Abstract
The importance of morphogenetic proteins (BMPs) and their antagonists in vascular development is increasingly being recognized. BMP-4 is essential for angiogenesis and is antagonized by matrix Gla protein (MGP) and crossveinless 2 (CV2), both induced by the activin receptor like-kinase 1 (ALK1) when stimulated by BMP-9. In this study, however, we show that CV2 preferentially binds and inhibits BMP-9 thereby providing strong feedback inhibition for BMP-9/ALK1 signaling rather than for BMP-4/ALK2 signaling. CV2 disrupts complex formation involving ALK2, ALK1, BMP-4, and BMP-9 required for the induction of both BMP antagonists. It also limits VEGF expression, proliferation, and tube formation in ALK1-expressing endothelial cells. In vivo, CV2 deficiency translates into a dysregulation of vascular BMP signaling, resulting in an abnormal endothelium with increased endothelial cellularity and expression of lineage markers for mature endothelial cells. Thus, mutual regulation by BMP-9 and CV2 is essential in regulating the development of the vascular endothelium.
Introduction
Vascular development is essential in embryogenesis, organogenesis, and wound healing, where the importance of bone morphogenetic protein 4 (BMP-4) and BMP-9 is increasingly being recognized.1-4 BMP-4 stimulates angiogenesis, and BMP-9 regulates maturation of endothelial cells (ECs), in part mediated by the VEGF. BMP-4 and BMP-9 are linked through the activin receptor–like kinase 1 (ALK1). BMP-4 induces ALK1, which subsequently is stimulated by its ligand BMP-9.2,4-6 Thus, ALK1 expression and activation are central steps in angiogenesis,4 which is further supported by the link between mutations in the ALK1 gene and hereditary hemorrhagic telangiectasia (HHT),7,8 a disease characterized by arteriovenous malformations (AVMs).
Matrix Gla protein (MGP) and Crossveinless 2 (CV2; also referred to as BMP-binding endothelial regulator [BMPER]) are 2 BMP-binding proteins9 expressed in ECs and able to modulate local BMP activity.5,10 MGP is induced by BMP-4 in ECs and antagonizes BMP-2, BMP-4, and BMP-7.2,11 It inhibits angiogenesis through feedback inhibition of BMP-4,9,12 and lack of MGP results in AVMs similar to ALK1 deficiency.11 MGP also functions as an inhibitor of vascular calcification in elastic arteries, atherosclerotic lesions, and diabetic vasculopathy.13-15 CV2 is a member of the Chordin family and binds to BMP-2, BMP-4, and BMP-6, but has been reported to act both as a BMP agonist and antagonist depending on the context.9,16 It is differentially expressed in endothelial precursor cells, and appears in that context to antagonize BMP signaling and EC differentiation.10,17 However, it is not clear how CV2 antagonizes EC differentiation, or how it functionally relates to, or potentially overlaps with the function of MGP in the vasculature.
Here, we show that CV2 preferentially binds and inhibits BMP-9, thereby providing strong feedback inhibition for BMP-9/ALK1 signaling rather than for BMP-4/ALK2 signaling. Furthermore, we show that induction of both MGP and CV2 depends on BMP-9/ALK1 signaling, with expression of MGP preceding that of CV2. CV2 disrupts complex formation between BMP-4 and BMP-9, and their receptors ALK2 and ALK1, thereby blocking further induction of the 2 BMP antagonists. In addition, CV2 limits expression of VEGF, proliferation, and tube formation in ALK1-expressing ECs. Lack of CV2 in mice translates into dysregulated vascular BMP signaling and an abnormal endothelium with increased cellularity and expression of EC markers. Together, our data suggest that MGP and CV2 are 2 BMP antagonists that work in tandem and provide important feedback inhibition for BMP-4 and BMP-9, respectively. BMP-4 and MGP regulate the availability of the ALK1 receptor, and BMP-9 and CV2 regulate its state of activation.
Methods
Animals
Alk1+/− mice on a C57BL6/J background were obtained from The Jackson Laboratory. Mgp+/− mice on a C57BL/6J background were obtained from Dr Cecilia Giachelli (University of Washington, Seattle, WA) with the permission of Dr Gerard Karsenty (Columbia University, New York, NY). Cv2+/− mice were obtained from Dr Edward De Robertis (University of California, Los Angeles, CA). All mice were fed a standard chow diet (Teklad rodent diet; Harlan Laboratories). The investigation conformed to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health), and had been reviewed and approved by the Institutional Review Board of the University of California, Los Angeles.
Cell culture and siRNA transfection
Human aortic endothelial cells (HAECs) and bovine aortic endothelial cells (BAECs) were prepared and cultured as previously described.5,18 For treatment, cells were seeded at a confluence of 50% to 80%, and treatments were added to the media 20-24 hours later. Transient transfections of HAECs with siRNA were performed with Lipofectamine 2000 (Invitrogen) using 60nM siRNA as previously described.5 Three separate siRNAs to ALK1 and SMAD1 (Silencer predesigned siRNA; Ambion) and scrambled siRNA with the same nucleotide content were tested. The siRNA that provided the most efficient inhibition (90%-95%) was used for experiments, and treatments were usually initiated 24 hours after the start of the transfection. Transient transfections of BAECs with an expression vector for HA-tagged ALK1 were performed with FuGENE 6 (Roche Applied Science) as previously described.5
RNA analysis
Coimmunoprecipitation
Coimmunoprecipitation using recombinant proteins or cell lysates was performed as previously described.20,21 We used recombinant human CV2, BMP-4, BMP-9, and ALK1-Fc chimeric protein (0.2 or 1 μg/mL; all from R&D Systems). We used Abs to CV2 (2 μg/mL; R&D Systems), BMP-9 and BMP-4 (both 0.4 μg/mL; R&D Systems), ALK1 and ALK2 (both 0.2 μg/mL; Santa Cruz Biotechnology), biotin (0.02 μg/mL; Cell Signaling Technology), and nonspecific IgG (0.2 μg/mL; Santa Cruz Biotechnology). Immunoprecipitated complexes were analyzed by immunoblotting as described in “Immunoblotting.”
Crosslinking and biotin labeling
Human recombinant CV2, BMP-9, and BMP-4 proteins were used in cross-linking experiments as previously described.21,22 CV2 (100 ng) was mixed with BMP-9 (50 ng) or BMP-4 (50 ng), chemically cross-linked with disuccinimidyl suberate (DSS) and the cross-linked products were analyzed by immunoblotting as described in the next section. In some experiments, up to 20-fold excess of BMP-4 was used, which corresponded to 1000 ng of BMP-4. Biotin labeling of BMP-9 was performed using the Biotin-XX Microscale protein labeling kit (Molecular Probes) as per the manufacturer's instructions.
Immunoblotting
Immunoblotting was performed as previously described.13 Equal amounts of cellular protein or culture medium were used. Blots were incubated with specific Abs to pSMAD1/5/8, pSMAD2/3 (both diluted to 0.4 μg/mL; Cell Signaling Technology), total SMAD, ALK1, ALK2, and ALK5 (all diluted to 0.4 μg/mL; Santa Cruz Biotechnology), BMP-4, BMP-9, and CV2 (all 0.2 μg/mL; R&D Systems), CD31 (PECAM-1; 2 μg/mL; Millipore), VE-cadherin (0.4 μg/mL; Santa Cruz Biotechnology), TGF-β1 (2 μg/mL; Sigma-Aldrich), and biotin (0.02 μg/mL; Cell Signaling Technology). β-actin (1:5000 dilution; Sigma-Aldrich) was used as loading control. For optimal detection of VEGF in culture media, VEGF was first immunoprecipitated with anti-VEGF Abs (Santa Cruz Biotechnology), as previously described19 and then analyzed by immunoblotting using specific Abs to VEGF (0.2 μg/mL; R&D Systems). The specificity of all Abs was verified before use for immunoblotting as previously described.13
Proteins associated with the plasma membrane in HAECs were separated from the intracellular proteins using biotinylation of cell-surface proteins and immunoblotting as previously described.23 Blots were incubated with specific Abs to pan-cadherin, calnexin, and cytochrome c oxidase (COX) IV (all obtained from Cell Signaling Technology and diluted 1:1000).
Immunofluorescence
Tissue sections were processed and stained as previously described in detail.13,14,24 Images were acquired with a microscope (Axiovert 200) equipped with imaging software (AxioVision 4.6; Carl Zeiss), using 4× and 20× objective lenses, at room temperature. A charge-coupled device camera (CoolSnap fx; Photometrics) was used for immunofluorescence staining, and a microscopy camera (AxioCam MRc; Carl Zeiss) was used for immunohistochemistry or immunocytochemistry staining. All images within the same panel were processed similarly using Adobe Photoshop CS3 (Version 10.0.1). We used specific Abs to MGP (provided by Dr Reidar Wallin, Wake Forest University, Winston-Salem, NC), pSMAD1/5/8, pSMAD2/3 (both from Cell Signaling Technology), total SMAD, ALK1, ALK2, ALK5, VEGF (all from Santa Cruz Biotechnology), and CV2 and BMP-4 (both from R&D Systems).
Proliferation assay and tube formation assays
HAECs were seeded in 24-well plates at a density of 100 000 cells/well, and allowed to attach for 4-6 hours. 3H-Thymidine was added at 1 μCi/mL for 2 days, and 3H-thymidine incorporation was determined as previously described.18 Tube formation assays in Matrigel Matrix (BD Biosciences) were performed as previously described.2
Statistical analysis
Data were analyzed for statistical significance by 2-way ANOVA with posthoc Tukey analysis. The analyses were performed using GraphPad Instat 3.0 software (GraphPad Software). P values < .05 were considered significant. All experiments were repeated a minimum of 3 times.
Results
BMP-9 induces CV2 expression in endothelial cells
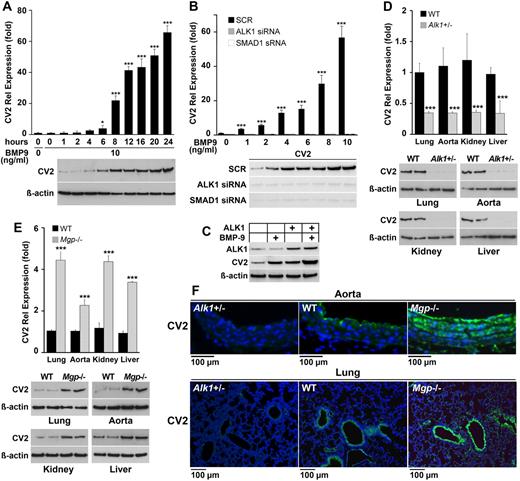
In previous studies in ECs, we found that BMP-4 rapidly induced expression of the ALK1 receptor by acting on the closely related ALK2 receptor.5,6 Once expressed, ALK1 is stimulated by BMP-9, which is a circulating BMP.25 Activation of BMP-9/ALK1 signaling resulted in a peak of MGP expression ∼ 6 hours later.2 MGP provides negative feedback inhibition for BMP-4 thereby limiting the ALK1 expression. We recently found that increased BMP activity in the vasculature also stimulated expression of CV2 in atherosclerotic mice.13 Because CV2 has been reported to antagonize EC differentiation, we hypothesized that BMP-9/ALK1 signaling affected CV2 expression. To test this hypothesis, we treated HAECs with BMP-9 (10 ng/mL) and collected RNA and protein for up to 24 hours. The result showed that BMP-9 strongly induced CV2 > 60-fold after 24 hours, as determined by real-time PCR and immunoblotting (Figure 1A). To test whether BMP-9 induced CV2 through SMAD1/5/8 activation, we depleted ALK1 or SMAD1 using siRNA in HAECs and compared with scrambled control siRNA. The siRNA depleted ALK1 and SMAD1 to < 20% of the original levels (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).5,6 The cells were subsequently treated with BMP-9 (0-10 ng/mL) for 24 hours, starting 24 hours after transfection. Depletion of ALK1 or SMAD1 siRNA both abolished the CV2 induction (Figure 1B). Furthermore, overexpression of ALK1 in BAECs enhanced the CV2 expression, especially when combined with treatment with BMP-9 (10 ng/mL) starting at the time of transfection, as shown by immunoblotting (Figure 1C). The results indicate that CV2 induction is mediated by ALK1 and SMAD1.
BMP-9 induces CV2 expression in endothelial cells. (A) Expression of CV2 after treatment of HAECs with BMP-0 (10 ng/mL) for 24 hours, as determined by real-time PCR and immunoblotting. β-actin is shown for comparison. (B) Expression of CV2 after transfection of scrambled siRNA (SCR) or siRNA to ALK1 or SMAD1 in HAECs followed by treatment with BMP-9 (0-10 ng/mL), as determined by real-time PCR and immunoblotting. Treatment was started 24 hours after transfection and lasted 24 hours. (C) Expression of ALK1 and CV2 in BAECs after transfection of an ALK1 expression vector and treatment with BMP-9 (10 ng/mL), as determined by immunoblotting. Treatment was started at the time of transfection and lasted 24 hours. (D-E) Expression of CV2 in lungs, aorta, kidney, and liver of (D) wild-type (WT) and Alk1+/− mice, and (E)WT and Mgp−/− mice, as determined by real-time PCR and immunoblotting (n = 3). (F) Vascular expression of CV2 (green fluorescence) in aorta and lungs of Alk1+/−, WT, and Mgp−/− mice, as determined by immunofluorescence. Nuclei are visualized by DAPI (blue fluorescence). Asterisks indicate statistically significant differences compared with (A) start time, (B) no BMP-9, or (D-E) WT tissue. *P < .05, ***P < .001, Tukey test.
BMP-9 induces CV2 expression in endothelial cells. (A) Expression of CV2 after treatment of HAECs with BMP-0 (10 ng/mL) for 24 hours, as determined by real-time PCR and immunoblotting. β-actin is shown for comparison. (B) Expression of CV2 after transfection of scrambled siRNA (SCR) or siRNA to ALK1 or SMAD1 in HAECs followed by treatment with BMP-9 (0-10 ng/mL), as determined by real-time PCR and immunoblotting. Treatment was started 24 hours after transfection and lasted 24 hours. (C) Expression of ALK1 and CV2 in BAECs after transfection of an ALK1 expression vector and treatment with BMP-9 (10 ng/mL), as determined by immunoblotting. Treatment was started at the time of transfection and lasted 24 hours. (D-E) Expression of CV2 in lungs, aorta, kidney, and liver of (D) wild-type (WT) and Alk1+/− mice, and (E)WT and Mgp−/− mice, as determined by real-time PCR and immunoblotting (n = 3). (F) Vascular expression of CV2 (green fluorescence) in aorta and lungs of Alk1+/−, WT, and Mgp−/− mice, as determined by immunofluorescence. Nuclei are visualized by DAPI (blue fluorescence). Asterisks indicate statistically significant differences compared with (A) start time, (B) no BMP-9, or (D-E) WT tissue. *P < .05, ***P < .001, Tukey test.
We then determined the expression of CV2 in ALK1-deficient (Alk1+/−) mice, which have ∼ 50% reduction in ALK1 levels.2 This revealed a lower expression of CV2 in the lungs, aorta, kidneys, and liver of Alk1+/− mice compared with wild-type mice, as determined by real-time PCR and immunoblotting (Figure 1D). Low expression of CV2 in the aorta and the lungs of Alk1+/− mice was also shown by immunofluorescence (Figure 1F left panels). In wild-type aorta, CV2 was detected in both the endothelium and the media. In wild-type lungs, CV2 was detected in both the airway epithelium and the vascular endothelium (Figure 1F middle panels). Because ALK1 is known to be up-regulated in Mgp−/− mice,11 we also examined CV2 expression in organs of Mgp−/− mice. We found that CV2 was elevated in the lungs, aorta, kidneys, and liver in Mgp−/− compared with wild-type mice as determined by real-time PCR and immunoblotting (Figure 1E), and that the distribution in the aorta and lungs was similar to that of wild-type mice (Figure 1F right panels).
Together, the results suggest that BMP-9 stimulates CV2 expression through ALK1 and SMAD1 activation, but with a longer time to peak expression than previously observed for MGP induction.2 Interestingly, the high CV2 levels in the Mgp−/− aortas did not prevent the increase in BMP activity and calcification previously demonstrated in the Mgp−/− aorta.13
CV2 directly binds and inhibits BMP-9
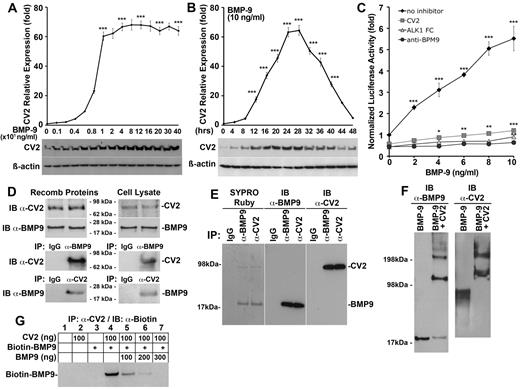
To further clarify the time course and dose-dependency of the BMP-9–induced CV2 expression, we first treated HAECs with BMP-9 (0-400 ng/mL). We found that the highest expression of CV2 was achieved with BMP-9 treatment at or above 10 ng/mL when treating for 24 hours, as determined by real-time PCR and immunoblotting (Figure 2A). To define the time course of CV2 induction, we treated HAECs with BMP-9 (10 ng/mL) for up to 48 hours. We found that CV2 expression peaked at 24-28 hours (Figure 2B), with a subsequent decrease in CV2 expression suggesting that BMP-9 lost activity during induction of CV2. This made us hypothesize that CV2 inhibited BMP-9 activity in a negative feedback loop. To test whether CV2 inhibited BMP-9, we transfected BAECs with a BMP-responsive luciferase reporter gene (BRE-luc), and treated the cells with BMP-9 (0-10 ng/mL) alone or together with nonspecific control IgG (300 ng/mL), CV2 (100 ng/mL), neutralizing anti–BMP-9 Abs (300 ng/mL), or ALK1-Fc, a soluble ALK1 fragment that binds BMP-9 (300 ng/mL). The result showed that CV2 efficiently blocked BMP-9–induced luciferase activity similar to anti–BMP-9 Abs and ALK1-Fc (Figure 2C). MGP, on the other hand, had no effect on the BMP-9 activity (data not shown).
CV2 binds and inhibits BMP-9. (A-B) Expression of CV2 in HAECs (A) in response to increasing BMP-9 concentrations (0-400 ng/mL) after 24 hours of treatment, and (B) in response to BMP-9 (10 ng/mL) for increasing time periods (0-48 hours), as determined by real-time PCR and immunoblotting. (C) Response of BMP-responsive luciferase reporter gene (BRE-luc) in BAECs treated with BMP-9 (0-10 ng/mL), alone or together with nonspecific control IgG (300 ng/mL), CV2 (100 ng/mL), soluble ALK1 fragments (300 ng/mL), or anti–BMP-9 Abs (300 ng/mL). (D) Interactions between CV2 and BMP-9 (50 ng of each protein) were examined using recombinant proteins (left) or cell lysates from HAECs (right) by immunoprecipitation (IP) followed by immunoblotting (IB) with nonspecific IgG, and anti-CV2, and anti–BMP-9 Abs as indicated. The starting material for the immunoprecipitation is shown in the top panels by immunoblotting. (E) Secreted CV2 and BMP-9 were coimmunoprecipitated from 10 mL of HAEC-conditioned serum-free medium using nonspecific IgG, or Abs against BMP-9 or CV2, and analyzed by SDS-PAGE and SYPRO Ruby protein stain (left), or by immunoblotting using anti–BMP-9 or anti-CV2 Abs (center and right). (F) Interactions between recombinant CV2 and BMP-9 were confirmed by chemical cross-linking followed by immunoblotting using anti–BMP-9 and anti-CV2 Abs. (G) CV2 and biotin–BMP-9 were immunoprecipitated with anti-CV2 Abs in the presence of increasing concentrations of unlabeled BMP-9, and the complexes were analyzed by IB using anti–biotin Abs. Asterisks indicate statistically significant differences compared with (A) no BMP-9, (B) start time, or (C-D) no BMP-9. *P < .05, **P < .01, ***P < .001, Tukey test.
CV2 binds and inhibits BMP-9. (A-B) Expression of CV2 in HAECs (A) in response to increasing BMP-9 concentrations (0-400 ng/mL) after 24 hours of treatment, and (B) in response to BMP-9 (10 ng/mL) for increasing time periods (0-48 hours), as determined by real-time PCR and immunoblotting. (C) Response of BMP-responsive luciferase reporter gene (BRE-luc) in BAECs treated with BMP-9 (0-10 ng/mL), alone or together with nonspecific control IgG (300 ng/mL), CV2 (100 ng/mL), soluble ALK1 fragments (300 ng/mL), or anti–BMP-9 Abs (300 ng/mL). (D) Interactions between CV2 and BMP-9 (50 ng of each protein) were examined using recombinant proteins (left) or cell lysates from HAECs (right) by immunoprecipitation (IP) followed by immunoblotting (IB) with nonspecific IgG, and anti-CV2, and anti–BMP-9 Abs as indicated. The starting material for the immunoprecipitation is shown in the top panels by immunoblotting. (E) Secreted CV2 and BMP-9 were coimmunoprecipitated from 10 mL of HAEC-conditioned serum-free medium using nonspecific IgG, or Abs against BMP-9 or CV2, and analyzed by SDS-PAGE and SYPRO Ruby protein stain (left), or by immunoblotting using anti–BMP-9 or anti-CV2 Abs (center and right). (F) Interactions between recombinant CV2 and BMP-9 were confirmed by chemical cross-linking followed by immunoblotting using anti–BMP-9 and anti-CV2 Abs. (G) CV2 and biotin–BMP-9 were immunoprecipitated with anti-CV2 Abs in the presence of increasing concentrations of unlabeled BMP-9, and the complexes were analyzed by IB using anti–biotin Abs. Asterisks indicate statistically significant differences compared with (A) no BMP-9, (B) start time, or (C-D) no BMP-9. *P < .05, **P < .01, ***P < .001, Tukey test.
To determine whether CV2 inhibits BMP-9 activity through protein-protein interactions, we first combined recombinant human CV2 (50 ng) and BMP-9 (50 ng), or lysates from HAECs that express both proteins. We performed immunoprecipitations of BMP-9 and CV2 with anti–BMP-9 or anti-CV2 Abs. The results revealed that BMP-9 and CV2 coimmunoprecipitated regardless of which Abs were used for immunoprecipitation (Figure 2D). Because BMP-9 and CV2 are extracellular proteins, we next examined whether secreted BMP-9 and CV2 also interact in culture media. Ten millilitres of serum-free culture media conditioned for 24 hours on HAECs were used for coimmunoprecipitation with anti–BMP-9 or anti-CV2 Abs. The precipitates were first analyzed by SDS-PAGE gel. SYPRO-Ruby protein staining showed 2 bands, which corresponded in size to BMP-9 (∼ 17 kDa) and CV2 (∼ 80 kDa) and reacted with anti–BMP-9 and anti-CV2 Abs, respectively (Figure 2E). We then confirmed the protein-protein interactions with cross-linking followed by immunoblotting with Abs to BMP-9 and CV2. In the presence of both recombinant proteins, 2 major bands migrating at ∼ 100 and 200 kDa were detected by both Abs, and corresponded to complexes containing either 1 or 2 molecular each of BMP-9 and CV2 (Figure 2F).
Finally, we performed competition experiments between biotin-labeled and unlabeled BMP-9. We combined biotin-BMP-9 (100 ng), CV2 (100 ng), and increasing amounts of unlabeled BMP-9 (0-300 ng), immunoprecipitated the complexes with anti-CV2 Abs, and examined biotin–BMP-9 by immunoblotting with antibiotin Abs. The results showed that biotin-BMP-9 bound to CV2 (Figure 2G lane 4), and that excess unlabeled BMP-9 outcompeted the biotin–BMP-9 (Figure 2G lanes 5-7).
Together, the results suggest that CV2 is induced by BMP-9, and inhibits BMP-9 activity by direct protein-protein interactions, thereby providing negative feedback inhibition for BMP-9. However, the delayed expression of CV2 (∼ 18 hours after MGP peak expression), would allow BMP-9 time to direct important steps in EC differentiation.
CV2 preferentially binds BMP-9
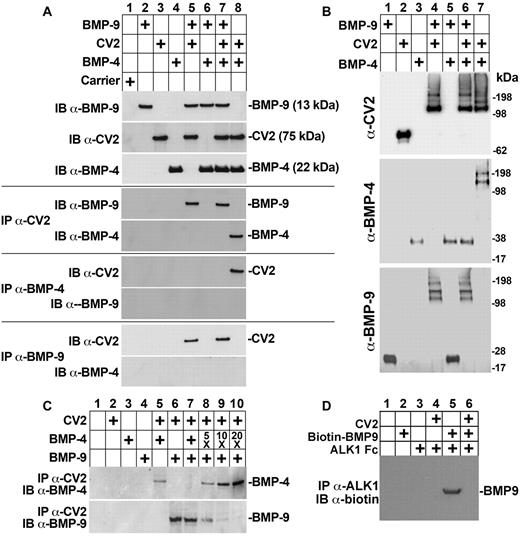
Previous studies have shown that CV2 binds to BMP-2, BMP-4, and BMP-6 with similar affinities.26 To determine whether CV2 preferentially binds BMP-9, we performed coimmunoprecipitation experiments using BMP-4. We combined CV2 (100 ng), BMP-9 (50 ng), and BMP-4 (50 ng) as indicated in Figure 3A, and immunoprecipitated with anti-CV2, anti–BMP-4, or anti–BMP-9 Abs. The precipitated complexes were analyzed by immunoblotting. If one of the Abs was used for immunoprecipitation, the other 2 Abs were used for immunoblotting. The results showed that CV2 and BMP-4 coimmunoprecipitated when BMP-9 was absent (Figure 3A lane 8), consistent with previous reports.26 However, BMP-9 outcompeted BMP-4 and coimmunoprecipitated with CV2 when both BMPs were present in equal amounts (Figure 3A compare lane 7 with lane 5), indicating that CV2 preferentially binds BMP-9. No interaction was detected between BMP-4 and BMP-9 (Figure 3A lane 6).
CV2 preferentially binds BMP-9. (A) Recombinant BMP-9 (50 ng), CV2 (100 ng), BMP-4 (50 ng), and carrier were combined as indicated at the top, and the presence of the respective protein was confirmed by immunoblotting (IB; top 3 blots). Interactions between the proteins were analyzed by immunoprecipitation (IP) followed by immunoblotting using the indicated Abs. (B) BMP-9 (50 ng), CV2 (100 ng), and BMP-4 (50 ng) were combined as indicated at the top, chemically cross-linked, and analyzed by immunoblotting using the indicated Abs. (C) CV2 (100 ng), BMP-4 (50, 250 ng, 500 ng, or 1000 ng), and BMP-9 (50 ng) were combined as indicated at the top, immunoprecipitated by anti-CV2 Abs and analyzed by immunoblotting using anti–BMP-4 and anti–BMP-9 Abs. (D) CV2 (100 ng), biotin-BMP-9 (100 ng), and ALK1-Fc (100 ng) were combined as indicated at the top, immunoprecipitated by anti-ALK1 Abs and analyzed by immunoblotting using antibiotin Abs.
CV2 preferentially binds BMP-9. (A) Recombinant BMP-9 (50 ng), CV2 (100 ng), BMP-4 (50 ng), and carrier were combined as indicated at the top, and the presence of the respective protein was confirmed by immunoblotting (IB; top 3 blots). Interactions between the proteins were analyzed by immunoprecipitation (IP) followed by immunoblotting using the indicated Abs. (B) BMP-9 (50 ng), CV2 (100 ng), and BMP-4 (50 ng) were combined as indicated at the top, chemically cross-linked, and analyzed by immunoblotting using the indicated Abs. (C) CV2 (100 ng), BMP-4 (50, 250 ng, 500 ng, or 1000 ng), and BMP-9 (50 ng) were combined as indicated at the top, immunoprecipitated by anti-CV2 Abs and analyzed by immunoblotting using anti–BMP-4 and anti–BMP-9 Abs. (D) CV2 (100 ng), biotin-BMP-9 (100 ng), and ALK1-Fc (100 ng) were combined as indicated at the top, immunoprecipitated by anti-ALK1 Abs and analyzed by immunoblotting using antibiotin Abs.
To validate our results, we performed cross-linking experiments. Recombinant CV2 (100 ng), BMP-4 (50 ng), and BMP-9 (50 ng) were combined as indicated in Figure 3B and cross-linked. Immunoblotting with anti-CV2 Abs revealed that BMP-4 and CV2 formed a complex in the absence of BMP-9 (Figure 3B lane 7). Incubation of BMP-9 and CV2 led to the formation of a similar complex (Figure 3B lane 4). However, incubation of all 3 proteins resulted in a complex recognized only by anti–BMP-9 and anti-CV2 Abs (Figure 3B lane 6), again indicating that CV2 preferentially binds BMP-9. Thus, addition of BMP-9 abolished the formation of a BMP-4–CV2 complex. No cross-linking was detected between BMP-4 and BMP-9 (Figure 3B lane 5).
Next, we tested whether excess BMP-4 would diminish the binding of BMP-9 to CV2. We combined BMP-9 (50 ng) and CV2 (100 ng) with increasing amounts of BMP-4 (50, 250, 500, or 1000 ng corresponding to 1-, 5-, 10-, or 20-fold excess of BMP-4), and immunoprecipitated with anti-CV2 Abs. The precipitated complexes were analyzed by immunoblotting with anti–BMP-4 or anti–BMP-9 Abs. The results revealed that increasing amounts of BMP-4 progressively diminished the binding between CV2 and BMP-9 (Figure 3C lanes 6-10). At 20-fold excess of BMP-4, only trace amounts of BMP-9 were detected.
Finally, we tested whether CV2 would interfere with the binding between BMP-9 and ALK1-Fc. We combined CV2 (100 ng), biotin–BMP-9 (100 ng), and ALK1-Fc (100 ng), and immunoprecipitated with anti-ALK1 Abs. Biotin–BMP-9 was detected by immunoblotting with antibiotin Abs, which showed that BMP-9 binds to ALK1 Fc (Figure 3D lane 5) and that CV2 blocks this interaction (Figure 3D lane 6). Taken together, the results suggest that CV2 preferentially binds BMP-9 but that excess BMP-4 can interfere with this interaction.
CV2 limits ALK2 and ALK1 from forming complexes with BMP-4 and BMP-9
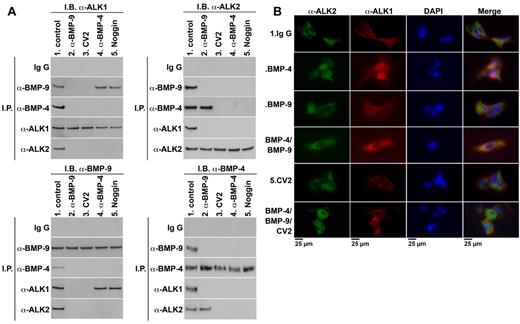
It has previously been suggested that BMP bound to CV2 is in equilibrium with complexes containing the type I BMP receptors.9 Therefore, we explored whether CV2 affected binding between BMP-4, BMP-9, and their respective type I receptors, ALK2 and ALK1. We compared interactions between these BMPs and receptors in ECs in the presence or absence of CV2. Noggin and neutralizing anti–BMP-9 and anti–BMP-4 Abs were used as alternative inhibitors. HAECs were treated for 8 hours with nonspecific control IgG (300 ng/mL), anti–BMP-9 Abs (300 ng/mL), CV2 (100 ng/mL), anti–BMP-4 Abs (300 ng/mL), or Noggin (100 ng/mL). BMP-4 and BMP-9 were expressed by the HAECs, and BMP-9 was also present in media that contained serum.25 Cell lysates were then immunoprecipitated with nonspecific IgG or Abs to BMP-4, BMP-9, ALK2, or ALK1. The precipitated complexes were analyzed by immunoblotting. If one of the Abs was used for immunoprecipitation, the other 3 Abs were used for immunoblotting. The results showed that BMP-4, BMP-9, ALK2, and ALK1 all coprecipitated in the absence of BMP antagonists (Figure 4A lane 1 in all panels), suggesting that they are part of the same complex. CV2, Noggin, anti–BMP-4, and anti–BMP-9 Abs restricted complex formation in different ways. Addition of anti–BMP-9 Abs abolished the complex formation between ALK1 and BMP-9 (Figure 4A lane 2 in all panels). Addition of Noggin or anti–BMP-4 Abs abolished formation of ALK2 and BMP-4 complexes (Figure 4A lane 4-5 in all panels). It suggests that both BMP-4 and BMP-9 are necessary for interactions between ALK2 and ALK1. However, addition of CV2 abolished all complexes (Figure 4A lane 3 in all panels), suggesting that CV2 may act on both BMP-9 and BMP-4 in the complex.
CV2 limits complex formation involving ALK2, ALK1, BMP-4, and BMP-9. (A) To compare interactions between BMP-4, BMP-9, and their receptors in the presence of different antagonists, HAECs were treated for 8 hours with nonspecific IgG (300 ng/mL), anti–BMP-9 Abs (300 ng/mL), CV2 (100 ng/mL), Noggin (100 ng/mL), or anti–BMP-4 Abs (300 ng/mL). Cell lysates were then immunoprecipitated (IP) with nonspecific IgG Abs, or Abs to BMP-9, BMP-4, ALK1, or ALK2. The precipitates were analyzed by immunoblotting using the Ab combinations indicated in the figure. (B) HAECs were treated for 8 hours with nonspecific control IgG (control; 300 ng/mL), BMP-4 (40 ng/mL), BMP-9 (10 ng/mL), BMP-4 and BMP-9 together, CV2 (100 ng/mL), or BMP-4 and BMP-9 together with CV2. The cells were costained for ALK2 (green fluorescence), ALK1 (red fluorescence). DAPI was used to visualize the nuclei.
CV2 limits complex formation involving ALK2, ALK1, BMP-4, and BMP-9. (A) To compare interactions between BMP-4, BMP-9, and their receptors in the presence of different antagonists, HAECs were treated for 8 hours with nonspecific IgG (300 ng/mL), anti–BMP-9 Abs (300 ng/mL), CV2 (100 ng/mL), Noggin (100 ng/mL), or anti–BMP-4 Abs (300 ng/mL). Cell lysates were then immunoprecipitated (IP) with nonspecific IgG Abs, or Abs to BMP-9, BMP-4, ALK1, or ALK2. The precipitates were analyzed by immunoblotting using the Ab combinations indicated in the figure. (B) HAECs were treated for 8 hours with nonspecific control IgG (control; 300 ng/mL), BMP-4 (40 ng/mL), BMP-9 (10 ng/mL), BMP-4 and BMP-9 together, CV2 (100 ng/mL), or BMP-4 and BMP-9 together with CV2. The cells were costained for ALK2 (green fluorescence), ALK1 (red fluorescence). DAPI was used to visualize the nuclei.
To visualize the interactions between ALK1 and ALK2 in HAECs by immunofluorescence, we added ligands and/or CV2. The cells were treated for 8 hours with nonspecific control IgG (control; 300 ng/mL), BMP-4 (40 ng/mL), BMP-9 (10 ng/mL), BMP-4 and BMP-9 together, CV2 (100 ng/mL), or BMP-4 and BMP-9 together with CV2. Immunofluorescence revealed that ALK1 and ALK2 colocalized when the cells were treated with a combination of BMP-4 and BMP-9 (Figure 4B row 4) compared with control (Figure 4B row 1), but showed only limited colocalization after addition of BMP-4 or BMP9 (Figure 4B row 2-3). CV2, however, prevented all colocalization of ALK1 and ALK2 regardless of the presence of BMP-4 and BMP-9 (Figure 4B row 5-6).
To examine whether ALK1 and ALK2 were associated with the plasma membrane, we fractionated HAECs into plasma membranes and intracellular content, and compared the location of ALK1 and ALK2 with that of markers for the plasma membrane (pan-cadherin), the endoplasmic reticulum (calnexin), and mitochondria (COX IV). The result revealed that ALK1 and ALK2 associated with the plasma membrane fraction (supplemental Figure 2), suggesting that interactions involving ALK1 and ALK2 occur at the cell surface.
Altogether, our results suggest that CV2 restricts interactions between the closely related receptors ALK1 and ALK2, potentially interacting with both BMP-9 and BMP-4, and that this interaction occurs at the plasma membrane. The formation of this particular receptor complex may be a key step in angiogenesis and EC maturation.
Differential induction of MGP and CV2 by BMP-4 and BMP-9
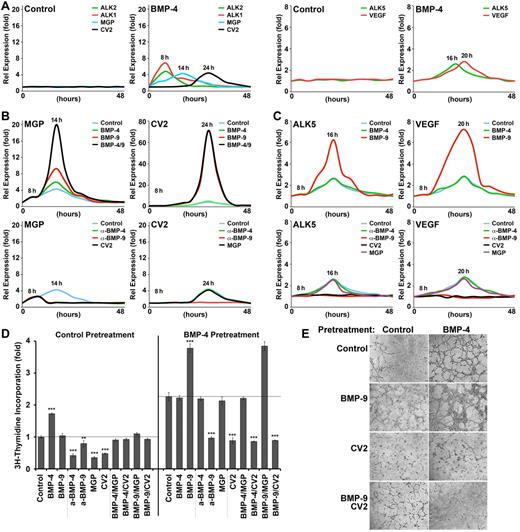
To better understand how BMP-4 and BMP-9 regulate the expression of MGP and CV2, we treated HAECs with combinations of BMP-4 and BMP-9, or their inhibitors, for up to 48 hours and monitored expression by real-time PCR. We first treated HAECs with control vehicle or BMP-4 (40 ng/mL), and collected samples for real-time PCR every 2 hours. We found that expression of ALK2 and ALK1 peaked ∼ 8 hours after initiation of the treatment with BMP-4, and expression of MGP and CV2 peaked after 14 and 24 hours, respectively (Figure 5A left panels), supporting distinct roles for MGP and CV2. No change in expression was seen after control treatment. Although BMP-9 was not added to the culture medium, it is present in serum added to the medium.25 Based on these results, we used 8 hours of pretreatment with BMP-4 to maximize the expression of ALK2 and ALK1 in subsequent experiments. We pretreated with BMP-4 for 8 hours, then removed the pretreatment and added control vehicle, BMP-4 (40 ng/mL), BMP-9 (10 ng/mL), or both BMP-4 and BMP-9. RNA samples were collected every 2 hours for real-time PCR.
Differential induction of MGP and CV2 by BMP-4 and BMP-9. (A) HAECs were treated with BMP-4 (40 ng/mL) or control vehicle and ALK1-Fc (300 ng/mL). RNA samples were collected every 2 hours for up to 48 hours, and expression of ALK2, ALK1, MGP, and CV2 (left panels), and ALK5 and VEGF (right panels) was determined. (B) HAECs were pretreated with BMP-4 and ALK1-Fc for 8 hours, and then treated with (top panels) control vehicle, BMP-4 (40 ng/mL), BMP-9 (10 ng/mL), or both BMP-4 and BMP-9, or (bottom panels) nonspecific IgG (300 ng/mL), anti–BMP-4 Abs (300 ng/mL), anti–BMP-9 Abs (300 ng/mL), or CV2 (100 ng/mL). RNA was collected every 2 hours for up to 48 hours. Expression of (left panels) MGP and (right panels) CV2 was determined by real-time PCR. (C) HAECs were pretreated with BMP-4 and ALK1-Fc for 8 hours, and then treated with (top panels) control vehicle, BMP-4, or BMP-9, or (bottom panels) nonspecific IgG, anti–BMP-4 Abs, anti–BMP-9 Abs, CV2, or MGP. RNA was collected every 2 hours for up to 48 hours. Expression of ALK5 (left panels) and VEGF (left panels) was determined by real-time PCR. (D) HAECs were pretreated for 8 hours with control vehicle (left) or BMP-4 (right), plated, and treated as indicated in the figure. Cell proliferation was determined after 48 hours by 3H-thymidine incorporation. (E) HAECs were pretreated for 8 hours with (left) control vehicle or (right) BMP-4, plated in Matrigel, and treated as indicated in the figure. Images were obtained after 6 hours. Asterisks indicate statistically significant differences compared with control treatment. *P < .05, **P < .01, ***P < .001, Tukey test.
Differential induction of MGP and CV2 by BMP-4 and BMP-9. (A) HAECs were treated with BMP-4 (40 ng/mL) or control vehicle and ALK1-Fc (300 ng/mL). RNA samples were collected every 2 hours for up to 48 hours, and expression of ALK2, ALK1, MGP, and CV2 (left panels), and ALK5 and VEGF (right panels) was determined. (B) HAECs were pretreated with BMP-4 and ALK1-Fc for 8 hours, and then treated with (top panels) control vehicle, BMP-4 (40 ng/mL), BMP-9 (10 ng/mL), or both BMP-4 and BMP-9, or (bottom panels) nonspecific IgG (300 ng/mL), anti–BMP-4 Abs (300 ng/mL), anti–BMP-9 Abs (300 ng/mL), or CV2 (100 ng/mL). RNA was collected every 2 hours for up to 48 hours. Expression of (left panels) MGP and (right panels) CV2 was determined by real-time PCR. (C) HAECs were pretreated with BMP-4 and ALK1-Fc for 8 hours, and then treated with (top panels) control vehicle, BMP-4, or BMP-9, or (bottom panels) nonspecific IgG, anti–BMP-4 Abs, anti–BMP-9 Abs, CV2, or MGP. RNA was collected every 2 hours for up to 48 hours. Expression of ALK5 (left panels) and VEGF (left panels) was determined by real-time PCR. (D) HAECs were pretreated for 8 hours with control vehicle (left) or BMP-4 (right), plated, and treated as indicated in the figure. Cell proliferation was determined after 48 hours by 3H-thymidine incorporation. (E) HAECs were pretreated for 8 hours with (left) control vehicle or (right) BMP-4, plated in Matrigel, and treated as indicated in the figure. Images were obtained after 6 hours. Asterisks indicate statistically significant differences compared with control treatment. *P < .05, **P < .01, ***P < .001, Tukey test.
The results revealed that expression of MGP was synergistically increased by the combination of BMP-4 and BMP-9 (Figure 5B top left). We also treated the HAECs with neutralizing anti–BMP-4 or anti–BMP-9 Abs (300 ng/mL), or with CV2 (100 ng/mL). All these treatments abolished the induction of MGP (Figure 5B bottom left). The results suggested that both BMP-4 and BMP-9 contributed to induction of MGP, and that CV2 blocked MGP induction. We then examined CV2 expression in HAECs after the same treatments. Only BMP-9 induced CV2 expression (Figure 5B top right). Neutralizing anti–BMP-9 Abs, but not neutralizing anti–BMP-4 Abs or MGP (100 ng/mL), abolished CV2 induction by BMP-9 (Figure 5B bottom right), suggesting that BMP9 and ALK1 induced CV2 independent of BMP-4 and ALK2.
Thus, the full complex of BMP-4, BMP-9, ALK1, and ALK2 appears to participate in MGP induction, whereas BMP-9 and ALK1 suffice for CV2 induction. Consequently, the presence of CV2 can block MGP expression, thereby allowing an increase in BMP-4 activity and re-induction of ALK1.
CV2 limited VEGF expression, proliferation, and tube formation in ECs exposed to BMP-4
Previous studies have shown that ALK5 (the TGF-β type 1 receptor) and VEGF are downstream targets of BMP-9/ALK1 signaling that participate in the regulation of EC proliferation.2 In the current experiments, peak expression of ALK5 and VEGF occurred after 16 and 20 hours of BMP-4 treatment, respectively (Figure 5A right panels). To explore the effect of CV2 on VEGF expression, we first determined whether expression of ALK5 and VEGF was enhanced by BMP-4 or BMP-9. HAECs were treated with BMP-4 or BMP-9, starting after 8 hours of BMP-4 pretreatment. Only BMP-9 induced ALK5 and VEGF (Figure 5C top panels). We then determined whether CV2 limited ALK5 and VEGF expression. HAECs were treated with CV2, neutralizing anti–BMP-4 or BMP-9 Abs, or MGP. We found that CV2 and anti–BMP-9 Abs, but not anti–BMP-4 Abs or MGP, abolished ALK5 and VEGF expression (Figure 5C bottom panels). The results suggested that BMP-9 and CV2 modulate ALK5 and VEGF expression.
We then compared the effects of BMP-9 and CV2 on EC proliferation. HAECs were pretreated with control vehicle or BMP-4, then treated with BMP-4, BMP-9, anti–BMP-4, anti–BMP-9, MGP, or CV2 for 48 hours as indicated in Figure 5D. Only BMP-4 increased proliferation in cells not pretreated with BMP-4. Anti–BMP-4, CV2, and MGP all inhibited proliferation (Figure 5D left). After pretreatment with BMP-4 and induction of ALK1, however, BMP-9 stimulated proliferation and anti–BMP-9 Abs and CV2 blocked it (Figure 5D right). BMP-4, anti–BMP-4, and MGP had no effect on proliferation at this stage. Thus, BMP-9 and CV2 efficiently modulate EC proliferation once ALK1 had been induced by BMP-4.
Tube formation by HAECs in Matrigel was consistent with the proliferation assays. After control pretreatment, the formation was mildly inhibited by CV2, an effect that was neutralized by additional BMP-9 (Figure 5E left). However, after BMP-4 pretreatment, addition of BMP-9 resulted in tubes with increased cellularity (Figure 5E right), and effect that was blocked by CV2. Depletion of CV2 by siRNA in the HAECs also resulted in thicker tubes, especially after BMP-4 pretreatment followed by BMP-9 (supplemental Figure 3).
The results suggest that BMP-4 and its inhibitors affect ECs before induction of ALK1, whereas BMP-9 and CV2 have a potent effect on ECs with ALK1 expression, thereby promoting proliferation at a later stage of EC maturation.
Dysregulation of the aortic endothelium in CV2-deficient mice
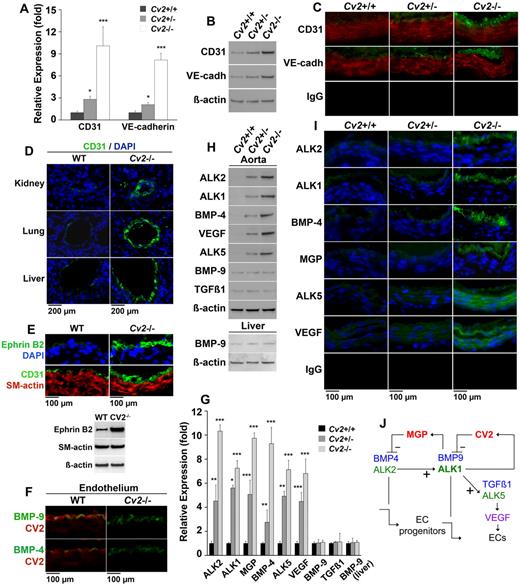
We predicted that the loss of CV2 in mice would result in abnormal endothelium and expression of EC lineage markers. Because Cv2−/− mice do not survive after birth due to tracheal abnormalities,27,28 we examined aortic tissue from embryonic day 18.5 embryos. Our results showed that aortic expression of CD31 and VE-cadherin, lineage markers for mature ECs, was elevated in Cv2−/− mice compared with Cv2+/− and wild-type mice, as determined by real-time PCR, immunoblotting, and immunofluorescence (Figure 6A-C). In addition, the CV2-deficient endothelium had an increased cellularity and appeared thicker than normal (Figure 6C).
Dysregulation of the aortic endothelium in CV2-deficient mice. (A-C) Aortic expression of CD31 and VE-cadherin in Cv2+/+ (wild-type), Cv2+/−, and Cv2−/− mice by (A) real-time PCR, (B) immunoblotting, and (C) immunofluorescence. β-actin is shown for comparison in the immunoblot (n = 3). (D) Vascular expression of CD31 in kidney, lung, and liver of wild-type and Cv2−/− mice by immunofluorescence. (E) Aortic expression of Ephrin B2, and CD31 and α-SM-actin in wild-type and Cv2−/− mice by immunofluorescence and immunoblotting. (F) Colocalization of (top) BMP-9 and CV2, and (bottom) BMP-4 and CV2 in the aortic endothelium of wild-type mice, but not Cv2−/− mice, by immunofluorescence. (G-I) Aortic expression of ALK2, ALK1, MGP, BMP-4, ALK5, VEGF, BMP-9, and TGF-β1, and liver expression of BMP-9 in Cv2+/+, Cv2+/−, and Cv2−/− mice by (G) real-time PCR, (H) immunoblotting, and (I) immunofluorescence, (n = 3). (J) Schematic working model for MGP and CV2 based on Shao et al2 and Yao et al.5,6,22 Nuclei are visualized by DAPI (blue fluorescence). Asterisks indicate statistically significant differences compared with Cv2+/+ (wild-type) mice. *P < .05, **P < .01, ***P < .001, Tukey test.
Dysregulation of the aortic endothelium in CV2-deficient mice. (A-C) Aortic expression of CD31 and VE-cadherin in Cv2+/+ (wild-type), Cv2+/−, and Cv2−/− mice by (A) real-time PCR, (B) immunoblotting, and (C) immunofluorescence. β-actin is shown for comparison in the immunoblot (n = 3). (D) Vascular expression of CD31 in kidney, lung, and liver of wild-type and Cv2−/− mice by immunofluorescence. (E) Aortic expression of Ephrin B2, and CD31 and α-SM-actin in wild-type and Cv2−/− mice by immunofluorescence and immunoblotting. (F) Colocalization of (top) BMP-9 and CV2, and (bottom) BMP-4 and CV2 in the aortic endothelium of wild-type mice, but not Cv2−/− mice, by immunofluorescence. (G-I) Aortic expression of ALK2, ALK1, MGP, BMP-4, ALK5, VEGF, BMP-9, and TGF-β1, and liver expression of BMP-9 in Cv2+/+, Cv2+/−, and Cv2−/− mice by (G) real-time PCR, (H) immunoblotting, and (I) immunofluorescence, (n = 3). (J) Schematic working model for MGP and CV2 based on Shao et al2 and Yao et al.5,6,22 Nuclei are visualized by DAPI (blue fluorescence). Asterisks indicate statistically significant differences compared with Cv2+/+ (wild-type) mice. *P < .05, **P < .01, ***P < .001, Tukey test.
To determine whether a thickened endothelium could be detected in other locations, we stained sections from multiple organs for CD31. This showed enhanced endothelial staining also in the vasculature of lungs, kidneys, and liver in the CV2-deficient mice, compared with wild-type (Figure 6D), suggesting that CV2 deficiency has a widespread effect on the endothelium. Furthermore, we examined the expression of Ephrin B2, a marker of arterial EC differentiation, in wild-type and CV2-deficient aortas. This revealed enhanced staining for Ephrin B2 similar to that of CD31 (Figure 6E), which was confirmed by immunoblotting (Figure 6E). It suggests that arterial differentiation is achieved in a larger number of ECs, pointing to an enhanced proliferation of more mature ECs, consistent with the proliferation experiments. Furthermore, we were unable to detect colocalization of CD31 and α-SM-actin (Figure 6E), arguing against endothelial-mesenchymal transitions.
To study the effect of CV2 deficiency on aortic BMP signaling, we first examined whether CV2 interacts with BMP-9 or BMP-4 in the endothelium by costaining for BMP-9 and CV2, or BMP-4 and CV2. This showed colocalization of BMP-9 and CV2, and to a lesser extent of BMP-4 and CV2, in wild-type aorta but not in CV2-deficient aorta (Figure 6F).
To assess changes in gene expression responsive to BMP activation, we first examined the expression of ALK2, ALK1, ALK5, VEGF, MGP, BMP-4, BMP-9, and TGFβ1 in the aortas of Cv2−/−, Cv2+/−, and wild-type mice. Based on our previous studies2,5,6 and data in Figure 5, we predicted that ALK2, ALK1, ALK5, VEGF, and MGP would be increased, which was indeed the case, as determined by real-time PCR and immunoblotting (Figure 6G-H). In addition, BMP-4 was increased, suggesting that CV2 may have a role in suppressing BMP-4 expression. No change was seen in the expression of BMP-9 or TGFβ1, consistent with their wide availability in the circulation and local tissues. ALK2, ALK1, BMP-4, and MGP were detected mostly in the endothelium by immunofluorescence, whereas ALK5 and VEGF were detected all through the vascular wall (Figure 6I).
Finally, we determined the effect on BMP activity by examining the levels of phosphorylated (p)SMAD1/5/8 (activated by BMPs), and (p)SMAD2/3 (activated by TGFβ) in aortic tissue of Cv2−/−, Cv2+/−, and wild-type mice. Both pSMAD1/5/8 and pSMAD 2/3 were increased in aortic tissues of Cv2−/− mice, as determined by immunoblotting and immunofluorescence (supplemental Figure 4). Although BMP-9 and CV2 were detected predominantly in the endothelium, the SMAD activation occurred throughout in the media, suggesting that endothelial BMP activity affects the media. Thus, the loss of CV2 increased the endothelial cellularity causing an apparent thickening of the endothelium, and expression of EC lineage markers, likely because of increased expression of BMP/TGFβ components, and enhanced BMP and TGFβ activity.
Discussion
In this study, we demonstrate for the first time that CV2 functions as an efficient inhibitor of BMP-9, thereby regulating BMP-9–induced EC proliferation and maturation. Binding of CV2 to BMP-9 disrupts a complex involving BMP-4, BMP-9, ALK2, and ALK1, and limits expression of MGP, CV2, ALK5, and VEGF, previously shown to be dependent on BMP-9/ALK1 signaling.2
Our data are consistent with a differential feedback inhibition of BMP-4 and BMP-9, where the MGP inhibition of BMP-4 precedes the CV2 inhibition of BMP-9 (see working model in Figure 6J), indicating that CV2 and MGP have different roles in the vasculature. Their different patterns of induction and feedback inhibition may be an example of stage-specific feedback inhibition in EC differentiation, where ALK1 is the defining step between 2 stages of EC maturation. This allows BMP-9 and CV2 to specifically regulate proliferation and differentiation in ALK1-expressing cells. Before ALK1 induction, BMP-4 has a modest effect on proliferation, which is counteracted by CV2, MGP, and anti–BMP-4 Abs. It supports previous reports that CV2 functions as a BMP-4 inhibitor depending on the cellular context and possibly BMP receptor availability. The time delay between the induction of ALK1 and that of CV2 would allow for completion of EC differentiation and an optimization of cell numbers. Stage-specific feedback inhibition of cell lineages has been previously described in the olfactory mucosa,29 and might help balance the number of undifferentiated versus differentiated ECs.
Lack of CV2 translates into an apparent endothelial thickening and enhancement of staining for CD31 and Ephrin B2 in the CV2-deficient mice, supporting an inhibitory role of CV2 in mature ECs. CV2 may also be important for lumen formation in the emerging vessels because endothelial overgrowth could impinge on the lumen. Indeed, the CV2-deficient mouse resembles in part the β1-integrin null mouse, which has a thickened endothelium and a loss of lumen.30 However, the CV2 null mouse appears to maintain a larger vascular lumen than the β1-integrin null mouse.
It has previously been proposed that BMP bound to CV2 is in equilibrium with a transient complex containing the BMP type I receptors, and that CV2 facilitates exchanges of BMPs between CV2 and the receptors.9 The high affinity of CV2 for BMP-9 in our experiments may affect such exchanges, potentially allowing CV2 to inhibit different BMPs depending on relative BMP concentrations, the type of receptors expressed, and the overall cellular context. It is known that CV2 binds to BMP-2 via its N-terminal VWF type C (VWC) domain 1 and is able to compete for all receptor types.31 Further studies are needed to determine whether the same region also binds BMP-9. CV2 is known to participate in the generation of a BMP signaling morphogenetic field during vertebral formation together with Chordin and twisted gastrulation,9,32 but it is not known whether these 2 proteins also contribute to EC regulation.
The regulation of MGP expression differed from that of CV2 expression. Induction of MGP required the presence of both BMP-4 and BMP-9, whereas induction of CV2 (as well as that of ALK5 and VEGF) was regulated by BMP-9/ALK1 independent of BMP-4/ALK2 signaling. This arrangement may explain previous reports that BMPER/CV2 is necessary for BMP-4 to activate ECs and to trigger SMAD1/5/8 activation.33 CV2 would allow for increased BMP-4 activity by inhibiting BMP-9–induced SMAD activation, and by decreasing MGP levels. It may also explain why BMP-4 is necessary for BMPER/CV2 activity33 ; BMP-4 induces ALK1, which allows the CV2 induction.
BMP-2/4 and MGP have been implicated in the patterning of the vasculature. In vitro, BMP-2 and MGP modulate cell aggregation in vascular mesenchymal cells.34 In vivo, excess MGP leads to loss of branching in the pulmonary arteries in mice,12 whereas lack of MGP leads to arteriovenous malformations.11 Considering that MGP is expressed before the induction of CV2, the patterning may precede EC commitment. BMP-4 could then induce angiogenesis by a cycle of ALK1 induction, MGP induction, patterning, and EC proliferation and maturation, which could be repeated as long as there is a source of BMP-4. Vascular outgrowth in the lungs may be an example of this; BMP-4 is found in proximity of the tips of the airways during lung development,35 and could assist in coordinating the vascular growth with the airway formation.
It is interesting that the majority of BMP-9 appears to be derived from the liver and found in the circulation,25 where it may act in concert with BMP-4 expressed locally in the tissue. Although BMP-9 is present in a ubiquitous fashion, it can only act where ALK1 has been induced. Similarly, TGF-β1 is frequently present in tissue, but can only act where ALK5 has been induced. The sequential BMP-4, BMP-9, and TGFβ1 actions allowed by such a receptor arrangement support the progression of EC commitment, proliferation, and maturation.
Activation of the 2 BMP circuits (Figure 6J) can be seen in vascular disease. Endothelial BMP-4 is induced by oscillatory shear stress and inflammatory cytokines36 and promotes the initial stages of atherosclerotic lesion development.13 It is also induced by hyperglycemia and diabetes.14 In both cases, expression of ALK1, ALK5, VEGF, MGP, and CV2 is stimulated,13,14 suggesting that the disease process activates the same system that is responsible for EC differentiation. An increase in MGP limits lesion formation and vascular calcification,13,14 thus supporting appropriate feedback inhibition as central for disease prevention. In our current study, CV2 was significantly enhanced in the MGP null mice, but did not prevent vascular calcification in the MGP-deficient mice (Figure 1F), supporting distinct functions for BMP-4 and BMP-9. Further studies will be needed to explore the effect of CV2 on the development of vascular disease, including vascular calcification and vascular malformations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Institutes of Health grants HL30568, HL81397, and ZDK1 GRB-J; and the American Heart Association (Western Affiliate and National Center).
National Institutes of Health
Authorship
Contribution: Y.Y. designed and performed research, analyzed data, and wrote the manuscript; M.J., A.L., M.R., A.H.W., and R.A. performed research and analyzed data; and K.I.B. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kristina I. Boström, MD, PhD, or Yucheng Yao, MD, PhD, Division of Cardiology, David Geffen School of Medicine at UCLA, Box 951679, Los Angeles, CA 90095-1679; e-mail: kbostrom@mednet.ucla.edu or yyao@mednet.ucla.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal