Transforming growth factor-β (TGF-β) is involved in vascular formation through activin receptor-like kinase (ALK)1 and ALK5. ALK5, which is expressed ubiquitously, phosphorylates Smad2 and Smad3, whereas endothelial cell (EC)–specific ALK1 activates Smad1 and Smad5. Because ALK5 kinase activity is required for ALK1 to transduce TGF-β signaling via Smad1/5 in ECs, ALK5 knockout (KO) mice were not able to give us the precise mechanisms by which TGF-β/ALK5/Smad2/3 signaling is implicated in angiogenesis. To delineate the role of Smad2/3 signaling in endothelium, the Smad2 gene in Smad3 KO mice was selectively deleted in ECs using Tie2-Cre transgenic mice, termed EC-specific Smad2/3 double KO (EC-Smad2/3KO) mice. EC-Smad2/3KO embryos revealed hemorrhage leading to embryonic lethality around E12.5. EC-Smad2/3KO embryos exhibited no abnormality of vasculogenesis and angiogenesis in both the yolk sac and the whole embryo, whereas vascular maturation was incomplete because of inadequate assembly of mural cells in the vasculature. Wide gaps between ECs and mural cells could be observed in the vasculature of EC-Smad2/3KO mice because of reduced expression of N-cadherin and sphingosine-1-phosphate receptor-1 (S1PR1) in ECs from those mice. These results indicated that Smad2/3 signaling in ECs is indispensable for maintenance of vascular integrity via the fine-tuning of N-cadherin, VE-cadherin, and S1PR1 expressions in the vasculature.
Introduction
Aberrant vascularization leads to a number of diseases including atherosclerosis, tumorigenicity, and retinopathy,1,2 whereas angiogenesis is essential during embryonic development as well as in adulthood. Angiogenesis is mediated by sprouting of new vessels from preexisting ones or by intussusceptive microvascular growth. In general, vascular formation is quiet in adulthood, although angiogenesis involved in wound healing, inflammation, ischemia, and the female reproductive cycle can be observed. Angiogenesis is divided into 2 phases: the activation phase and the resolution phase. The balance between physiologic stimulators (eg, vascular endothelial growth factor (VEGF), fibroblast growth factor 2 (FGF-2), angiopoietins, and hypoxia) and inhibitors (eg, angiostatin, endostatin, and interferon-α) is strategic to tuning of the angiogenic switch. Proliferation of endothelial cells (ECs), increase in vascular permeability, and degradation of extracellular matrix components can be observed during the activation phase. Consequently, ECs make new capillary sprouts. In the resolution phase, the proliferation and migration of ECs ceases and is followed by reconstitution of the basement membrane and maturation of the vessels.3
Transforming growth factor-β (TGF-β) is a pivotal cytokine that contributes to the behaviors and activities of most cells from the embryonic to the adult stage. The TGF-β signal is initiated when the ligand binds to its own TGF-β type II receptor (TβRII); thereafter, the TGF-β type I receptor (TβRI or activin receptor-like kinase [ALK]5) is phosphorylated by constitutively active TβRII kinase, and then the TβRI kinase becomes active. In general, the activated TβRI kinase phosphorylates receptor-regulated Smads (R-Smads) at their extreme carboxyl-terminal serine residues. Activated R-Smads form heteromeric complexes with Smad4, which translocate into the nucleus where they control gene expression via interaction with other transcription factors, coactivators, and corepressors.4 However, in ECs, TGF-β binds to the EC-restricted TβRI, ALK1, which induces Smad1/5 phosphorylation to potentiate angiogenic reactions. In contrast, ALK5, which is ubiquitously expressed in most cells, promotes phosphorylation of Smad2/3 and inhibits proliferation, tube formation, and migration in ECs.5
Genetic studies in mice revealed the importance of TGF-β signaling in angiogenesis.6 Because ALK1 requires ALK5 kinase activity for phosphorylation of Smad1/5, neither Smad2/3 nor Smad1/5 is phosphorylated on TGF-β stimulation in ECs established from ALK5 knockout (KO) mice.7 Because ALK5KO mice showed embryonic lethality at E10.5 because of angiogenic defects,8 the phenotype seen in ALK5 KO mice seems to be a result of combinational loss of these 2 pathways. To clarify the exact role of the TGF-β/ALK1/Smad1/5 pathway, we previously generated ALK5 knockin (KI) mice whose ECs maintain the TGF-β/ALK1/Smad1/5 pathway.9 However, the ALK5KI mice showed a phenotype that was quite similar to that of ALK5 KO mice because of loss of the ALK5/Smad2/3 pathway throughout the body. Therefore, we were unable to elaborate the role of TGF-β/Smad2/3 signaling in ECs.
In this study, we used functional and genetic approaches with mice in which the Smad2 gene is conditionally deleted in ECs using Tie2-Cre transgenic mice on a Smad3−/− background to elicit the definitive function of TGF-β/ALK5/Smad2/3 signaling in ECs during vascular development. Unlike other KO mice lacking TGF-β signaling components, these conditional KO mice showed fragile vascular networks. This finding provides new evidence for the involvement of TGF-β signaling in vascular integrity.
Methods
Generation of EC-Smad2/3KO mice
Smad2fl/fl mice10 were crossed with Smad3+/− mice11 to generate Smad2fl/fl;Smad3+/− mice. Subsequently, Tie2-Cre transgenic mice12 were mated with Smad2fl/fl;Smad3+/− mice. Then, we obtained Smad2fl/fl;Smad3+/−;Tie2-Cre male and Smad2fl/fl;Smad3+/− female mice. These mice were further mated together for generation of Smad2fl/fl;Smad3−/−;Tie2-Cre (EC-Smad2/3KO) mice. ROSA26 reporter (R26R) mice were purchased from The Jackson Laboratory. The generation of ALK5fl/fl mice previously reported.8 Smad4fl/fl mice were kindly obtained from Dr Deng (National Institutes of Health).13 The mice were housed in the animal facilities of the Laboratory Animal Resource Center at the University of Tsukuba under specific pathogen-free (SPF) conditions at constant temperature and humidity and fed a standard diet. Treatment of the mice was in accordance with the institutional guidelines of the Animal Care and Use Program of the University of Tsukuba.
Immunofluorescence and histology
Embryos were dissected and fixed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) overnight. For immunofluorescence, the embryos were processed for cryosectioning as previously described.9 Then, the embryos were sectioned at a thickness of 5 μm at −22°C. After being blocked with 5% swine serum in PBS, the sections were incubated with antiplatelet endothelial cell adhesion molecule 1 (PECAM-1; BD Biosciences; 1:200), Cy3-conjugated anti–α-smooth muscle actin (SMA; Sigma Clone 1A4; 1:200), or N-cadherin antibody (Lifespan, 1:200) at 4°C overnight. Subsequently, the sections were washed 3 times with PBS and incubated with fluorescence-conjugated secondary antibodies at room temperature for 1 hour if necessary. After all samples were mounted with Fluorescent Mounting Medium (Dako Demark), they were visualized using an immunofluorescence microscope (Axiovert 200M; Carl Zeiss) with 63×/1.4 oil objective lenses (Carl Zeiss) at room temperature. Images were acquired with AxioCam MRm 60-C1 (Carl Zeiss) and processed with the AxioVision Rel 4.4 (Carl Zeiss) and Adobe Photoshop 7.0.1 software (Adobe). In Figure 2E, samples were visualized using a confocal microscope TCS-SP5 (Leica) with PL APO CS 10×/0.40HC objective lenses (Leica) at room temperature. Images were processed with the LAS AF Version 2.3 (Leica) and Adobe Photoshop 7.0.1 software (Abobe). For histology, the paraffin-embedded tissues were sectioned at a thickness of 2.5 μm, deparaffinized in xylene, and rehydrated in graded ethanol solutions. Tissue samples were stained with hematoxylin and eosin (H&E) according to standard methods.9 For immunohistochemisty, deparaffinized sections were immersed in 10mM sodium citrate (pH 6.2) for 20 minutes at 121°C with 121 kPa for the restoration of antigenicity. After blocking, the sections were incubated with anti–claudin-5 antibody (Zymed, 1:200). The sections were washed 3 times with PBS, and subsequently colored by Dako EnVision system/HRP (Dako Cytomation). Images were taken using a microscope (Olympus). Samples were observed using the upright microscope (BX51; Olympus) with UPlanSApo 40×/0.90 lenses (Olympus) at room temperature. Images were acquired with DP70 (Olympus) and processed with DPcontroller 2002-2004 (Olympus) and Adobe Phtotshop 7.0.1 software (Adobe).
Whole-mount immunostaining
Embryos were fixed in 4% PFA/PBS overnight. After being washed with PBS containing 0.2% Triton X-100 (PBST), the samples were blocked with 2% skimmed milk in PBST and incubated with anti–PECAM-1 (1:200) antibody overnight at 4°C. After being washed, the samples were incubated overnight at 4°C with 1:200 dilutions of the secondary antibody. Subsequently, the samples were washed, and the horseradish peroxidase (HRP) color reaction was carried out for 10 minutes with diaminobenzidine (SK-4100; Vector Laboratories). Images were taken using a stereomicroscope (model MZ FL III; Leica Microscopy Systems). Samples were observed using a stereomicroscope (MZ FL III; Leica) with APO 0.63×/WD100 mm.S8AP0 lenses (Leica) at room temperature. Images were acquired with EC3 (Leica) and processed with the LAS EZ (Version 1.7.0; Leica) and Adobe Photoshop 7.0.1 software (Adobe).
In situ hybridization
Whole-mount in situ hybridization9 was performed using a digoxigenin (DIG)–labeled RNA probe against sphingosine-1-phosphate receptor (S1PR)1 mRNA. Antisense and sense RNA probes corresponding to S1PR1 cDNA were synthesized using T7 RNA polymerase. The probes were visualized in dark blue with NBT/BCIP (Roche Diagnostics). Samples were observed using a stereomicroscope (MZ FL III; Leica) with APO 0.63×/WD100 mm.S8AP0 lenses (Leica) at room temperature. Images were acquired with EC3 (Leica) and processed with the LAS EZ (Version 1.7.0; Leica) and Adobe Photoshop 7.0.1 software (Adobe).
Endothelial cell cultures and reagents
Western blotting
OMD assay
Omphalomesenteric ducts (OMDs) were isolated from E10.5 embryos. The ducts were divided into two 1-mm-long pieces. Then, each duct was infected with adenoviruses carrying LacZ, Smad2, or Smad3 (1 × 108 colony-forming unit [CFU]) in 20 μL of medium overnight using the hanging drop method.16 The ducts were then embedded in collagen gel (Nitta gelatin) and cultured for 6 days in 5% DMEM supplemented with 2.5 ng/mL VEGF (Wako). The medium was refreshed every other day. Images were taken using a confocal microscope (Leica Microscopy Systems).
Results
Mice harboring conditional deletion of Smad2 and Smad3 in ECs display embryonic lethality
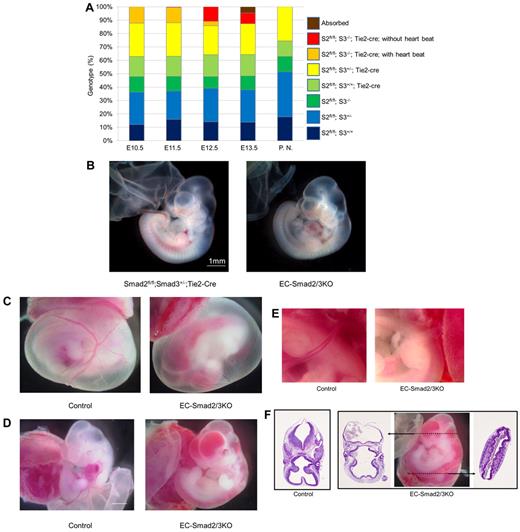
Mice deficient in Smad2 display a failure in gastrulation and developmental defects before E8.5,17 whereas Smad3 KO mice are viable.11 Double heterozygous mice that possess one copy of both Smad2 and Smad3 genes were born at the Mendelian ratio and were fully viable and fertile. On the other hand, loss of Smad3 with one wild-type copy of Smad2 resulted in embryonic lethality at E9.5.18 We crossed Smad2fl/fl;Smad3+/−;Tie2-Cre male mice with Smad2fl/fl;Smad3+/− female mice to generate EC-specific deletion of Smad2 based on Smad3 KO mice (EC-Smad2/3KO mice), we expected approximately 12.5% of the pups to be EC-Smad2/3KO pups at birth (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). However, no viable EC-Smad2/3KO mice were born using this combination of mating, indicating that the disruption of both Smad2 and Smad3 genes in ECs results in embryonic lethality. However, Smad2fl/fl; Smad3+/−; Tie2-Cre mice were viable with no abnormality. Thus, the one allele of the Smad3 gene in ECs was enough for mice to survive into adulthood. To determine when EC-Smad2/3KO embryos die during development, we collected embryos at a number of embryonic stages after gestation to analyze the genotype using thise mating system. The expected Mendelian ratio in the EC-Smad2/3KO mice was found at E10.5 to E12.5, although most of the EC-Smad2/3KO embryos at E12.5 showed no heart beat (Figure 1A). The EC-Smad2/3KO embryos at E10.5 seemed phenotypically normal without visible vessel formation disorder (Figure 1B), whereas at E11.5, blood vessels in the yolk sacs revealed a highly branched vasculature with no blood (Figure 1C). Furthermore, massive intraembryonic bleeding was obvious in the brain and neural tube of the EC-Smad2/3KO embryos (Figure 1D). In addition, the umbilical cords were also ischemic (Figure 1E). Histologic analysis with H&E staining revealed widespread hemorrhage in the midbrain of the EC-Smad2/3KO embryos, with nucleated erythrocytes in the cerebral ventricles and neural lumen (Figure 1F). At E13.5, we could find EC-Smad2/3KO embryos, but they were not alive. They were absorbed or showed unrecognizable organ features (supplemental Figure 1B). Thus, EC-specific deletion of both Smad2 and Smad3 genes exhibited embryonic lethality around E11.5-12.5 with severe bleeding.
EC-specific disruption of Smad2 and Smad3 results in embryonic lethality around E12.5. (A) Genotype analysis of offspring. The exact numbers of the embryos are shown in supplemental Table 1. PN indicates postnatal mice; S2, Smad2; and S3, Smad3. (B) Gross morphology of Smad2fl/fl;Smad3+/−;Tie2-Cre (left panel) and EC-Smad2/3KO embryos (right panel) at E10.5. (C-E) Gross morphology of control and EC-Smad2/3KO embryos at E11.5. In panel C, vitelline vessels are clearly visible in the yolk sacs of both the control (left panel) and the EC-Smad2/3KO embryos (right panel), although no blood is detected in the EC-Smad2/3KO embryo. In panel D, embryos lacking both Smad2 and Smad3 exhibit evidence of vasodilatation and diffuse bleeding in the head, trunk, and intersomitic regions. (E) High magnitude view around the umbilical cords reveals avascularity in the EC-Smad2/3KO embryos (right panel). (F) Transverse sections of control and EC-Smad2/3KO embryos at E11.5. EC-Smad2/3KO embryos were sliced at the positions indicated by broken lines of arrows. The sections were then stained with H&E to show profuse bleeding in the ventricles of the brain and the neural tube of EC-Smad2/3KO embryos. In current study, all embryos whose genotype is Smad2fl/fl;Smad3−/−;Tie2-Cre show severe hemorrhage at E11.5.
EC-specific disruption of Smad2 and Smad3 results in embryonic lethality around E12.5. (A) Genotype analysis of offspring. The exact numbers of the embryos are shown in supplemental Table 1. PN indicates postnatal mice; S2, Smad2; and S3, Smad3. (B) Gross morphology of Smad2fl/fl;Smad3+/−;Tie2-Cre (left panel) and EC-Smad2/3KO embryos (right panel) at E10.5. (C-E) Gross morphology of control and EC-Smad2/3KO embryos at E11.5. In panel C, vitelline vessels are clearly visible in the yolk sacs of both the control (left panel) and the EC-Smad2/3KO embryos (right panel), although no blood is detected in the EC-Smad2/3KO embryo. In panel D, embryos lacking both Smad2 and Smad3 exhibit evidence of vasodilatation and diffuse bleeding in the head, trunk, and intersomitic regions. (E) High magnitude view around the umbilical cords reveals avascularity in the EC-Smad2/3KO embryos (right panel). (F) Transverse sections of control and EC-Smad2/3KO embryos at E11.5. EC-Smad2/3KO embryos were sliced at the positions indicated by broken lines of arrows. The sections were then stained with H&E to show profuse bleeding in the ventricles of the brain and the neural tube of EC-Smad2/3KO embryos. In current study, all embryos whose genotype is Smad2fl/fl;Smad3−/−;Tie2-Cre show severe hemorrhage at E11.5.
We further compared the EC-Smad2/3KO embryos with embryos for EC-specific deletion of either ALK5 (EC-ALK5KO embryos) or of Smad4 (EC-Smad4KO embryos; supplemental Figure 2), none of which can relay TGF-β signaling in endothelium. Both embryos were analogous to the EC-Smad2/3KO embryos although the EC-ALK5KO embryos survived one-half day longer than the EC-Smad2/3KO embryos (supplemental Tables 1-2). On the other hand, EC-Smad4KO embryos died between E11.5 and E12.5, comparable with the results reported by Lan et al,19 although vascular disorganization was recognized at E9.5.
To verify that the activity of Cre recombinase driven by the Tie2 promoter is EC-specific, we crossed Tie2-Cre transgenic mice with Rosa26 reporter mice.20 Subsequently, the embryos obtained were stained with X-gal. LacZ activity was restricted in the vasculature (supplemental Figure 3) at E10.5.
Vascular abnormality of EC-Smad2/3KO embryos at E10.5
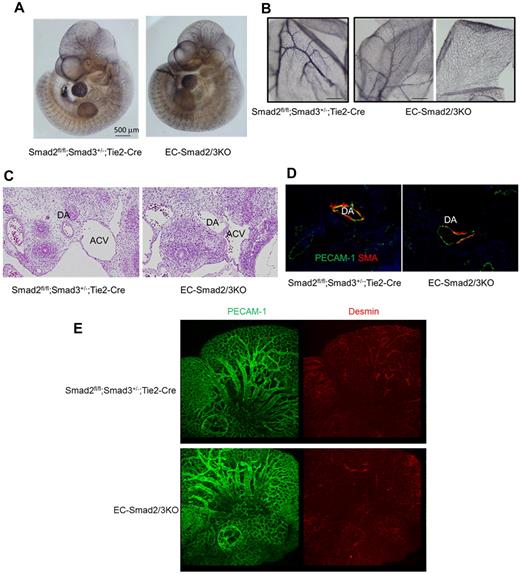
To further analyze the vascular structure of the EC-Smad2/3KO embryos at E10.5, the morphology of the vasculature was characterized by whole-mount immunohistochemical staining using the antibody against PECAM-1. The mutant embryos showed a normal vascular network comparable with that of the control embryos. High magnification views showed capillary sprouts in the head of the mutant embryos (Figure 2A). However, parts of the small vessels in the yolk sacs appeared to be dilated, rugged structures compared with the wild-type embryos (Figure 2B). Thus, EC-Smad2/3KO embryos show partial defects in the vasculature at E10.5.
Vascular abnormality in EC-Smad2/3KO embryos at E10.5. (A) Staining of whole embryos at E10.5 with anti–PECAM-1 antibody. The left and right panels show Smad2fl/fl;Smad3+/−;Tie2-Cre and EC-Smad2/3KO embryos, respectively. (B) Staining of yolk sacs at E10.5 with anti–PECAM-1 antibody. The left and right panels show Smad2fl/fl;Smad3+/−;Tie2-Cre and EC-Smad2/3KO embryos, respectively. (C) Transverse sections of embryos at E10.5 including the heart were stained with H&E. DA indicates dorsal aorta; and ACV, anterior cardinal vein. (D) Transverse sections of aortae from E10.5 embryos stained with anti–PECAM-1 (green) and anti-αSMA (red) antibodies. The coverage of ECs with SMCs is shown in yellow. (E) Whole-head staining of embryos at E10.5 with anti–PECAM-1 (green) and antidesmin antibodies (red).
Vascular abnormality in EC-Smad2/3KO embryos at E10.5. (A) Staining of whole embryos at E10.5 with anti–PECAM-1 antibody. The left and right panels show Smad2fl/fl;Smad3+/−;Tie2-Cre and EC-Smad2/3KO embryos, respectively. (B) Staining of yolk sacs at E10.5 with anti–PECAM-1 antibody. The left and right panels show Smad2fl/fl;Smad3+/−;Tie2-Cre and EC-Smad2/3KO embryos, respectively. (C) Transverse sections of embryos at E10.5 including the heart were stained with H&E. DA indicates dorsal aorta; and ACV, anterior cardinal vein. (D) Transverse sections of aortae from E10.5 embryos stained with anti–PECAM-1 (green) and anti-αSMA (red) antibodies. The coverage of ECs with SMCs is shown in yellow. (E) Whole-head staining of embryos at E10.5 with anti–PECAM-1 (green) and antidesmin antibodies (red).
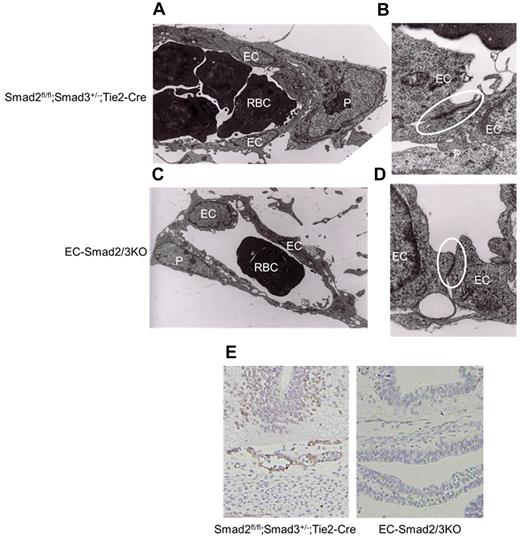
To further analyze the oddity of EC-Smad2/3KO embryos at E10.5, the H&E-stained transverse sections of EC-Smad2/3KO and control embryos were compared (Figure 2C). Apparently, the dorsal aorta (DA) and the anterior cardinal vein (ACV) in the control embryos showed a taut structure, whereas the DA and ACV in the EC-Smad2/3KO embryos were misshapen. When we stained the DA with anti–PECAM-1 and anti–α-smooth muscle actin (αSMA) antibody, we could observe the endothelial layers (stained green) surrounded by αSMA-positive mural cells (stained red) in the DA of the control embryos (Figure 2D). In contrast, the DA of the EC-Smad2/3KO embryos was partially covered with mural cells. Further analysis demonstrated that the recruitment of pericytes to microvessels in the brain could not be completely achieved in EC-Smad2/3KO embryos when antidesmin antibody was used (Figure 2E). Our data indicate that EC-Smad2/3KO embryos can achieve vessel sprouting and penetration, but not vascular maturation. It is known that platelet-derived growth factor B (PDGF-B) produced from ECs can promotes recruitment of pericytes to ECs.3 To examine whether PDGF-B expression in ECs from EC-Smad2/3 KO mice decreases, we performed semiquantitive reverse transcription-polymerase chain reaction (RT-PCR) using established MEECs from EC-Smad2/3KO (S2/3KO) and Smad2fl/fl; Smad3+/+ embryos (S2fl/fl). As seen in supplemental Figure 4A, the expression of PDGF-B in S2/3KO MEECs is lower than that in S2fl/fl MEECs. Thus, one possible reason why vessels from EC-Smad2/3KO embryos are partially covered with mural cells might be because of inadequate secretion of PDGF-B from ECs. To further understand how vascular leakage takes place in EC-Smad2/3KO embryos, we made sections in the midbrain of EC-Smad2/3KO embryos at E11.5 for analysis by transmission electron microscopy. The capillaries in the control mice were taut and the pericytes continuously and tightly attached to the outer surface of the ECs, whereas the capillaries in the EC-Smad2/3KO mice were slack and plenty of gaps had formed between the ECs and pericytes (Figure 3A-C). Furthermore, the tight junction between ECs was less developed in EC-Smad2/3KO mice (Figure 3B-D, supplemental Figure 5).
Collapsing vascular structure in the midbrain. Electron microscopic analysis of brain capillaries from Smad2fl/fl;Smad3+/−;Tie2-Cre (A-B) and EC-Smad2/3KO (C-D) embryos at E11.5. Panels A and C, 2000× magnification; panel B and D, 50 000× magnification. Samples were observed using an electron microscope (H7000; Hitachi) operated with 75 kV at 20°C. Each area surrounded by the circle reveals tight junction between ECs. (E) Transverse sections of midbrains from E10.5 embryos were stained with anti–claudin-5 antibody as well as with hematoxylin. EC indicates endothelial cell; P, pericyte; and RBC, red blood cell.
Collapsing vascular structure in the midbrain. Electron microscopic analysis of brain capillaries from Smad2fl/fl;Smad3+/−;Tie2-Cre (A-B) and EC-Smad2/3KO (C-D) embryos at E11.5. Panels A and C, 2000× magnification; panel B and D, 50 000× magnification. Samples were observed using an electron microscope (H7000; Hitachi) operated with 75 kV at 20°C. Each area surrounded by the circle reveals tight junction between ECs. (E) Transverse sections of midbrains from E10.5 embryos were stained with anti–claudin-5 antibody as well as with hematoxylin. EC indicates endothelial cell; P, pericyte; and RBC, red blood cell.
To confirm the molecular basis of the abnormality, we examined the expression of claudin-5, one of the tight junction markers in ECs, in the vasculature. As seen in Figure 3E, the expression of claudin-5 was not detectable in the sections from the EC-Smad2/3KO embryos, in contrast to the sections from the wild-type embryos. Taken together, the lack of both Smad2 and Smad3 in ECs can be predicted to impair vascular stability.
Rescue of either Smad2 or Smad3 improves vascular sprouting
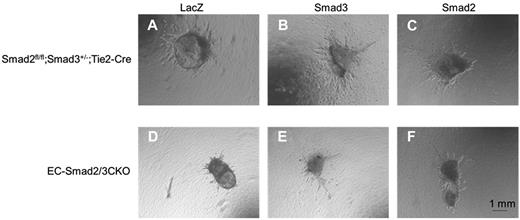
Because the vasculature in EC-Smad2/3KO embryos was impaired, we investigated whether the vasculature in EC-Smad2/3KO embryos can be improved by overexpression of either Smad2 or Smad3. For this purpose, we isolated the OMDs from embryos at E10.5 instead of the thoracic aorta. The OMDs were then embedded in collagen gels and cultured with medium containing VEGF. Vascular sprouting from the OMDs of the control embryos could be observed after the OMDs were infected with adenoviral LacZ, Smad2, or Smad3 (Figure 4A-C), whereas no vascular sprouting from the LacZ-infected OMDs of EC-Smad2/3KO embryos was detected (Figure 4D). However, relief provided by Smad2 or Smad3 in the OMDs of EC-Smad2/3KO embryos improved the elongation of the vasculature in collagen gels (Figure 4E-F, supplemental Figure 6). These results indicated that the introduction of either Smad2 or Smad3 in OMDs lacking both Smad2 and Smad3 genes can rescue the ability of vascular sprouting in OMDs of EC-Smad2/3KO embryos.
Improvement of vascular sprouting from OMDs of EC-Smad2/3KO embryos. OMD assay was performed using Smad2fl/fl;Smad3+/−;Tie2-Cre (A-C) and EC-Smad2/3KO OMDs (D-F). (A-D) Infection with adenoviral LacZ. (B-E) Infection with adenoviral Smad3. (C-F) Infection with adenoviral Smad2.
Improvement of vascular sprouting from OMDs of EC-Smad2/3KO embryos. OMD assay was performed using Smad2fl/fl;Smad3+/−;Tie2-Cre (A-C) and EC-Smad2/3KO OMDs (D-F). (A-D) Infection with adenoviral LacZ. (B-E) Infection with adenoviral Smad3. (C-F) Infection with adenoviral Smad2.
Decreased expression of sphingosine 1 phosphate receptor 1 (S1PR1) and N-cadherin because of lack of both Smad2 and Smad3 genes in ECs
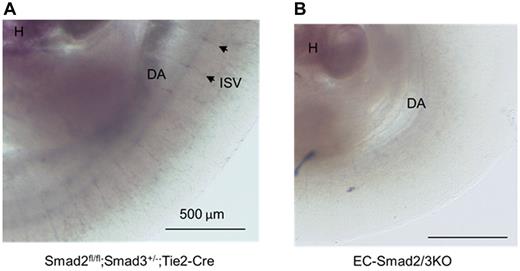
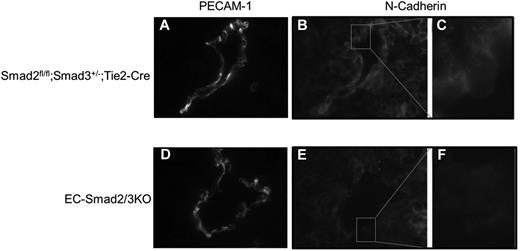
A number of knockout mice are known to exhibit defects in vascular formation during embryonic development. Among them, the phenotype of EC-Smad2/3KO mice is comparable with S1PR1KO mice. S1PR1, abundantly expressed in ECs, is structurally related to G protein–coupled receptors. The lysosphingolipid sphingosine-1-phosphate (S1P) binds to S1PR1 to promote cell–cell adhesion. Mice deficient in the S1PR1 gene exhibited embryonic hemorrhage, resulting in intrauterine death between E12.5 and E14.5. Although vasculogenesis and angiogenesis seemed normal in S1PR1KO embryos, vascular maturation was incomplete because of defects in the recruitment of vascular smooth muscle cells/pericytes to ECs.21 Thus, we investigated the expression of S1PR1 in the vasculature of mutant embryos. When we performed in situ hybridization using S1PR1 antisense mRNA as a probe, we could detect the expression of S1PR1 mRNA in the intrasomitic vessels of the control embryos (Figure 5A), but not in the EC-Smad2/3KO embryos (Figure 5B). Because lack of S1PR1 in mice leads to aberrant localization of N-cadherin in endothelial cells,22 we checked the expression of N-cadherin in the DA. As expected, the expression of N-cadherin could be detected along the vessels of the Smad2fl/fl;Smad3+/−;Tie2-Cre embryos (Figure 6A-C). However, expression of N-cadherin was reduced along the vessels of EC-Smad2/3KO embryos (Figure 6E-F). In addition, we also tried to investigate the expression of a tight junction protein VE-cadherin in vessels. As seen in supplemental Figure 7, we observed the decreased expression of VE-cadherin in aorta from EC-Smad2/3KO embryos. Thus, we supposed that the N-cadherin and VE-cadherin in endothelial cells from EC-Smad2/3KO embryos are either mislocalized or lost (or reduced).
In situ hybridization of S1PR1 mRNA expression in E10.5 embryos. (A) Smad2fl/fl;Smad3+/−;Tie2-Cre embryo. (B) EC-Smad2/3 KO embryo. DA indicates dorsal aorta; H, heart; and ISV, intrasomitic vessel. Arrowheads indicate the representative ISVs expressing S1PR1 mRNA.
In situ hybridization of S1PR1 mRNA expression in E10.5 embryos. (A) Smad2fl/fl;Smad3+/−;Tie2-Cre embryo. (B) EC-Smad2/3 KO embryo. DA indicates dorsal aorta; H, heart; and ISV, intrasomitic vessel. Arrowheads indicate the representative ISVs expressing S1PR1 mRNA.
Immunohistochemical analysis of N-cadherin expression in DA of E10.5 embryos. Transverse frozen sections of Smad2fl/fl;Smad3+/−;Tie2-Cre (A-C) and EC-Smad2/3KO embryos (D-F) were stained with anti–PECAM-1 (A-D) and anti–N-cadherin antibodies (B-C,E-F). In panels C and F, the square areas in panels B and E are shown at higher magnifications, respectively.
Immunohistochemical analysis of N-cadherin expression in DA of E10.5 embryos. Transverse frozen sections of Smad2fl/fl;Smad3+/−;Tie2-Cre (A-C) and EC-Smad2/3KO embryos (D-F) were stained with anti–PECAM-1 (A-D) and anti–N-cadherin antibodies (B-C,E-F). In panels C and F, the square areas in panels B and E are shown at higher magnifications, respectively.
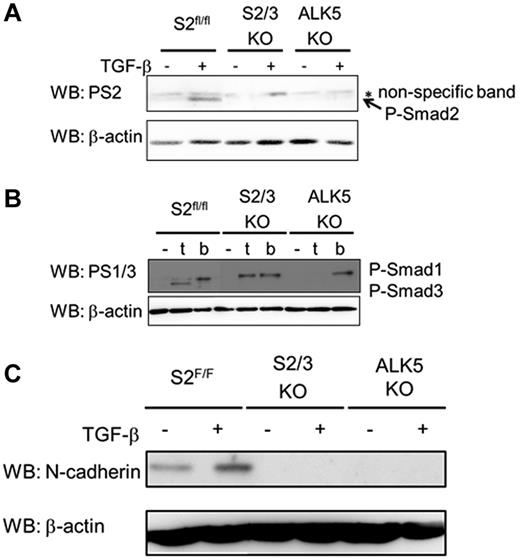
To investigate the possibilities discussed in the preceding paragraph, we used S2/3KO MEECs and ALK5KO MEECs. As a positive control, we also used S2fl/fl MEECs. Because S1PR1 expression in ECs from EC-Smad2/3KO embryos was not detected, we examined the expression of S1PR1 in S2/3KO MEECs. However, the expression of S1PR1 mRNA in S2/3KO MEECs was detected comparable with S2fl/fl MEECs. Consistently, TGF-β did not affect the expression of S1PR1 mRNA in S2fl/fl and S2/3KO MEECs (supplemental Figure 4C). In addition, we examined the expression of other S1PR receptor family (S1PR2 to S1PR5). The expression of S1PR2, S1PR3, and S1PR4, but not that of S1PR5 could be observed in S2fl/fl MEECs. On the other hand, we could detect the expression of S1PR2 and S1PR4 mRNAs in S2/3KO MEECs (supplemental Figure 4D). Although we do not currently know why the expression of S1PR3 mRNA cannot be detected in S2/3KO MEECs, it will be needed to consolidate if Smad2/3 signal is indispensable for the expression of S1PR3 mRNA in ECs. S2fl/fl MEECs showed Smad2 phosphorylation on TGF-β stimulation (Figure 7A), whereas the phosphorylation of Smad2 in S2/3KO MEECs and ALK5KO MEECs was not detected when the cells were stimulated with TGF-β. We already reported that TGF-β induces Smad1/5/8 phosphorylation in ECs because of the activation of the TGF-β/ALK1 pathway.5 As mentioned in the preceding paragraph, TGF-β promoted the phosphorylation of Smad1/5/8 in both S2fl/fl MEECs and S2/3KO MEECs although no detectable phosphorylation in ALK5KO MEECs could be seen on TGF-β stimulation (Figure 7B). Using these cell lines, we confirmed the effect of Smad2/3 signaling on induction of N-cadherin. In addition to detecting expression of N-cadherin in S2fl/fl MEECs, we also found that TGF-β stimulation potentiated its expression in S2fl/fl MEECs. However, the expression of N-cadherin was completely lost in both EC-Smad2/3KO and EC-ALK5 KO MEECs even though cells were stimulated with TGF-β. Collectively, Smad2/3 signaling regulates vascular integrity by induction of S1PR1 and regulates N-cadherin expression (Figure 7C).
TGF-β/Smad2/3 signaling induces N-cadherin expression. (A) TGF-β–induced Smad2 phosphorylation in ECs. MEECs were stimulated with TGF-β for 1 hour. The filters were incubated with anti-phosphorylated Smad2 (PS2; top panel) and anti–β-actin antibodies (bottom panel). P-Smad2, phosphorylated Smad2. (B) Detection of phosphorylated Smad1 and phosphorylated Smad3 on TGF-β (t) or BMP (b) stimulation. MEECs were stimulated with TGF-β or BMP6 for 1 hour. The filters were incubated with anti-phosphorylated Smad1/3 (PS1/3; top panel), which specifically recognize phosphorylated Smad1 and Smad3, and anti–β-actin antibodies (bottom panel). P-Smad1 indicates phosphorylated Smad1; and P-Smad3, phosphorylated Smad3. (C) TGF-β–induced N-cadherin expression in MEECs. MEECs were stimulated with TGF-β for 48 hour. The filters were incubated with anti–N-cadherin (top panel) and anti–β-actin antibodies (bottom panel).
TGF-β/Smad2/3 signaling induces N-cadherin expression. (A) TGF-β–induced Smad2 phosphorylation in ECs. MEECs were stimulated with TGF-β for 1 hour. The filters were incubated with anti-phosphorylated Smad2 (PS2; top panel) and anti–β-actin antibodies (bottom panel). P-Smad2, phosphorylated Smad2. (B) Detection of phosphorylated Smad1 and phosphorylated Smad3 on TGF-β (t) or BMP (b) stimulation. MEECs were stimulated with TGF-β or BMP6 for 1 hour. The filters were incubated with anti-phosphorylated Smad1/3 (PS1/3; top panel), which specifically recognize phosphorylated Smad1 and Smad3, and anti–β-actin antibodies (bottom panel). P-Smad1 indicates phosphorylated Smad1; and P-Smad3, phosphorylated Smad3. (C) TGF-β–induced N-cadherin expression in MEECs. MEECs were stimulated with TGF-β for 48 hour. The filters were incubated with anti–N-cadherin (top panel) and anti–β-actin antibodies (bottom panel).
Discussion
Genetic studies in humans as well as analysis in genetically modified mice have demonstrated that a number of genes involved in TGF-β signaling play key roles in vascular formation during both fetal development and adulthood. We indeed showed that TGF-β possesses a bilateral character in endothelium: a role in activation via the ALK1/Smad1/5 pathway and a role in resolution via the ALK5/Smad2/3 pathway. Our previous studies using ALK5 KI mice revealed that ALK5 kinase activity is required for vascular maturation in vivo via both the TGF-β/ALK1/Smad1/5 and the TGF-β/ALK5/Smad2/3 pathways.9,23 To further distinguish between the TGF-β/ALK1/Smad1/5 and TGF-β/ALK5/Smad2/3 pathways, we generated mice lacking both Smad2 and Smad3 genes in ECs because embryos lacking the Smad2 gene die at E7.5 before gastrulation.17 To our surprise, embryos lacking both Smad2 and Smad3 in the endothelium could show normal vascular development at E10.5 when ALK5KO mice become lethal because of their vascular abnormality.8 However, obvious hemorrhage could be detected in EC-Smad2/3KO embryos after E10.5. Consequently, no EC-Smad2/3KO embryos could survive beyond E12.5. To confirm the activity of cre recombinase in ECs around E9.5, mice carrying Tie2-Cre were mated with Rosa26 reporter mice. As expected, enzymatic activity could be seen in the endothelium (supplemental Figure 3).
EC-Smad4KO and EC-ALK5KO mice showed lethal phenotypes before and after the death of EC-Smad2/3KO embryos, respectively (supplemental Figure 2). Indeed, both Smad2 and Smad3 are not only downstream intracellular signal molecules for TGF-β signaling but also for activin and nodal signals.23,24 Thus, we supposed that activin-mediated ALK4 and/or nodal-activated ALK7 (or ALK4) compensate for the deficiency of ALK5 in ECs and transduce Smad2/3 signaling,23 whereby EC-ALK5KO embryos can be predicted to survive longer than EC-Smad2/3KO embryos. On the other hand, EC-Smad4KO embryos exhibit severe hemorrhage at E9.5 because Smad4 is required for all canonical TGF-β family signaling. Although we could not recognize any differences between the control and EC-Smad2/3KO yolk sacs at E10.5 on the macroscale, subtle abnormality in the vessels of the yolk sacs of EC-Smad2/3KO embryos at E10.5 could be observed (Figure 2B). Thus, the vascular integrity is gradually dysregulated in the yolk sac of EC-Smad2/3KO embryos despite the absence of bleeding at E10.5. Indeed, wide gaps between ECs and mural cells of EC-Smad2/3KO embryos at E10.5 could be seen in the ultrastructural analysis by electron microscopy (Figure 3). It has been reported that TGF-β directly induces the production of PDGF-B and VEGF in ECs and mural cells.25,–27 VEGF influences the growth and differentiation of both cell types, whereas PDGF-B promotes mural cell migration and maturation.28 One possible mechanism for defect of the vascular integrity in EC-Smad2/3KO embryos might be the decrease in angiogenic cytokines.
Vascular sprouting can be experimentally detected using the aorta ring assay as an ex vivo assay.29 However, it was not possible to gain enough length of aorta from embryos at E10.5. Therefore, instead of using the aorta, we isolated OMDs from the embryos to show vascular sprouting ex vivo, which is a novel method to detect angiogenic responses. Obviously, vascular sprouting from the control embryos could be viewed in the presence, but not in the absence of VEGF (Figure 4, data not shown). Because rescue by adenoviral infection of either Smad2 or Smad3 into OMDs of EC-Smad2/3KO embryos improved vascular sprouting ex vivo, either Smad2 or Smad3 is probably enough for ECs to exhibit the angiogenic reaction in the presence of VEGF, although Smad2, unlike Smad3, is known to lack DNA binding activities.
Among genetically modified mice that exhibit vascular defects, the phenotype of EC-Smad2/3KO mice was quite similar to that of S1PR1KO mice.21 Vasculogenesis and angiogenesis appeared normal in both genetically modified mice although their vascular maturation was incomplete because of inadequate recruitment of mural cells to the vessel walls. Thus, both embryos suffer from hemorrhage that leads to death after E12.5. We speculated that S1PR1 expression in ECs decreased in EC-Smad2/3KO mice. In fact, we could not detect any transcripts of the S1PR1 gene along the intrasomites of EC-Smad2/3KO embryos at E10.5 although S1PR1 mRNA could be observed in the control embryos (Figure 5). In contrast, the expression of S1PR1 mRNA could be detected in S2/3KO MEECs comparable with the S2fl/fl MEECs (supplemental Figure 4C). Thus, we speculated the following possibility; mural cells stimulated with TGF-β which is secreted from ECs produces other cytokines(s) that suppresses the expression of S1PR1 in ECs. We will need to elucidate this possibility in the future experiments. Lee et al demonstrated that overexpression of S1PR1 enhances the expression of N-cadherin and promotes adhesion junctions.30 When we checked the expression of N-cadherin in ECs, it was reduced in the vasculature of EC-Smad2/3KO embryos compared with that of the control embryos. In addition to the reduced expression of the adhesion junction marker N-cadherin, ECs of the EC-Smad2/3KO embryos revealed lower expression of a tight junction marker, claudin-5, than did ECs of the control embryos. Consistent with the decreased (or undetectable) level of junction proteins in ECs, we could observe wide gaps between ECs and mural cells in EC-Smad2/3KO embryos (Figures 3A-B). To confirm that the expression of N-cadherin is influenced by TGF-β, we established ECs from EC-Smad2/3KO embryos. The ECs were then stimulated with TGF-β. Consistent with the immunohistochemistry findings, no N-cadherin was detected in the ECs from EC-Smad2/3KO embryos even though the ECs had been stimulated with TGF-β. In contrast, the expression of N-cadherin in the control ECs was enhanced on TGF-β stimulation. In the process of epithelial-mesenchymal transition, the expression of N-cadherin is known to be enhanced by TGF-β.31 We are not sure whether N-cadherin is a direct target gene for TGF-β signaling because the expression of N-cadherin was not increased immediately after TGF-β stimulation. Recently, Li et al reported that endothelial Smad4 maintains vascular integrity via the activation of the N-cadherin promoter,32 which is a key factor for recruitment of mural cells. The expression of N-cadherin might be required for well-developed junctions between ECs and mural cells. It was reported that S1P/S1PR1 signaling is indispensable for TGF-β signaling in EMT.33 Thus, it would be interesting to investigate the effect of TGF-β signaling in S1PR1-deficient embryos in future.
In summary, Smad2 and Smad3 in ECs are indispensable for maintenance of vascular integrity via the regulation of N-cadherin and S1PR1 expressions in the vasculature to control vessel maturation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ms F. Miyamasu for excellent English proofreading, Prof Urban Deutsch for polyoma middle T expressing system, and Prof. P. ten Dijke for valuable suggestions. Observation with the confocal microscope was done at NIMS Nanotechnology Innovation Station under the support from “Nanotechnology Network Project” of the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan.
This research was supported by Grants-in-aid for Young Scientists A (F.I.) and Grants-in-aid for Scientific Research (17390073, 20012007, 21390115, and 21590328; M.K. and S.I.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; a Grant-in-Aid for JSPS Fellows from the Japan Society for the Promotion of Science (F.I.); Grants-in-aid from the Research Committee for Hereditary Small Vessel Disease, the Ministry of Health, Labour, and Welfare of Japan (F.I.); the Takeda Science Foundation (F.I., S.I.); the Yasuda Medical Foundation (M.K.); the Naito Foundation (S.I.); and the Uehara Memorial Foundation (F.I.).
Authorship
Contribution: F.I., T.A., Y.M., K.I., T.T., T.K., and S.I. performed the experiments; M.F., M.W., U.D., and S.K. provided genetically modified mice and critical reagents; F.I. and S.I. designed most aspects of the research, interpreted the data, and drafted the paper; and M.K. reviewed the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Susumu Itoh, Laboratory of Biochemistry, Showa Pharmaceutical University, 3-3165 Higashi-Tamagawagakuen, Machida, Tokyo 194-8543, Japan; e-mail: sitoh@ac.shoyaku.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal