Abstract
The CLL3 trial was designed to study intensive treatment including autologous stem cell transplantation (autoSCT) as part of first-line therapy in patients with chronic lymphocytic leukemia (CLL). Here, we present the long-term outcome of the trial with particular focus on the impact of genomic risk factors, and we provide a retrospective comparison with patients from the fludarabine-cyclophosphamide-rituximab (FCR) arm of the German CLL Study Group (GCLLSG) CLL8 trial. After a median observation time of 8.7 years (0.3-12.3 years), median progression-free survival (PFS), time to retreatment, and overall survival (OS) of 169 evaluable patients, including 38 patients who did not proceed to autoSCT, was 5.7, 7.3, and 11.3 years, respectively. PFS and OS were significantly reduced in the presence of 17p- and of an unfavorable immunoglobulin heavy variable chain mutational status, but not of 11q-. Five-year nonrelapse mortality was 6.5%. When 110 CLL3 patients were compared with 126 matched patients from the FCR arm of the CLL8 trial, 4-year time to retreatment (75% vs 77%) and OS (86% vs 90%) was similar despite a significant benefit for autoSCT in terms of PFS. In summary, early treatment intensification including autoSCT can provide very effective disease control in poor-risk CLL, although its clinical benefit in the FCR era remains uncertain. The trial has been registered with www.clinicaltrials.gov as NCT00275015.
Introduction
Before the advent of fludarabine combination therapies and rituximab, autologous stem cell transplantation (autoSCT) was developed as potentially effective and eventually curative treatment for chronic lymphocytic leukemia (CLL). Triggered by promising single center pilot data from the United States and Europe,1-8 a phase II multicenter trial performed in the late 1990s suggested that autoSCT, when used as part of first-line therapy, could have the potential of effective disease control in CLL, although there was little evidence that it could provide complete disease eradication in a substantial proportion of patients.9 A subsequent multinational phase III trial from the prerituximab era demonstrated that autoSCT indeed doubles progression-free survival (PFS) and treatment-free survival compared with conventional fludarabine-based or similarly effective first-line chemotherapy in younger patients with CLL.10,11 However, the current standard for upfront treatment in fit patients with CLL is fludarabine-cyclophosphamide-rituximab (FCR)12,13 that showed a significant survival benefit compared with fludarabine-cyclophosphamide without rituximab (FC).14
The CLL3 trial was designed in 1996 to study the feasibility and efficacy of early autoSCT in poor-risk CLL in a phase II multicenter setting. Compared with the British pilot study and the 2 separate randomized trials published to date,10,15 unique features of the CLL3 study were (1) eligibility of patients who were high risk by biologic criteria but still had no classic treatment indication, (2) a Dexa-BEAM intensification step to improve mobilization efficacy and disease control before autoSCT, and (3) vigorous ex vivo B-cell depletion of the graft that could be performed in a large proportion of patients. The purpose of the analysis presented here was to provide the long-term outcome of the trial with particular focus on the impact of genomic risk factors, and a retrospective comparison with patients from the FCR arm of the German CLL Study Group (GCLLSG) CLL8 trial14 who were matched for age, Binet stage, leukocyte count, FISH risk group, and immunoglobulin heavy variable chain (IGHV) mutation status, thereby filling the gap of randomized studies restricted to the pre-FCR era. The results suggest that intensive therapy including autoSCT, when used as planned part of first-line treatment, can provide very effective disease control and excellent survival in poor-risk CLL, although it does not seem to have curative potential.
Methods
Patients
Patients eligible for the CLL3 trial were those with Binet stage B or C CLL, or poor-risk Binet stage A disease as defined by a lymphocyte doubling time of less than 12 months and/or a non-nodular bone marrow infiltration pattern16 plus a serum thymidine kinase level of 7 U/L or higher and/or a β-2 microglobulin (β2M) serum level of 3.5 mg/L or higher.17 Patients needed to have a PCR amplifiable clonal CDR3 rearrangement and had to be between 18 and 60 years old with normal organ function and an Eastern Cooperative Oncology Group performance status of 1 or better. Patients with Richter's transformation and those with more than 1 prior chemotherapy regimen or with previous chemotherapy for more than 6 months were excluded. Up to 20% of the patient population described here has been part of previous reports with a much shorter follow-up.18-20
Treatment
Study treatment consisted of initial cytoreduction with up to 4 cycles of cyclophosphamide (750 mg/m2), doxorubicine (50 mg/m2), vincristin (1.4 mg/m2), and prednisone (CHOP), fludarabine monotherapy, or FC according to investigator's choice to reduce the peripheral lymphocyte count below 10/nL, followed by 1 or 2 cycles of Dexa-BEAM for further remission induction and peripheral blood stem cell (PBSC) mobilization. The Dexa-BEAM regimen included dexamethasone (3 × 8 mg day [d] 1-10), N,N′-bis(2-chloroethyl)-N-nitrosourea (60 mg/m2 d2), etoposide (75 mg/m2 d4-7), cytarabine (100 mg/m2 q12h d4-7), and melphalan (20 mg/m2 d3). Patients received filgrastim or lenograstim daily from d8 after start of Dexa-BEAM until the last day of PBSC collection. PBSC products were subjected to ex vivo B-cell depletion using immunomagnetic devices approved for this purpose during the time of study conduct (Clinimacs; Miltenyi Biotech; Isolex, Baxter Immunotherapy; or Isolex with consecutive negative selection with B-cell antibodies18 ), and cryopreserved. Collection target was 2 × 106/kg CD34+ cells after purging plus an unpurged backup with at least 2 × 106/kg CD34+ cells. As a final step, patients underwent myeloablative therapy consisting of 12-Gy hyperfractionated total body irradiation and 120 mg/kg cyclophosphamide, followed by reinfusion of the purged PBSC product.18
Trial objectives and assessments
As per protocol, the primary objective of this open, nonrandomized, multicenter phase II clinical trial was to study the safety and feasibility of early autoSCT in patients with poor-risk CLL. Secondary objectives were assessment of clinical and molecular response, PFS, and overall survival (OS). Response evaluation was done according to National Cancer Institute criteria.21 Clinical follow-up and assessment of remission status consisting of clinical status; blood counts; marrow biopsy; flow cytometry of blood and marrow; molecular minimal residual disease (MRD) monitoring of blood and marrow; and imaging (CT scan or ultrasound) was performed 1, 3, 6, and 12 months after autoSCT and every 6 to 12 months thereafter.
The protocol including the study-specific informed consent form in accordance with the Declaration of Helsinki was approved by all responsible institutional review boards (Primary institutional review board: Ethics Committee of the University of Kiel, approval A122/97). The trial has been registered with the National Cancer Institute (protocol identifier NCT00275015).
Serologic, genetic, and MRD analyses
Assessments of serum thymidine kinase and β2M, genomic aberrations, IGHV mutational status, and MRD by ASO primer IgH real-time quantitative PCR were performed in the central reference laboratories of the GCLLSG, as described previously.17,22-24 Only results obtained in the GCLLSG central laboratories were considered for the analyses performed in this study. All results refer to samples obtained at study entry.
Comparison of autoSCT (CLL3) with FCR (CLL8)
To put the results of autoSCT into perspective, the outcome of patients treated in the CLL3 trial (autoSCT) was compared with that of patients treated on the FCR arm of the GCLLSG CLL8 trial within the framework of a retrospective cohort analysis according to the intent to treat principle.14 To be eligible for this cohort analysis, patients from CLL3 and from the FCR arm of CLL8 had to meet the following criteria at baseline: age of 60 years or younger, chemotherapy-naive, and complete central genomic workup (FISH and IGHV status) available. Patients with deletion 17p were excluded. Primary objective for this secondary study was to find evidence whether autoSCT could provide superior PFS and time to CLL-specific retreatment (TTRT) over FCR.
Statistical analyses
Pearson χ2 test was used to compare categorical factors between 2 groups of patients. Survival times from start of study treatment (CLL3) or randomization (CLL8) were calculated using the Kaplan-Meier method for OS, PFS, and TTRT, whereas cumulative incidence estimates were calculated in a competing risk framework for nonrelapse mortality (NRM). Events relevant for PFS were clinical progression, disease recurrence, or death from any cause. Events relevant for TTRT were any CLL-specific treatments after or off protocol-defined treatment. Events determining NRM were all deaths before clinical progression or disease recurrence. Kaplan-Meier curves were compared with the logrank test. Proportional hazards models (Cox regression) were fitted to investigate effects of prognostic factors for OS, PFS, and TTRT, with stepwise backward selection of variables using the likelihood ratio test. Patients undergoing allogeneic transplantation for CLL relapse were censored at allografting. Calculations were performed with SPSS (release 18.0; SPSS). Significance levels were set at .05. The CLL3 database was closed on November 30, 2009.
Results
Patients
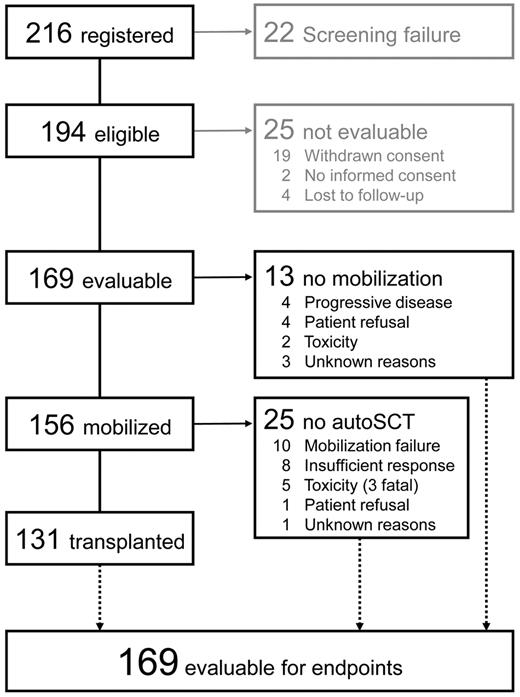
Between December 1996 and June 2002, 216 patients were registered by 52 centers from Germany and Austria. Of these, 22 patients had to be excluded because of ineligibility, and 25 patients were not evaluable for various reasons (Figure 1).
In the 169 evaluable patients, the median time from diagnosis was 13 months (0-156 months). Although the majority was chemotherapy naive at study entry, 15 patients (9%) had received previous chemotherapy for a maximum duration of 6 months (chlorambucil [12], cyclophosphamide-based [2], and fludarabine [1]). Details on genomic aberrations and other patient characteristics are listed in Table 1.
Patient characteristics at study entry (all eligible patients, n = 169)
| Characteristic . | Value . |
|---|---|
| Sex, female/male (%) | 28/141 (17/83) |
| Age, y | 51 (27-60) |
| IGHV unfavorable (%) | 87/91 (68) |
| Unmutated* (%) | 84/129 (65) |
| V3-21 mutated (%) | 4/129 (3) |
| FISH karyotype (hierarchical model) | |
| 17p- ± others (%) | 4/160 (2.5) |
| 11q- ± others (except 17p-) (%) | 40/160 (25) |
| +12 ± others (except 17p-, 11q-) (%) | 20/160 (12.5) |
| 13q- only (%) | 48/160 (30) |
| Others (%) | 20/160 (12.5) |
| Normal FISH karyotype (%) | 28/160 (17) |
| Binet stage A, B, C† (%) | 26, 94, 46 (16, 56, 28) |
| Time from diagnosis, mo | 13 (0-156) |
| Chemotherapy naïve (%) | 152/167 (91) |
| Serum thymidine kinase > 7 U/L (%) | 97/115 (84) |
| β2M > 3.5 mg/L (%) | 47/115 (41) |
| Lymphocyte doubling time < 12 mo (%) | 55/104 (53) |
| White blood count > 50/nL (%) | 92/162 (57) |
| Characteristic . | Value . |
|---|---|
| Sex, female/male (%) | 28/141 (17/83) |
| Age, y | 51 (27-60) |
| IGHV unfavorable (%) | 87/91 (68) |
| Unmutated* (%) | 84/129 (65) |
| V3-21 mutated (%) | 4/129 (3) |
| FISH karyotype (hierarchical model) | |
| 17p- ± others (%) | 4/160 (2.5) |
| 11q- ± others (except 17p-) (%) | 40/160 (25) |
| +12 ± others (except 17p-, 11q-) (%) | 20/160 (12.5) |
| 13q- only (%) | 48/160 (30) |
| Others (%) | 20/160 (12.5) |
| Normal FISH karyotype (%) | 28/160 (17) |
| Binet stage A, B, C† (%) | 26, 94, 46 (16, 56, 28) |
| Time from diagnosis, mo | 13 (0-156) |
| Chemotherapy naïve (%) | 152/167 (91) |
| Serum thymidine kinase > 7 U/L (%) | 97/115 (84) |
| β2M > 3.5 mg/L (%) | 47/115 (41) |
| Lymphocyte doubling time < 12 mo (%) | 55/104 (53) |
| White blood count > 50/nL (%) | 92/162 (57) |
Unmutated (> 98% homology to germline).
At initiation of first-line treatment.
Treatment
One hundred sixty-one of 167 patients with information available underwent initial cytoreduction with a CLL standard regimen: CHOP, 93 (56%; median, 3 cycles; range, 2-6); fludarabine monotherapy, 14 (8%; median, 3 cycles; range, 1-5); and FC, 54 (32%; median, 3 cycles; range, 1-6).
Whereas 13 patients (8%) did not undergo mobilization because of various reasons (Figure 1), 156 patients proceeded to Dexa-BEAM mobilization. The median CD34+ yield was 12 (0-81) × 106/kg within 2 (0-6) leukaphereses. One hundred ten patients (70%) collected 5 × 106/kg CD34+ cells or more. Thirteen of 156 patients (8%) failed to collect 2 × 106/kg CD34+ cells or more; precluding autoSCT in 10 of them. FC cytoreduction tended to be associated with a reduced mobilization yield (Table 2). Collection products were subjected to immunomagnetic B-cell depletion in 106 patients (Isolex + negative selection, 46; Isolex alone, 22; MACS, 36; and other, 2). Of the 55 patients for whom postpurging B-cell counts were available, 23 (42%) were reported to have no flow cytometry-detectable B cells in the final product. The autografts of the remaining 32 patients contained 2.07 × 103/kg (0.03-336 × 103/kg) B cells after purging. AutoSCT was performed in 131 of 169 patients (78%), whereas 22% did not proceed to transplantation as detailed in Figure 1. Of note, all of the 4 patients with 17p- could not be transplanted because of progressive disease (3) or mobilization failure (1). In contrast, 11q- did not affect the probability of reaching autoSCT compared with the remaining patients (33 of 40 patients [83%] vs 98 of 125 patients [78%]; P = .58).
Mobilization yields by cytoreductive regimen
| Cytoreductive regimen . | None . | CHOP . | Fludarabine . | FC . |
|---|---|---|---|---|
| No. of patients undergoing Dexa-BEAM mobilization | 6 | 87 | 14 | 49 |
| Median CD34 yield × 106/kg (range) | 21.6 (12-36.1) | 14.5 (0-52.7) | 12 (0-81) | 9.8 (0-60.3)* |
| No. of patients failing CD34+ mobilization target (2 × 106/kg) | 0 | 4 | 2 | 7† |
| Cytoreductive regimen . | None . | CHOP . | Fludarabine . | FC . |
|---|---|---|---|---|
| No. of patients undergoing Dexa-BEAM mobilization | 6 | 87 | 14 | 49 |
| Median CD34 yield × 106/kg (range) | 21.6 (12-36.1) | 14.5 (0-52.7) | 12 (0-81) | 9.8 (0-60.3)* |
| No. of patients failing CD34+ mobilization target (2 × 106/kg) | 0 | 4 | 2 | 7† |
P = .064 (vs CHOP).
P = .057 (vs CHOP).
Median time to neutrophil recovery more than 0.5/nL after autoSCT was 10 days (8-16 days) in 95 patients who received G-CSF and 15 days (11-37 days) in 18 patients without G-CSF stimulation. Time to an unsupported platelet count more than 20/nL was 10 days (5-40 days). One patient failed to engraft but could be reconstituted with the unpurged backup. Median duration of hospitalization after reinfusion was 15 days (10-59 days).
Nonhematologic toxicity
Grade 3 or 4 nonhematologic toxicity was reported in 10 patients (6%) during cytoreduction and in 39 patients (25%) during mobilization. Dexa-BEAM–related severe adverse events (SAEs) were infections in 28 patients (18%). Sixty episodes of grade 3 or 4 nonhematologic adverse events were documented in 51 patients (39%) after autoSCT, largely because of infection (20 patients) and gastrointestinal toxicity (36 patients; supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Secondary malignancies
Altogether, 20 secondary malignancies were observed, with the most frequent malignancies being treatement-related myelodysplastic syndromes (t-MDS)/treatment-related acute myelogenous leukemia (t-AML; n = 6) and gastrointestinal cancers (n = 3), translating into a 10-year incidence of 19% (95% CI, 5%-39%). There was no significant difference in the 10-year incidence of any secondary malignancy among individuals treated with and without autoSCT (P = .73). However, all cases of t-MDS/t-AML occurred after autoSCT, yielding a 10-year incidence rate of t-MDS/t-AML of 8% (95% CI, 0%-39%). Whereas 10 secondary malignancies were observed in the absence of CLL relapse, 10 occurred after CLL recurrence, of which 8 (including 3 t-MDS/AML cases) were observed only after CLL-specific retreatment (supplemental Table 3). OS after onset of secondary neoplasm was 11 months.
Causes of death and survival
Altogether, 57 patients died, 44 subsequent to disease progression, and 13 of nonrelapse causes (infections related to Dexa-BEAM, 3; infections related to autoSCT, 3; t-MDS/AML, 2; secondary cancers, 2; suicide, 1; and unknown, 2; supplemental Table 2). The cumulative incidence of NRM was 2% after 1 year, 4% after 2 years, 6.5% after 5 years, and 14% after 10 years. With a median follow-up of 8.7 years (0.3-12.3 years), median OS of all 169 patients was 11.3 years (Figure 2).
PFS and OS of CLL3 patients. PFS (left) and OS (right) of all 169 evaluable patients registered for CLL3 (A-B) according to IGHV status (C-D) and FISH karyotype (hierarchical model; E-F).
PFS and OS of CLL3 patients. PFS (left) and OS (right) of all 169 evaluable patients registered for CLL3 (A-B) according to IGHV status (C-D) and FISH karyotype (hierarchical model; E-F).
Disease control and MRD
Best response (measured 3 months after autoSCT or at time of going off study in those who did not complete the study treatment) was complete response in 103 patients and partial response in 29 patients, giving an overall response rate of 92% (complete response, 72%) of all 143 patients evaluable for response.
Pre- and posttransplant molecular MRD results were available in 52 patients. Median MRD levels at study entry, at 3 months and at 12 months after autoSCT, were 0.615 (0.022-41), 0.00012 (undetectable, 0.0093), and 0.00011 (undetectable, 0.02). Six patients were MRD-negative at both posttransplant time points. Whereas 2 of these patients had clinical disease recurrence 7.3 and 8.5 years after study entry, respectively, 4 remained relapse-free with 4.3-, 6.8-, 8.8-, and 10.9-year follow-up.
Considering all 169 patients, clinical disease progression or relapse after study entry was observed in 99 patients, resulting in median PFS of 5.7 years (Figure 2). The median time from study entry to CLL-specific retreatment or death was 7.3 years.
Retreatment
Retreatment on CLL progression was not part of the protocol and was left at the discretion of the individual investigators. CLL-specific retreatment was documented for 77 patients. Of these, 21 (27%) received an FCR-like salvage regimen, whereas 37 patients (48%) were treated with FC-like regimens. The remaining patients were salvaged with monotherapy (chlorambucil, fludarabine, bendamustin, rituximab; n = 6), lymphoma-type regimens (n = 8), and/or alemtuzumab (n = 12). There was no significant survival difference between patients salvaged with FCR and FC, respectively. Twenty of the 77 retreated patients (26%) underwent allogeneic SCT. With a median observation time of surviving patients of 5.7 years (0.7-8.7 years), 5-year OS after start of first salvage regimen of allografted patients was 77% (95% CI, 50%-100%; supplemental Figures 1-3). The median OS from start of salvage of all 77 patients was 3.1 years.
Prognostic factor analyses
By univariate logrank comparisons, PFS was significantly affected by a high leukocyte count (median PFS, 5.0 vs 7.7 years; P = .002), unfavorable IGHV (4.9 years vs not reached; P < .0001), and 17p- (median PFS for 17p-, 11q-, +12, 13q- single, and other karyotypes, 0.7 vs 5.5, 4.8, 7.3, and 6.7 years). Except for 17p- (P < .0001 for 17p- vs all other FISH categories), all differences between the individual FISH categories were not significant (Figure 2). Similarly, Binet stage, sex, and age had no significant impact on PFS. Patients receiving CHOP cytoreduction tended to have a better PFS than patients treated with FC (7.3 vs 4.9 years; P = .056). In the 55 patients with information available, PFS of patients receiving a graft without detectable B cells was not significantly longer than that of patients reconstituted with MRD-positive grafts (7.3 vs 6.5 years; P = .24; supplemental Figure 4). Multivariate analysis confirmed the adverse effects of unfavorable IGHV and 17p- but not of high leukocyte count and FC cytoreduction on PFS (Table 3).
CLL3 trial: multivariate prognostic factor analysis (Cox backward; n = 121)
| End point . | Variable . | P . | HR . | Lower CL . | Upper CL . |
|---|---|---|---|---|---|
| PFS | 17p- | < .001 | 19.08 | 5.53 | 65.83 |
| IGHV unfavorable | < .001 | 3.35 | 1.87 | 5.99 | |
| OS | 17p- | < .001 | 8.35 | 2.56 | 27.22 |
| IGHV unfavorable | .006 | 3.11 | 1.37 | 7.02 | |
| Binet stage B | .076 | 6.12 | 0.83 | 45.29 | |
| Binet stage C | .025 | 10.25 | 1.33 | 78.98 | |
| Age (/y) | .067 | 1.047 | 0.997 | 1.099 |
| End point . | Variable . | P . | HR . | Lower CL . | Upper CL . |
|---|---|---|---|---|---|
| PFS | 17p- | < .001 | 19.08 | 5.53 | 65.83 |
| IGHV unfavorable | < .001 | 3.35 | 1.87 | 5.99 | |
| OS | 17p- | < .001 | 8.35 | 2.56 | 27.22 |
| IGHV unfavorable | .006 | 3.11 | 1.37 | 7.02 | |
| Binet stage B | .076 | 6.12 | 0.83 | 45.29 | |
| Binet stage C | .025 | 10.25 | 1.33 | 78.98 | |
| Age (/y) | .067 | 1.047 | 0.997 | 1.099 |
Covariates considered for all end points were 17p- vs other karyotypes; IGHV unfavorable vs favorable; Binet stage A, B, C; leukocyte count > 50/nL vs less; sex; cytoreductive regimen (FC, F, CHOP, none); and age. Only covariates remaining in the final models are listed.
HR indicates hazard ratio.
Predictors of a shorter OS according to univariate comparisons were Binet stage (median OS not reached for Binet A vs B 10.3 years vs C 10.6 years; P = .043), age (hazard ratio per year 1.034; P = .098), high leukocyte count (10.3 years vs not reached; P = .03), unfavorable IGHV (8.3 years vs not reached; P = .04), and 17p- (1.0 year vs > 10 years for all other FISH categories; P < .0001; Figure 2). Survival was not significantly affected by CHOP vs FC (11.3 vs 9.2 years; P = .14). Of these variables, 17p-, IGHV, Binet stage, and age remained in the final model of the multivariate Cox analysis (Table 3).
Comparative survival analyses of CLL3 and CLL8(FCR) trial populations
One hundred ten of 169 patients from the CLL3 trial and 126 of 408 patients from the CLL8(FCR) trial met the inclusion criteria as described under “Patients” for this posthoc secondary study. Both cohorts were well matched for age, time from diagnosis to study entry, serum thymidine kinase levels, FISH risk group, and IGHV status. However, CLL3 patients had significantly higher β2M serum levels, were significantly more likely to be male and to be in Binet A at study entry, and were less likely to have a high leukocyte count (Table 4).
Characteristics of patients eligible for CLL3/CLL8(FCR) comparison
| Characteristic . | CLL3 . | CLL8(FCR) . | P . |
|---|---|---|---|
| n | 110 | 126 | |
| Trial entry (calendar year) | 2000 (1996-2002) | 2005 (2003-2006) | < .001 |
| Sex, female/male (%) | 15/95 (14/86) | 37/89 (29/71) | .004 |
| Age, y | 52 (30-60) | 53 (30-60) | .049 |
| IGHV unfavorable (%) | 70 (64) | 85 (68) | .54 |
| FISH karyotype (hierarchical model) | .18 | ||
| 11q- ± others (%) | 26 (24) | 38 (30) | |
| +12 ± others (except 11q-) (%) | 16 (15) | 9 (7) | |
| 13q- single (%) | 34 (31) | 46 (37) | |
| Others (including normal) (%) | 34 (31) | 33 (26) | |
| Binet stage A, B, C (%) | 16, 62, 31 (15, 57, 28) | 4, 83, 39 (3, 66, 31) | .007 |
| Time from diagnosis, mo | 12 (0-156) | 18 (0-160) | .18 |
| Serum thymidine kinase, U/L | 15.0 (3.0-469) | 18.9 (2.8-259) | .18 |
| β2M, mg/L | 3.3 (0-10) | 2.7 (1.2-7.3) | .008 |
| White blood count > 50/nL (%) | 60/110 (55) | 88/126 (70) | .015 |
| Proportion of patients undergoing complete study treatment (%) | 90/110 (82) | 104/126 (83) | 1.0 |
| Observation time of surviving patients, y | 8.3 (0.4-11.5) | 4.7 (0.3-6.2) |
| Characteristic . | CLL3 . | CLL8(FCR) . | P . |
|---|---|---|---|
| n | 110 | 126 | |
| Trial entry (calendar year) | 2000 (1996-2002) | 2005 (2003-2006) | < .001 |
| Sex, female/male (%) | 15/95 (14/86) | 37/89 (29/71) | .004 |
| Age, y | 52 (30-60) | 53 (30-60) | .049 |
| IGHV unfavorable (%) | 70 (64) | 85 (68) | .54 |
| FISH karyotype (hierarchical model) | .18 | ||
| 11q- ± others (%) | 26 (24) | 38 (30) | |
| +12 ± others (except 11q-) (%) | 16 (15) | 9 (7) | |
| 13q- single (%) | 34 (31) | 46 (37) | |
| Others (including normal) (%) | 34 (31) | 33 (26) | |
| Binet stage A, B, C (%) | 16, 62, 31 (15, 57, 28) | 4, 83, 39 (3, 66, 31) | .007 |
| Time from diagnosis, mo | 12 (0-156) | 18 (0-160) | .18 |
| Serum thymidine kinase, U/L | 15.0 (3.0-469) | 18.9 (2.8-259) | .18 |
| β2M, mg/L | 3.3 (0-10) | 2.7 (1.2-7.3) | .008 |
| White blood count > 50/nL (%) | 60/110 (55) | 88/126 (70) | .015 |
| Proportion of patients undergoing complete study treatment (%) | 90/110 (82) | 104/126 (83) | 1.0 |
| Observation time of surviving patients, y | 8.3 (0.4-11.5) | 4.7 (0.3-6.2) |
With a median observation time of surviving patients of 8.3 and 4.7 years, respectively, PFS of the CLL3 group was significantly longer than that of the CLL8(FCR) group (median, 6.2 vs 4.3 years; P = .009; Figure 3). This effect remained significant after multivariate adjustment for potential confounders (Binet stage, age, IGHV status, FISH karyotype, leukocyte count; Table 5). However, TTRT and OS were not different between autoSCT and FCR (median, 7.7 years vs not reached; P = .91; and 4-year OS 86% [95% CI, 80%-93%] vs 90% [95% CI, 84%-95%]; P = .39; Figure 3). Unfavorable IGHV status was the only factor significantly affecting TTRT in multivariate analysis, whereas OS was adversely influenced by unfavorable IGHV, Binet C stage, and age but not by treatment (Table 5). Subgroup analyses did not reveal a significant TTRT benefit for autoSCT in any Binet stage, IGHV and FISH category. NRM was not significantly different between autoSCT and FCR (4-year NRM, 7% [95% CI, 1.9%-12.2%] vs 4% [95% CI, 0.5%-7.9%]; P = .68).
Patient analyses. PFS (A), TTRT (B), OS (C), and NRM (D) of 110 CLL3 patients (green) and 126 CLL8(FCR) patients (blue) matched for age and absence of pretreatment.
Patient analyses. PFS (A), TTRT (B), OS (C), and NRM (D) of 110 CLL3 patients (green) and 126 CLL8(FCR) patients (blue) matched for age and absence of pretreatment.
CLL3/CLL8(FCR) comparison: multivariate prognostic factor analysis (Cox backward; n = 234)
| End point . | Variable . | P . | HR . | Lower CL . | Upper CL . |
|---|---|---|---|---|---|
| PFS | IGHV unfavorable | < .001 | 3.81 | 2.39 | 6.05 |
| Treatment CLL8(FCR) | .004 | 1.79 | 1.21 | 2.65 | |
| TTRT | IGHV unfavorable | < .001 | 3.88 | 2.22 | 6.77 |
| OS | IGHV unfavorable | .001 | 3.23 | 1.62 | 6.44 |
| Binet stage B | .083 | 5.85 | 0.79 | 43.1 | |
| Binet stage C | .014 | 12.62 | 1.69 | 94.42 | |
| Age (y) | .017 | 1.053 | 1.009 | 1.098 |
| End point . | Variable . | P . | HR . | Lower CL . | Upper CL . |
|---|---|---|---|---|---|
| PFS | IGHV unfavorable | < .001 | 3.81 | 2.39 | 6.05 |
| Treatment CLL8(FCR) | .004 | 1.79 | 1.21 | 2.65 | |
| TTRT | IGHV unfavorable | < .001 | 3.88 | 2.22 | 6.77 |
| OS | IGHV unfavorable | .001 | 3.23 | 1.62 | 6.44 |
| Binet stage B | .083 | 5.85 | 0.79 | 43.1 | |
| Binet stage C | .014 | 12.62 | 1.69 | 94.42 | |
| Age (y) | .017 | 1.053 | 1.009 | 1.098 |
Covariates considered for all end points were treatment CLL8(FCR) vs CLL3; FISH karyotype (11q-, +12, 13q- single, all other categories pooled); IGHV unfavorable vs favorable; Binet stage A, B, C; leukocyte count > 50/nL vs less; and age. Only covariates remaining in the final models are listed.
HR indicates hazard ratio.
Discussion
The CLL3 trial was designed in the mid-1990s before fludarabine was approved for treatment of CLL and before validated genomic prognostic markers were available. Based on promising pilot data from single center studies, the hypothesis was that high-dose treatment including myeloablative total body irradiation could eventually cure the disease, in particular if administered early.1,4 The rationale behind it was that this could have been the first successful strategy to change the natural course of CLL in the absence of effective drugs at that time. Apart from its size and central genomic workup and MRD monitoring, unique features of the CLL3 study were the eligibility of high-risk patients without classic treatment indication, and the Dexa-BEAM intensification step, which was implemented to improve mobilization efficacy and disease control before autoSCT. Altogether, the CLL3 design represents the most aggressive autoSCT approach investigated in the “earliest” patient population to date.
The PBSC yield observed here compares favorably with that obtained in other studies relying largely on mobilization with cyclophosphamide and G-CSF.9,10,25 This resulted not only in a relatively high proportion of patients proceeding to transplant (78% in CLL3 vs 56% in the MRC pilot trial9 and 72% in the European Group for Blood & Marrow Transplantation (EBMT) trial, which included patients in remission only10 ) but also allowed ex vivo purging in 68% of all patients subjected to mobilization. This proportion is considerably higher than reported for the MRC trial (15 of 88; 17%).9 In contrast to previous studies, we were unable to demonstrate a correlation between purging efficacy and posttransplant disease control.26 However, because this was not a predefined end point and purging as well as graft MRD assessment was not standardized, our results do not allow valid conclusions on this issue.
On the reverse side, Dexa-BEAM was relatively toxic and caused nonhematologic SAEs in 25% of the patients, including 3 fatalities. This contributed to NRM associated with the study intervention, which was below 10% at 5 years and thereby within the anticipated range (and not significantly different from that observed in the CLL8 [FCR] patients). However, with 25% and 39% nonhematologic SAEs after Dexa-BEAM and autoSCT, respectively, and 100% grade 4 hematotoxicity in all autografted patients, the treatment strategy investigated here seems to be clearly more toxic than current standard treatments, such as FCR.14 The risk of secondary malignancies was within the expected range.
Because of the absence of a plateau in the PFS curve and the failure to achieve MRD negativity in the majority of patients assessed, it seems that leukemia eradication can obviously not be achieved by early myeloablative treatment in the majority of patients with poor-risk CLL as defined here. This is particularly evident in the patients with unfavorable IGHV status: As demonstrated here for the first time prospectively on a large homogeneously autografted patient cohort analyzed by intent-to-treat, unmutated IGHV remained a strongly adverse prognostic factor for all survival end points examined. This is in keeping with previous results from retrospective studies19 and with recent data from the French part of the EBMT trial.11 In contrast, it seems that autoSCT can at least partially overcome the poor prognostic impact of 11q-. Similar effects, however, have been observed with chemoimmunotherapy.14 Contradictory to our results, 11q- was found to be an adverse factor for relapse in the EBMT study, but this was based on only 5 patients in the autoSCT arm.10
Nevertheless, PFS in CLL3 was encouraging and at least comparable with results reported from previous autoSCT studies.2,9,10 Indeed, compared with a matched population from the CLL8(FCR) arm by intent to treat (ie, including all patients who did not undergo autoSCT because of CLL progression or other reasons), PFS of CLL3 patients was significantly better, thereby resembling results from the EBMT trial. In the prospective randomized EBMT study, autoSCT as consolidation in first or second remission after standard chemotherapy (ie, excluding all patients with refractory disease) was shown to halve the risk of relapse compared with observation.10 Similar PFS advantages for autoSCT over conventional chemotherapy were observed in a recently published much smaller randomized trial by the French GOELAMS group.15 However, virtually no patient of the control arm in the EBMT and French trials was treated with FCR which is the current “standard” for first-line treatment in fit patients.
Surprisingly, and in contrast to the EBMT trial, the substantial PFS benefit of CLL3 over CLL8(FCR) did not translate into a longer TTRT, neither in the population as a whole nor in any genomic subset. The most likely explanation for this is the more intense follow-up monitoring applied in the CLL8 compared with CLL3: In CLL8, patients underwent follow-up examinations every 3 months for the first 3 years, every 6 months for the next 2 years, and yearly thereafter.14 In contrast, mandatory follow-up visits were scheduled only every 12 months after the first year in CLL3. This may have resulted in the fact that “soft” events such as disease progression may have remained longer undetected in CLL3, whereas “hard” events such as retreatment were reported on time. (In both trials retreatment after disease progression was undefined.)
However, the fact that OS in CLL3 was at least similar to that after CLL8(FCR) suggests that not only is autoSCT is an excellent treatment option but also that the efficacy of salvage regimens given to relapsed autografted patients is not compromised compared with patients having failed first-line FCR.
It has to be kept in mind that this was a retrospective comparison of 2 separate prospective trials from different times and that the inclusion criteria for CLL3 and CLL8 were not exactly the same (favoring CLL3 by including asymptomatic patients and significantly more patients in Binet stage A). Moreover, there was a substantial difference in observation time between the 2 trials. Nevertheless, both populations were well comparable in terms of the most important prognostic factors in CLL, such as age and centrally determined IGHV status and FISH karyotype. Moreover, bias introduced by imbalances in stage and biologic risk factors could be minimized by multivariate adjustment.
In conclusion, the CLL3 trial demonstrates with the largest patient population and the longest follow-up available for autoSCT studies to date that “early” autoSCT is a very effective therapy, but it does not seem to be curative even if performed with Dexa-BEAM intensification and vigorous ex vivo purging. Although its toxicity is manageable, it is substantial, thereby making it difficult to recommend autoSCT as appropriate first-line consolidation in the FCR era. One might argue that combination of FCR with autoSCT could improve disease control and eventually eradicate the leukemic clone. However, it seems to be not very likely that simple intensification of traditional cytotoxic approaches can achieve this goal. Instead, novel approaches targeting essential signaling pathways of clonal B cells in concert with the chemoimmunotherapies already available hold promise to achieve better and longer lasting control of the malignant clone in CLL.27,28
The online version of this article contains a data supplement.
Presented in part in abstract form at the 51st Annual Meeting of the American Society of Hematology, New Orleans, LA, December 8, 2009; at the 11th Conference on Malignant Lymphoma, Lugano, Switzerland, June 17, 2011; and at the XIV International Workshop on CLL (iwCLL), Houston, TX, October 29, 2011.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all GCLLSG centers participating in the CLL3 and CLL8 trials. The CLL3 trial has been registered with the US National Cancer Institute (protocol identity NCT00275015).
This study was supported by grants from the Deutsche Jose-Carreras Leukämiestiftung e.V. Projects R02/18 and R05/02 (M.R., P.D., and M.K.).
Authorship
Contribution: P.D. designed and performed research, provided patients and samples, gave administrative support, analyzed data, and wrote the paper; H.D. designed research and gave administrative support; F.M. collected patient data, performed research, and contributed to writing the paper; R.B. performed the statistical analysis; M.R. contributed analytical tools and analyzed data; A.-M.F. collected patient data; H.G., W.K., M.S., M.P., U.D., M. Hensel, and J.S. provided patients and samples; G.B. provided patients and samples and designed research; D.W. and A.B. contributed analytical tools and analyzed data; M.K. provided patients and samples, gave administrative support, contributed analytical tools, and analyzed data; N.S. provided patients and samples and designed research; M. Hallek provided patients and samples and gave administrative support; and S.S. designed and performed research, provided patients and samples, gave administrative support, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: P.D. served as an advisory board member and did consultancy work for Baxter; received speaking honoraria from Bayer, Roche, and Novartis; and received research funding from Bayer. M.K. served as an advisory board member and did consultancy work for Roche, and received research funding from Roche and Novartis. S.S. received speaking honoraria and research funding from Amgen, Bayer, Celgene, GlaxoSmithKline, and Roche. The remaining authors declare no competing financial interests.
For a complete list of the CLL3 investigators, see the online supplemental Appendix.
Correspondence: Peter Dreger, Department of Medicine V, University of Heidelberg, Im Neuenheimer Feld 410, 69120 Heidelberg, Germany; e-mail: peter.dreger@med.uni-heidelberg.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal