during development will enable us to discern them in vivo and track their fate and function. In so doing, we can monitor and quantify their cell-cell and cell-matrix associations within the tissue-specific microenvironments in which they are generated throughout gestation. Such information will lend to the elucidation of the mechanism(s) by which hemogenic endothelial cells develop and generate HSCs, as well as determine whether distinct microenvironments give rise to functionally distinct multilineage HSCs/progenitor cells. This information will not only further our understanding of hemato-vascular development but may also point to a cell population that can be prospectively isolated from adult tissues, or generated in vitro from pluripotent human stem cells (reviewed by Iacobas et al50 ), thus circumventing current limitations to the production and expansion of functional HSCs in vitro.
Authorship
Contribution: K.K.H. generated the concepts, created the figures, and wrote the manuscript.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Karen K. Hirschi, Yale Cardiovascular Research Center, Yale Stem Cell Center, Yale University School of Medicine, 300 George St, Rm 770J, New Haven, CT 06511; e-mail: karen.hirschi@yale.edu.
References
National Institutes of Health


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal