Abstract
The Medical Research Council Myeloma IX Trial (ISRCTNG8454111) examined traditional and thalidomide-based induction and maintenance regimens and IV zoledronic acid (ZOL) and oral clodronate (CLO) in 1960 patients with newly diagnosed multiple myeloma. Overall survival (OS) and skeletal-related event (SRE) data have been reported for the overall trial population. The present analysis investigated optimal therapy regimens for different patient populations in Myeloma IX. Patients were assigned to intensive or nonintensive treatment pathways and randomized to induction cyclophosphamide, vincristine, doxorubicin, and dexamethasone (CVAD) versus cyclophosphamide, thalidomide, and dexamethasone (CTD; intensive) or melphalan and prednisolone versus attenuated oral CTD (CTDa; nonintensive). Patients were also randomized to ZOL or CLO. In the nonintensive pathway, CTDa produced better responses and lower SRE rates than melphalan and prednisolone. ZOL improved OS compared with CLO independently of sex, stage, or myeloma subtype, most profoundly in patients with baseline bone disease or other SREs. In patients treated for ≥ 2 years, ZOL improved OS compared with CLO from randomization (median not reached for either; P = .02) and also from first on-study disease progression (median, 34 months for ZOL vs 27 months for CLO; P = .03). Thalidomide-containing regimens had better efficacy than traditional regimens, and ZOL demonstrated greater benefits than CLO.
Introduction
During the last 2 decades, therapy for patients with multiple myeloma (MM) has evolved rapidly with the integration of novel approaches (eg, immunomodulating agents and targeted therapies) to enhance remission rates and prolong survival. Although the 5-year survival rates for patients with MM have improved, reaching approximately 36% in recent studies, considerable room for improvement remains, especially among elderly or poor-performance-status patients.1 Current therapy options for patients with MM include both novel and standard chemotherapy regimens. Proteasome inhibitors, immunomodulating agents, and corticosteroids are routinely used in approximately 75% of patients with newly diagnosed MM,2 and approximately 33% receive bisphosphonate therapy.2
Most clinical treatment guidelines in MM now recommend bisphosphonate therapy in all patients with symptomatic MM regardless of whether they have obvious bone lesions, and in this setting, zoledronic acid (ZOL) is becoming the preferred agent.3 Although there is no consensus on the optimal duration of bisphosphonate therapy, most guidelines recommend at least 2 years of treatment in patients with osteolytic bone disease or osteopenia.4-6 Overall, the decision for a longer duration of treatment is usually at the discretion of the treating physician; however, in previous Medical Research Council (MRC) studies, oral clodronate (CLO) was continued indefinitely or at least until disease progression.7
The MRC Myeloma IX trial (N = 1960) included an examination of the effects of ZOL, a nitrogen-containing bisphosphonate of high potency (as demonstrated in preclinical studies), versus oral CLO in addition to antimyeloma therapy (intensive and nonintensive pathways) in patients with newly diagnosed MM. Previous results from Myeloma IX have been reported,8-10 and analyses by bisphosphonate therapy have shown that ZOL significantly improved progression-free survival (PFS) by 12% (hazard ratio [HR] = 0.88; P = .018) and reduced the risk of death by 16% (HR = 0.84; P = .012) compared with CLO, prolonging overall survival (OS) by 5.5 months.11 The observed OS benefit with ZOL versus CLO remained significant after adjusting for the improved prevention of potentially life-limiting skeletal-related events (SREs; HR = 0.85; P = .018).11 Furthermore, ZOL significantly reduced the risk of SREs compared with CLO (HR = 0.74; P = .0004) in patients with (HR = 0.77, P = .0038) and without (HR = 0.53; P = .0068) bone disease or other SREs at baseline and reduced the incidence of all types of SREs.12 Both bisphosphonates were administered at least until disease progression.
In the present analysis of Myeloma IX, we investigated which of the 4 combinations of therapeutic options in each of the treatment pathways provided better outcomes in terms of PFS, OS, and SREs and examined the potential benefits of longer-term bisphosphonate therapy beyond 2 years. In addition, exploratory analyses (eg, by prognostic variables or duration of bisphosphonate therapy) were used to investigate whether treatment benefits were consistent across patient subsets.
Methods
Patient eligibility criteria
Adult patients (18 years of age or older) with newly diagnosed and histologically confirmed MM were eligible for inclusion, as described previously.11,12 Patients with evidence of bone lesions on axial skeletal survey or fracture at baseline were defined as having myeloma bone disease. Exclusion criteria included previous or concurrent active malignancies, acute renal failure (defined as serum creatinine greater than 500μM unresponsive to 72 hours of rehydration, urine output less than 400 mL/d, and/or requirement for dialysis), and previous treatment for MM (except local radiotherapy, bisphosphonates, or low-dose corticosteroids).
The trial was approved by the North West Multicenter Research Ethics Committee and by local review committees at all participating centers. All patients provided written informed consent in accordance with the Declaration of Helsinki.
Treatment
Patients were allocated to 2 main treatment pathways (intensive and nonintensive) as described previously.11,12 Pathway selection had no rigid age cutoff and was based on performance status, clinician judgment, and patient preference. In the intensive pathway, patients were randomized to 4-6 21-day cycles of either CVAD (oral cyclophosphamide 500 mg/wk, vincristine 0.4 mg/d combined with doxorubicin 9 mg/m2/d as a 4-day continuous infusion, and dexamethasone 40 mg/d on days 1-4 and 12-15) or oral CTD (cyclophosphamide 500 mg/wk, thalidomide 100 mg/d and increasing to 200 mg/d as tolerated, and dexamethasone 40 mg/d on days 1-4 and 12-15). After completion of induction therapy (4-6 cycles, depending on response), patients underwent peripheral blood stem-cell mobilization and harvest, high-dose melphalan treatment (200 mg/m2), and autologous stem cell transplantation (ASCT). In the nonintensive pathway, patients were randomized to 6-9 28-day cycles of either oral MP (melphalan 7 mg/m2/d and prednisolone 40 mg/d, both on days 1-4) or attenuated oral CTD (CTDa; cyclophosphamide 500 mg/wk, thalidomide 50 mg/d initially and increasing to 200 mg/d as tolerated, and dexamethasone 20 mg/d on days 1-4 and 15-18). Treatment was continued until best response (minimum of 6 cycles, maximum of 9 cycles). In each pathway, patients were randomized to oral CLO (1600 mg/d) or IV ZOL (4 mg every 3-4 weeks with induction chemotherapy and every 4 weeks thereafter). Dose adjustment for patients with impaired renal function at baseline and dose delays in patients with on-study creatinine elevations were implemented for ZOL per the prescribing information. After first-line therapy, eligible patients were randomized to thalidomide maintenance therapy (50 mg/d initially, increasing to 100 mg/d if tolerated) or no thalidomide maintenance. Bisphosphonates and maintenance therapy were to be continued at least until disease progression.
Assessment of study outcomes
The primary end points were PFS, OS, and overall response rate. Secondary end points included SREs (defined as fractures, spinal cord compression, radiation or surgery to bone, and new osteolytic lesions [counted as an SRE first and then as disease progression]) and safety.
Safety was evaluated by standard laboratory assessments (as described previously) and continuous adverse event monitoring. Recommendations for monitoring and maintaining oral health to reduce the risk of osteonecrosis of the jaw and to identify and manage suspected cases were provided to trial investigators starting in June 2006 after the recommendations of Weitzman et al were released.13
Data on SREs were collected every 3 months until disease progression. Disease was assessed after each cycle of induction chemotherapy (before high-dose melphalan and ASCT in the intensive pathway) and every 3 months thereafter, with an assessment at 100 days after ASCT in the intensive pathway. Treatment response was monitored centrally at the University of Birmingham (Birmingham, United Kingdom) from serum and urine protein and free light-chain studies or, if results of such studies were missing, by review of local laboratory results that were verified by an independent response-assessment panel. Complete response (CR) was defined as negative immunofixation (100% M-protein reduction), and very good partial response (VGPR) was defined as at least 90% M-protein reduction with positive immunofixation.14
A diagnosis of bone lesions was not a study-entry requirement. Baseline bone disease was screened using axial skeletal surveys and was defined as prior fractures at any site or osteolytic lesions; however, neither prior spinal cord compression nor radiation or surgery to bone was considered bone disease. Some exploratory analyses of ZOL versus CLO included any prior SRE (including spinal cord compression and radiation or surgery to bone) in addition to baseline bone disease. Fluorescence in-situ hybridization analysis of CD138-selected plasma cells was performed using standard methodology for a subset of patients (n = 1181). Gene expression was assessed using the Affymetrix system in a subset of patients (n = 261).
Statistical analyses
Analyses were based on the treatments that patients were randomized to receive and on the intention-to-treat population, defined as all randomized patients with histologically confirmed MM who provided written informed consent. Time to first SRE was assessed using the cumulative incidence function, and OS was estimated using the Kaplan-Meier method. A Cox analysis was used to assess the HR and associated 95% confidence intervals for SRE risk, adjusting for effects of chemotherapy regimen, minimization factors, and SRE history at baseline (stratified by pathway).15 Subgroup analyses by baseline demographics and disease characteristics were performed. Correlations between bone disease and subgroups were tested using the Fisher exact test. The log-rank test was used to compare both Kaplan-Meier curves and cumulative incidence curves.
Statistical analyses were performed with SAS Version 9.2 software (SAS Institute) and Digital Visual Fortran Version 6.0A software (DIGITAL). All tests were 2-sided and at the 5% significance level without adjustment for multiplicity. This trial is registered at www.controlled-trials.com as ISRCTN68454111.
Results
Baseline characteristics
Of 1970 patients enrolled between May 14, 2003, and November 20, 2007, 1960 were included in the intention-to-treat population (Figure 1).11 Baseline demographics and disease characteristics were different between the intensive and nonintensive pathways and were generally balanced between treatment groups within each pathway (Table 1). The median number of cycles of chemotherapy administered was 4 (range, 0-8) in the CVAD group, 5 (range, 0-9) in the CTD group, and 6 in both the MP (range, 0-18) and CTDa (range, 0-11) groups.
Trial profile. *Patients were included in the safety population. †Two patients on ZOL received a conditioning regimen other than high-dose melphalan and underwent subsequent ASCT. ‡One patient in the nonintensive pathway who was excluded because no consent was received was then included in the randomization to receive maintenance treatment. Some of the 23 patients in the intensive pathway who did not start CVAD or CTD did receive high-dose melphalan plus ASCT, but none was included in the randomization to receive maintenance treatment. None of the 12 patients in the nonintensive pathway who did not start MP or CTDa was included in the randomization to receive maintenance treatment. BD indicates bortezomib and dexamethasone; CVAD, cyclophosphamide, vincristine, doxorubicin, and dexamethasone; CTD, cyclophosphamide, thalidomide, and dexamethasone; CTDa, attenuated CTD; and MP, melphalan and prednisolone. Reprinted from The Lancet, 376, Morgan GJ, Davies FE, Gregory WM, et al, First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial, 1989-1999, copyright 2010, with permission from Elsevier.11
Trial profile. *Patients were included in the safety population. †Two patients on ZOL received a conditioning regimen other than high-dose melphalan and underwent subsequent ASCT. ‡One patient in the nonintensive pathway who was excluded because no consent was received was then included in the randomization to receive maintenance treatment. Some of the 23 patients in the intensive pathway who did not start CVAD or CTD did receive high-dose melphalan plus ASCT, but none was included in the randomization to receive maintenance treatment. None of the 12 patients in the nonintensive pathway who did not start MP or CTDa was included in the randomization to receive maintenance treatment. BD indicates bortezomib and dexamethasone; CVAD, cyclophosphamide, vincristine, doxorubicin, and dexamethasone; CTD, cyclophosphamide, thalidomide, and dexamethasone; CTDa, attenuated CTD; and MP, melphalan and prednisolone. Reprinted from The Lancet, 376, Morgan GJ, Davies FE, Gregory WM, et al, First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial, 1989-1999, copyright 2010, with permission from Elsevier.11
Baseline demographics and disease characteristics (ITT population)
| Variable . | Nonintensive pathway (n = 849) . | Intensive pathway (n = 1111) . | ||||||
|---|---|---|---|---|---|---|---|---|
| MP (n = 423) . | CTDa (n = 426) . | CVAD (n = 556) . | CTD (n = 555) . | |||||
| ZOL . | CLO . | ZOL . | CLO . | ZOL . | CLO . | ZOL . | CLO . | |
| (n = 212) . | (n = 211) . | (n = 214) . | (n = 212) . | (n = 278) . | (n = 278) . | (n = 277) . | (n = 278) . | |
| Median age, y (range) | 73 (59-89) | 74 (57-88) | 74 (61-87) | 73 (58-85) | 59 (31-74) | 58 (39-72) | 58 (33-71) | 59 (33-78) |
| Sex, n (%) | ||||||||
| Female | 95 (44.8) | 97 (46.0) | 96 (44.9) | 88 (41.5) | 100 (36.0) | 108 (38.8) | 101 (36.5) | 110 (39.6) |
| Male | 117 (55.2) | 114 (54.0) | 118 (55.1) | 124 (58.5) | 178 (64.0) | 170 (61.2) | 176 (63.5) | 168 (60.4) |
| ISS stage, n (%) | ||||||||
| I | 37 (17.5) | 27 (12.8) | 26 (12.1) | 20 (9.4) | 59 (21.2) | 65 (23.4) | 70 (25.3) | 81 (29.1) |
| II | 63 (29.7) | 93 (44.1) | 76 (35.5) | 80 (37.7) | 93 (33.5) | 98 (25.3) | 105 (37.9) | 84 (30.2) |
| III | 92 (43.4) | 73 (34.6) | 81 (37.9) | 87 (41.0) | 98 (35.3) | 85 (30.6) | 76 (27.4) | 84 (30.2) |
| Data unavailable | 20 (9.4) | 18 (8.5) | 31 (14.5) | 25 (11.8) | 28 (10.1) | 30 (10.8) | 26 (9.4) | 29 (10.4) |
| Hyperdiploidy, n (%)* | ||||||||
| Yes | 56 (26.4) | 71 (33.6) | 61 (28.5) | 61 (28.8) | 70 (25.2) | 93 (33.5) | 89 (32.1) | 78 (28.1) |
| No | 41 (19.3) | 31 (14.7) | 46 (21.5) | 45 (21.2) | 62 (22.3) | 68 (24.5) | 71 (25.6) | 64 (23.0) |
| Data unavailable | 115 (54.2) | 109 (51.7) | 107 (50.0) | 106 (50.0) | 146 (52.5) | 117 (42.1) | 117 (42.2) | 136 (48.9) |
| t(4;14), n (%)* | ||||||||
| Yes | 12 (5.7) | 9 (4.3) | 6 (2.8) | 17 (8.0) | 18 (6.5) | 17 (6.1) | 20 (7.2) | 21 (7.6) |
| No | 90 (42.5) | 100 (47.4) | 102 (47.7) | 98 (46.2) | 118 (42.4) | 152 (54.7) | 144 (52.0) | 129 (46.4) |
| Data unavailable | 110 (51.9) | 102 (48.3) | 106 (49.5) | 97 (45.8) | 142 (51.1) | 109 (39.2) | 113 (40.8) | 128 (46.0) |
| t(11;14), n (%)* | ||||||||
| Yes | 16 (7.5) | 8 (3.8) | 14 (6.5) | 16 (7.5) | 18 (6.5) | 28 (10.1) | 27 (9.7) | 19 (6.8) |
| No | 86 (40.6) | 101 (47.9) | 95 (44.4) | 98 (46.2) | 118 (42.4) | 140 (50.4) | 137 (49.5) | 130 (46.8) |
| Data unavailable | 110 (51.9) | 102 (48.3) | 105 (49.1) | 98 (46.2) | 142 (51.1) | 110 (39.6) | 113 (40.8) | 129 (46.4) |
| Bone disease or other SRE, n (%)† | ||||||||
| Yes (SRE+) | 138 (65.1) | 146 (69.2) | 137 (64.0) | 130 (61.3) | 199 (71.6) | 210 (75.5) | 194 (70.0) | 196 (70.5) |
| No (SRE−) | 72 (34.0) | 60 (28.4) | 71 (33.2) | 75 (35.4) | 77 (27.7) | 65 (23.4) | 82 (29.6) | 76 (27.3) |
| Data unavailable | 2 (0.9) | 5 (2.4) | 6 (2.8) | 7 (3.3) | 2 (0.7) | 3 (1.1) | 1 (0.4) | 6 (2.2) |
| Variable . | Nonintensive pathway (n = 849) . | Intensive pathway (n = 1111) . | ||||||
|---|---|---|---|---|---|---|---|---|
| MP (n = 423) . | CTDa (n = 426) . | CVAD (n = 556) . | CTD (n = 555) . | |||||
| ZOL . | CLO . | ZOL . | CLO . | ZOL . | CLO . | ZOL . | CLO . | |
| (n = 212) . | (n = 211) . | (n = 214) . | (n = 212) . | (n = 278) . | (n = 278) . | (n = 277) . | (n = 278) . | |
| Median age, y (range) | 73 (59-89) | 74 (57-88) | 74 (61-87) | 73 (58-85) | 59 (31-74) | 58 (39-72) | 58 (33-71) | 59 (33-78) |
| Sex, n (%) | ||||||||
| Female | 95 (44.8) | 97 (46.0) | 96 (44.9) | 88 (41.5) | 100 (36.0) | 108 (38.8) | 101 (36.5) | 110 (39.6) |
| Male | 117 (55.2) | 114 (54.0) | 118 (55.1) | 124 (58.5) | 178 (64.0) | 170 (61.2) | 176 (63.5) | 168 (60.4) |
| ISS stage, n (%) | ||||||||
| I | 37 (17.5) | 27 (12.8) | 26 (12.1) | 20 (9.4) | 59 (21.2) | 65 (23.4) | 70 (25.3) | 81 (29.1) |
| II | 63 (29.7) | 93 (44.1) | 76 (35.5) | 80 (37.7) | 93 (33.5) | 98 (25.3) | 105 (37.9) | 84 (30.2) |
| III | 92 (43.4) | 73 (34.6) | 81 (37.9) | 87 (41.0) | 98 (35.3) | 85 (30.6) | 76 (27.4) | 84 (30.2) |
| Data unavailable | 20 (9.4) | 18 (8.5) | 31 (14.5) | 25 (11.8) | 28 (10.1) | 30 (10.8) | 26 (9.4) | 29 (10.4) |
| Hyperdiploidy, n (%)* | ||||||||
| Yes | 56 (26.4) | 71 (33.6) | 61 (28.5) | 61 (28.8) | 70 (25.2) | 93 (33.5) | 89 (32.1) | 78 (28.1) |
| No | 41 (19.3) | 31 (14.7) | 46 (21.5) | 45 (21.2) | 62 (22.3) | 68 (24.5) | 71 (25.6) | 64 (23.0) |
| Data unavailable | 115 (54.2) | 109 (51.7) | 107 (50.0) | 106 (50.0) | 146 (52.5) | 117 (42.1) | 117 (42.2) | 136 (48.9) |
| t(4;14), n (%)* | ||||||||
| Yes | 12 (5.7) | 9 (4.3) | 6 (2.8) | 17 (8.0) | 18 (6.5) | 17 (6.1) | 20 (7.2) | 21 (7.6) |
| No | 90 (42.5) | 100 (47.4) | 102 (47.7) | 98 (46.2) | 118 (42.4) | 152 (54.7) | 144 (52.0) | 129 (46.4) |
| Data unavailable | 110 (51.9) | 102 (48.3) | 106 (49.5) | 97 (45.8) | 142 (51.1) | 109 (39.2) | 113 (40.8) | 128 (46.0) |
| t(11;14), n (%)* | ||||||||
| Yes | 16 (7.5) | 8 (3.8) | 14 (6.5) | 16 (7.5) | 18 (6.5) | 28 (10.1) | 27 (9.7) | 19 (6.8) |
| No | 86 (40.6) | 101 (47.9) | 95 (44.4) | 98 (46.2) | 118 (42.4) | 140 (50.4) | 137 (49.5) | 130 (46.8) |
| Data unavailable | 110 (51.9) | 102 (48.3) | 105 (49.1) | 98 (46.2) | 142 (51.1) | 110 (39.6) | 113 (40.8) | 129 (46.4) |
| Bone disease or other SRE, n (%)† | ||||||||
| Yes (SRE+) | 138 (65.1) | 146 (69.2) | 137 (64.0) | 130 (61.3) | 199 (71.6) | 210 (75.5) | 194 (70.0) | 196 (70.5) |
| No (SRE−) | 72 (34.0) | 60 (28.4) | 71 (33.2) | 75 (35.4) | 77 (27.7) | 65 (23.4) | 82 (29.6) | 76 (27.3) |
| Data unavailable | 2 (0.9) | 5 (2.4) | 6 (2.8) | 7 (3.3) | 2 (0.7) | 3 (1.1) | 1 (0.4) | 6 (2.2) |
ISS indicates International Staging System; and ITT, intention-to-treat.
Genetic analysis by FISH was performed in a subset of patients (n = 1184).
Defined as fractures, spinal cord compression, radiation or surgery to bone, or new osteolytic lesions.
Efficacy analyses
Intensive pathway.
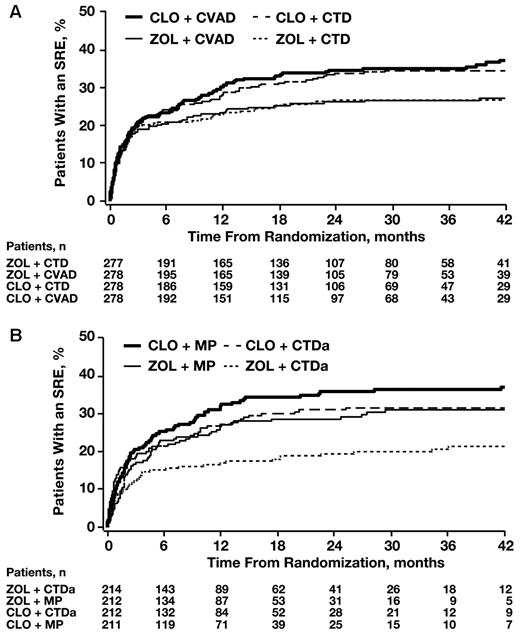
In the intensive pathway (n = 1111), a larger number of patients achieved CR or VGPR after induction therapy with CTD versus CVAD (43.2% vs 27.5%, respectively; Fisher exact P < .0001). Similar numbers of patients achieved at least VGPR in the ZOL and CLO groups (36.0% vs 34.7%, respectively; P = .66). There was no significant difference in the risk of SREs between the CTD and the CVAD groups (HR = 1.03; Cox P = .80). ZOL significantly reduced the incidence and risk of SREs in the intensive pathway overall (27.9% vs 36.3%; HR = 0.76; Cox P = .017) and in each chemotherapy subgroup (Figure 2A). The risk of death was numerically lower in the ZOL group versus the CLO group in this pathway, but the comparison was not powered for statistical significance (HR = 0.84, Cox P = .085). Risk of progression or death (ie, PFS) was also numerically lower in the ZOL group versus the CLO group (HR = 0.90; Cox P = .173).
Time to first SRE by treatment pathway. (A) Intensive treatment pathway. (B) Nonintensive treatment pathway.
Time to first SRE by treatment pathway. (A) Intensive treatment pathway. (B) Nonintensive treatment pathway.
Nonintensive pathway.
In the nonintensive pathway (n = 849), a larger number of patients achieved CR or VGPR with CTDa versus MP (30.0% vs 4.0%, respectively; Fisher exact P < .0001). Furthermore, CTDa significantly reduced the risk of a first SRE versus MP (HR = 0.74; Cox P = .0205). In each of these groups, more ZOL-treated than CLO-treated patients achieved CR or VGPR (MP: 6.1% vs 1.9%, respectively; CTDa: 33.6% vs 26.4%, respectively; overall logistic regression P = .018). Overall, ZOL significantly prolonged time to first SRE compared with CLO and reduced the incidence of SREs in each group (Figure 2B). The greatest SRE reduction was observed in the CTDa + ZOL group and the least reduction in the MP + CLO group, suggesting that the combination of the more active agents resulted in better outcomes. The risk of death was significantly lower with ZOL versus CLO (HR = 0.83; Cox P = .049), and the risk of disease progression or death (ie, PFS events) was numerically lower in ZOL- versus CLO-treated patients (HR = 0.87; Cox P = .065).
Maintenance therapy and follow-up.
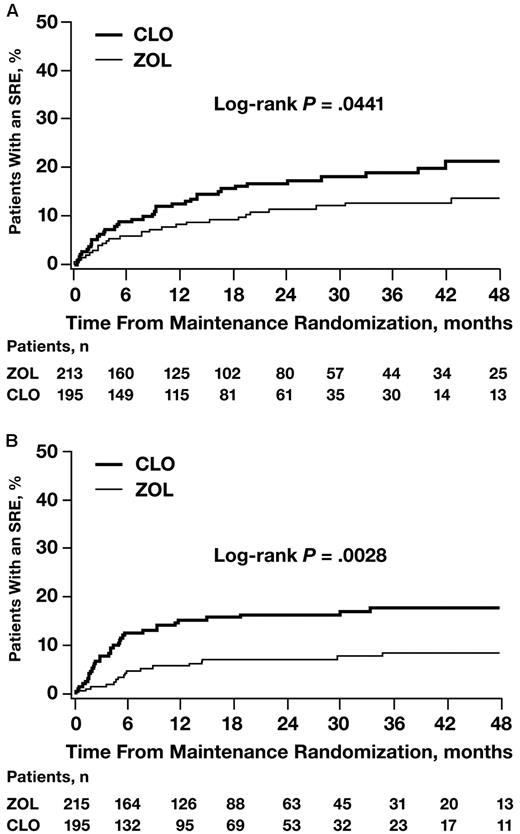
Among patients randomized to thalidomide maintenance or no thalidomide maintenance therapy (n = 820), ZOL significantly reduced the risk of SREs versus CLO regardless of whether patients received thalidomide (Figure 3A) or no thalidomide (Figure 3B).
Time to first SRE by maintenance therapy. (A) Patients randomized to maintenance therapy. (B) Patients randomized to no maintenance therapy.
Time to first SRE by maintenance therapy. (A) Patients randomized to maintenance therapy. (B) Patients randomized to no maintenance therapy.
Exploratory subset analyses by demographics, disease characteristics, and duration of therapy
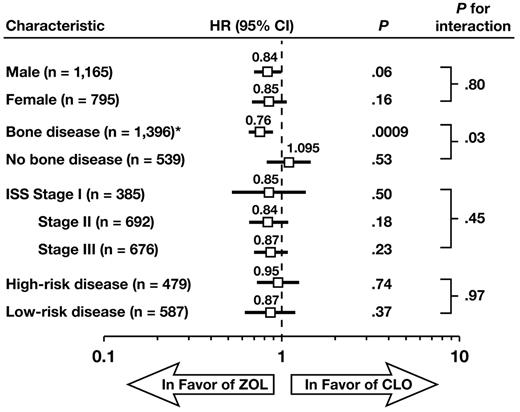
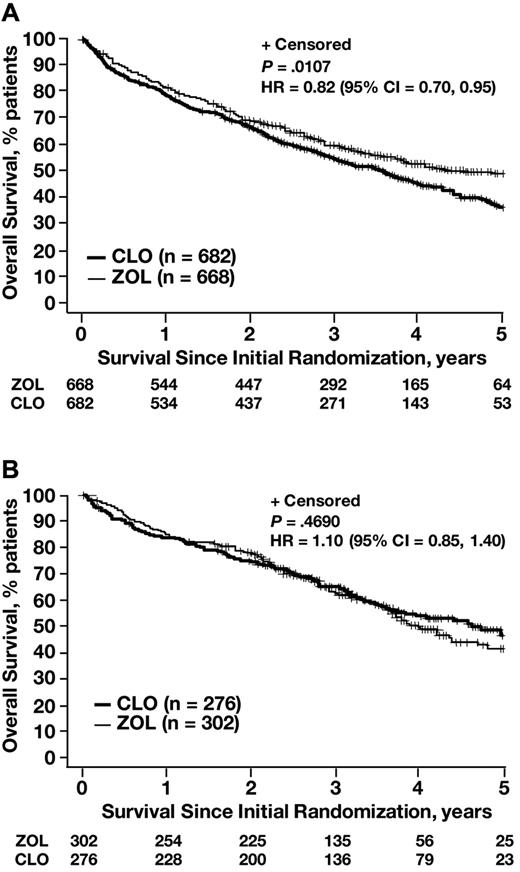
In exploratory analyses, the OS benefit with ZOL versus CLO was consistent regardless of treatment regimen, sex, International Staging System stage, and myeloma subtypes (high-risk and low-risk), but varied significantly by baseline bone disease status (P = .03 for the interaction; Figure 4). Bone disease at baseline was defined as prior fractures at any site or osteolytic lesions and excluded prior spinal cord compression or radiation or surgery to bone. In the overall trial population (regardless of bisphosphonate therapy), patients with documented bone disease at baseline had a significantly shorter OS (median, 45.5 months; n = 1401) compared with patients without bone disease at baseline (median, 51.6 months; n = 540; P = .009). In subset analyses, ZOL significantly reduced the risk of death compared with CLO in patients with confirmed bone disease or other SRE at baseline (designated as SRE+ and defined as osteolytic lesions, fracture, spinal cord compression, or radiation or surgery to bone; HR = 0.82; log-rank P = .0107; Figure 5), but not in patients without bone disease or other SRE at baseline (SRE−; HR = 1.10; log-rank P = .469; Figure 5).
Survival by sex, stage, baseline bone disease status, and myeloma subtype. Forest plot of OS for ZOL versus CLO. *Bone disease was defined as osteolytic lesions, fracture, spinal cord compression, or radiation or surgery to bone. Patients with high-risk disease included those with the following mutations: t(4;14), t(14;16), t(14;20), del(17p13) or +1q21 (both pathways) or del(1p32) (intensive pathway). Patients with low-risk disease had the following mutation: t(11;14). CI indicates confidence interval; and ISS, International Staging System.
Survival by sex, stage, baseline bone disease status, and myeloma subtype. Forest plot of OS for ZOL versus CLO. *Bone disease was defined as osteolytic lesions, fracture, spinal cord compression, or radiation or surgery to bone. Patients with high-risk disease included those with the following mutations: t(4;14), t(14;16), t(14;20), del(17p13) or +1q21 (both pathways) or del(1p32) (intensive pathway). Patients with low-risk disease had the following mutation: t(11;14). CI indicates confidence interval; and ISS, International Staging System.
Kaplan-Meier analyses of OS with ZOL versus CLO in patients with or without bone disease or other SRE at baseline. (A) Patients with bone disease or other SRE at baseline. (B) Patients without bone disease or other SRE at baseline. CI indicates confidence interval.
Kaplan-Meier analyses of OS with ZOL versus CLO in patients with or without bone disease or other SRE at baseline. (A) Patients with bone disease or other SRE at baseline. (B) Patients without bone disease or other SRE at baseline. CI indicates confidence interval.
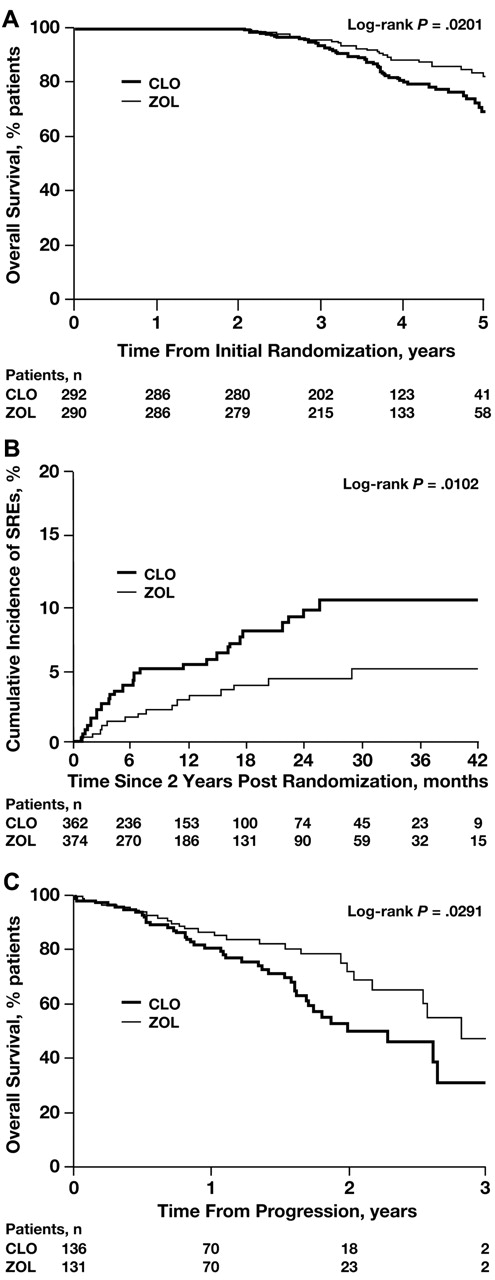
Among patients who received at least 2 years of bisphosphonate therapy (n = 582), ZOL significantly improved OS from initial randomization compared with CLO (medians not reached; HR = 0.60; log-rank P = .02; Figure 6A). In the same group of patients, ZOL also significantly reduced the incidence of SREs compared with CLO (log-rank P = .0102; Figure 6B). Moreover, ZOL also significantly improved OS after patients experienced a first disease progression event compared with CLO (34 vs 27 months, respectively; HR = 0.58; log-rank P = .03; Figure 6C). Further, an exploratory analysis of OS offset by cumulative 12-month intervals showed consistent, albeit statistically nonsignificant, reductions in the incidence of death with ZOL compared with CLO. These improvements in survival with ZOL versus CLO were observed in patients surviving for at least 1 year after randomization (HR = 0.90; P = .23), 2 years after randomization (HR = 0.89; P = .27), 3 years after randomization (HR = 0.81; P = .19), and 4 years after randomization (HR = 0.83; P = .48). A significant, consistent reduction in SRE incidence using similar 12-month intervals for ZOL versus CLO has been described previously.12
Kaplan-Meier analyses of OS and SRE incidence with ZOL versus CLO in patients who received at least 2 years of bisphosphonate therapy. (A) OS overall. (B) SRE incidence overall. (C) OS from progression.
Kaplan-Meier analyses of OS and SRE incidence with ZOL versus CLO in patients who received at least 2 years of bisphosphonate therapy. (A) OS overall. (B) SRE incidence overall. (C) OS from progression.
Safety analyses
Overall, serious adverse events were consistent with the established safety profiles of the agents and procedures used in patients with MM (Table 2) and have been described previously in detail.11,12 As noted in a preceding publication of Myeloma IX data, venous thrombotic events (VTEs) were more common in the ZOL group compared with the CLO group.11 In the present analysis, long-term tolerability beyond 2 years of bisphosphonate therapy was good overall, and patients with at least 2 years of bisphosphonate therapy (n = 582) experienced 12 (2.1%) cases of osteonecrosis of the jaw (ZOL, 12 cases in 12 [4.1%] patients; CLO, 0 cases in 0 [0.0%] patients), 26 fractures in 24 (4.1%) patients (ZOL, 10 fractures in 9 [3.1%] patients; CLO, 16 fractures in 15 [5.1%] patients), and 5 cases of acute renal failure in 5 (0.9%) patients (ZOL, 3 [1.0%] patients; CLO, 2 [0.7%]).
Adverse events of interest (safety population)
| Adverse event, n (%) . | Nonintensive pathway (n = 851) . | Intensive pathway (n = 1111) . | ||||||
|---|---|---|---|---|---|---|---|---|
| MP (n = 424) . | CTDa (n = 427) . | CVAD (n = 556) . | CTD (n = 555) . | |||||
| ZOL . | CLO . | ZOL . | CLO . | ZOL . | CLO . | ZOL . | CLO . | |
| (n = 213) . | (n = 211) . | (n = 215) . | (n = 212) . | (n = 278) . | (n = 278) . | (n = 277) . | (n = 278) . | |
| Acute renal failure | 15 (7) | 13 (6) | 13 (6) | 14 (7) | 14 (5) | 17 (6) | 15 (5) | 16 (6) |
| ONJ* | 10 (5) | 0 (0)† | 4 (2) | 1 (< 1) | 13 (5) | 2 (1)† | 8 (3) | 0 (0)† |
| Thromboembolic event | 10 (5) | 10 (5) | 43 (20) | 25 (12)† | 59 (21) | 41 (15) | 45 (16) | 41 (15) |
| Infection SAE | 4 (2) | 4 (2) | 12 (6) | 14 (7) | 28 (10) | 37 (13) | 24 (9) | 25 (9) |
| All SAEs | 97 (46) | 81 (38) | 115 (53) | 117 (55) | 167 (60) | 155 (56) | 160 (58) | 125 (45)† |
| TESAEs | 27 (13) | 18 (9) | 63 (29) | 67 (32) | 74 (27) | 69 (25) | 84 (30) | 72 (26) |
| Adverse event, n (%) . | Nonintensive pathway (n = 851) . | Intensive pathway (n = 1111) . | ||||||
|---|---|---|---|---|---|---|---|---|
| MP (n = 424) . | CTDa (n = 427) . | CVAD (n = 556) . | CTD (n = 555) . | |||||
| ZOL . | CLO . | ZOL . | CLO . | ZOL . | CLO . | ZOL . | CLO . | |
| (n = 213) . | (n = 211) . | (n = 215) . | (n = 212) . | (n = 278) . | (n = 278) . | (n = 277) . | (n = 278) . | |
| Acute renal failure | 15 (7) | 13 (6) | 13 (6) | 14 (7) | 14 (5) | 17 (6) | 15 (5) | 16 (6) |
| ONJ* | 10 (5) | 0 (0)† | 4 (2) | 1 (< 1) | 13 (5) | 2 (1)† | 8 (3) | 0 (0)† |
| Thromboembolic event | 10 (5) | 10 (5) | 43 (20) | 25 (12)† | 59 (21) | 41 (15) | 45 (16) | 41 (15) |
| Infection SAE | 4 (2) | 4 (2) | 12 (6) | 14 (7) | 28 (10) | 37 (13) | 24 (9) | 25 (9) |
| All SAEs | 97 (46) | 81 (38) | 115 (53) | 117 (55) | 167 (60) | 155 (56) | 160 (58) | 125 (45)† |
| TESAEs | 27 (13) | 18 (9) | 63 (29) | 67 (32) | 74 (27) | 69 (25) | 84 (30) | 72 (26) |
ONJ indicates osteonecrosis of the jaw; SAE, serious adverse event; and TESAE, treatment-emergent SAE.
ONJ cases were confirmed by an independent adjudication committee.
P ≤ .05 as determined by the Fisher exact test.
Discussion
The results from MRC Myeloma IX emphasize the importance of considering all components of treatment used in the management of MM. Clearly, agents previously considered as supportive care (eg, bisphosphonates) may have a profound effect on survival outcomes, and future clinical trials in MM will need to control for such factors in their design. In addition, the efficacy of antimyeloma regimens can clearly influence SRE risk, which will be an important consideration for analyzing clinical trials of antiresorptive therapies in this setting in the future.
During the past decade, therapy options for MM have evolved rapidly and, since the initiation of the Myeloma IX trial in 2003, have grown to include novel agents such as lenalidomide and bortezomib.3,16 However, the antimyeloma agents used in Myeloma IX remain important components of regimens that are still in common use. For example, poor-performance-status patients typically receive a combination of an alkylating agent, steroid, and nontraditional agent, particularly an immunomodulatory drug (eg, MPT, MPV, MPR, or CTDa), as standard therapy, and bortezomib is increasingly being added to established regimens in this setting. Younger, fitter, standard-risk patients are treated with high-dose induction therapy (including novel agents) and ASCT. Similarly, bortezomib is now being integrated into such induction regimens and also into maintenance therapy strategies.3,16 This more intensive strategy may abrogate some of the risk associated with poor cytogenetics in patients with high-risk disease.
Bisphosphonates are a valuable, although currently underused, component of therapy that are used to treat bone destruction and to prevent SREs and treat hypercalcemia of malignancy in MM and other oncology settings. A recent meta-analysis of randomized clinical trials (N = 4970) found that the bisphosphonate ZOL is superior to CLO, pamidronate, and ibandronate in preventing SREs in patients with MM.17 In addition to reducing the risk of SREs, preclinical and clinical evidence suggests that nitrogen-containing bisphosphonates such as ZOL have immunomodulatory effects and may have direct antimyeloma activity.18 Subgroup analyses from a previous MRC study of CLO in patients with MM suggested a possible survival benefit with bisphosphonate therapy in patients without skeletal fractures at baseline.19 Together with the preclinical evidence of the antimyeloma activity of ZOL, this observation provided the rationale for the MRC Myeloma IX study to investigate whether these potential antimyeloma properties could affect survival. In the present analysis, OS benefits of ZOL versus CLO in patients with newly diagnosed MM were found to be independent of sex and disease stage, and the only heterogeneity detected was bone disease/SRE status at baseline (defined as confirmed osteolytic lesions or any SRE), for which ZOL significantly improved OS compared with CLO in SRE+ but not SRE− patients. The beneficial effect of ZOL may be explained in part by the distinctly different biology observed in patients with bone disease at baseline, which predisposes them to both an increased risk of SREs and to worse survival outcomes compared with patients without bone disease at baseline.20 In this setting, the synergistic effects of ZOL and antimyeloma therapy appear to influence patient survival by slowing disease progression independently of SRE prevention. This is consistent with our previous report showing a survival benefit with ZOL that was independent of the reduction in SRE risk.11
In addition to the early effects on OS, improvements in OS with ZOL versus CLO were also apparent in patients receiving bisphosphonates for 2 years or more, and ZOL continued to improve OS even among patients who experienced disease progression. However, although statistically significant, patient numbers in the exploratory subgroup analyses that explored outcomes in patients who received more than 2 years of bisphosphonate therapy were low, and these data should be interpreted with caution. Although treatment guidelines have generally recommended 2 years of bisphosphonate therapy,4-6 our present results suggest that continuing ZOL therapy beyond 2 years may extend the potential survival benefit and continue to provide SRE-prevention benefits compared with CLO.
Understanding how the SRE-preventing efficacy of bisphosphonates can be affected by baseline disease characteristics may help to better identify patients who will benefit the most from therapy. In Myeloma IX, ZOL more effectively reduced SREs than CLO in both patients with and without bone disease or other SREs at baseline and in both patients receiving thalidomide maintenance and those receiving no maintenance therapy. In addition, ZOL significantly reduced the risk of SREs compared with CLO in patients with lower-risk disease, but apparently not in patients with high-risk disease, suggesting that rapid disease progression in high-risk patients may compete with SRE risk. This is supported by the finding that the incidence of first on-study SREs was lower in the high-risk group versus the low-risk group and by the lower incidence of bone disease reported in patients with adverse cytogenetics. However, because SRE data were only collected until disease progression, the effects of bisphosphonates on SREs after disease progression were not captured in this study.
The Myeloma IX data illustrate that there is a complex relationship between myeloma bone disease and the MM disease course. Although patients receiving intensive pathway treatment regimens containing CTD had better response rates than those receiving CVAD, there was no observable difference in SRE risk between these groups, perhaps reflecting a dominant effect of myeloablative therapy and ASCT on bone disease. In contrast, patients receiving nonintensive pathway treatment regimens containing CTDa not only had improved response, but also had reduced SRE risk compared with those receiving MP-containing regimens. This reduction in SRE risk may be explained in part by the more successful reduction in myeloma activity with the CTDa regimen; however, the potential for improved antimyeloma synergy between CTDa and the bisphosphonates may have also contributed to the reduction in SREs. Overall, the results from Myeloma IX show that regimens containing CTD or CTDa plus ZOL had greater effects on improving response rates, prolonging survival and reducing the risk of SREs.
The safety of bisphosphonate therapy in combination with multidrug regimens is an important consideration in patients with MM receiving long-term therapy. Results of Myeloma IX are consistent with the renal safety profile that has been reported for the combination of ZOL plus thalidomide maintenance therapy.21 We reported previously a significant difference in VTEs in the ZOL group versus the CLO group.11 The risk of VTEs is highly dependent on the combination chemotherapy regimen (especially those containing immunomodulatory drugs) and the level of prophylactic anticoagulation therapy. For example, the rates of VTEs were higher in the nonintensive pathway among patients receiving CTDa versus MP. Furthermore, VTEs among patients receiving CVAD were associated with delivery of medications through the indwelling catheter, which is no longer a standard of care at MRC institutions. Nonetheless, these data suggest that proactive use of anticoagulation therapy is warranted in patients with MM at elevated risk because of therapy- or disease-related factors. Overall, the Myeloma IX safety data suggest that ZOL can be used with a broad range of antimyeloma regimens; however, further studies with novel combination regimens are warranted.
To date, clinical treatment guidelines have been inconsistent regarding the duration of bisphosphonate therapy in patients with MM. Long-term follow-up of patients receiving bisphosphonate maintenance therapy will be required to inform best practices. Data accruing from follow-up of patients in Myeloma IX will likely contribute to this, and have already suggested that treatment to progression beyond 2 years may be appropriate.
The online version of this article contains a data supplement.
Presented in part at the 52nd American Society of Hematology Annual Meeting and Exposition, Orlando, FL, December 6, 2010; at the 2011 American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 5 and 6, 2011; and at the European Multidisciplinary Cancer Congress, Stockholm, Sweden, September 24, 2011.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the patients, investigators, and staff who participated in the MRC Myeloma IX Trial; staff at the Clinical Trials Research Unit, University of Leeds, Leeds, United Kingdom, for trial coordination and data management; staff at the Department of Immunology, University of Birmingham, Birmingham, United Kingdom; staff at Wessex Regional Genetics Laboratory, University of Southampton, Southampton, United Kingdom; staff at Haematological Malignancy Diagnostic Service, Leeds Teaching Hospitals National Health Service Trust, Leeds, United Kingdom; staff at Institute of Cancer Research, London, United Kingdom, for central laboratory investigations; David Bowen for independent safety oversight; the MRC Leukemia Data Monitoring and Ethics Committee; the MRC Leukemia Trial Steering Committee; the National Cancer Research Institute Haematological Oncology Clinical Studies Group; Myeloma United Kingdom; the National Institute for Health Research for support, through the National Cancer Research Network; the support of the Biomedical Research Center at the Royal Marsden Hospital; lead investigators, as described previously11 ; and Michael Hobert, PhD, ProEd Communications, for medical editorial assistance with this report.
Financial support for the MRC Myeloma IX trial and linked studies was obtained from the MRC, the Biomedical Research Center at the Royal Marsden Hospital, and Leukaemia & Lymphoma Research, with additional funding in the form of unrestricted educational grants from Novartis, Schering Health Care, Chugai, Pharmion, Celgene, and Ortho Biotech, mainly to support trial coordination and laboratory studies. Financial support for medical editorial assistance was provided by Novartis.
Authorship
Contribution: G.J.M., G.H.J., and J.A.C. served as the chief investigators; G.J.M., F.E.D., S.E.B., and J.A.C. designed the trial and developed the protocol; W.M.G. and A.J.S. developed the statistical analysis plan; G.J.M., F.E.D., R.G.O., A.J.A., G.H.J., and J.A.C. recruited the patients; S.E.B., M.T.D., and R.G.O. verified the response data; F.E.D. and G.H.J. reviewed the safety data; F.E.D., M.T.D., R.G.O., and A.J.A. performed the central laboratory investigations; S.E.B. coordinated the data collection and regulatory and governance requirements; G.J.M., F.E.D., W.M.G., A.J.S., S.E.B., and J.A.C. wrote the manuscript, generated tables and figures, and interpreted the data; G.J.M. wrote the first draft of the manuscript; and all authors reviewed, revised, and approved the final manuscript.
Conflict-of-interest disclosure: G.J.M. has participated in advisory boards for, received payment for lectures and development of educational presentations from, and received travel support from Celgene, Novartis, Merck, and Johnson & Johnson. F.E.D. has participated in advisory boards and spoken at meetings for Celgene, Ortho Biotech, and Novartis and has received travel support to attend meetings from Celgene and Ortho Biotech. R.G.O. has received speaker's fees and travel support from Celgene and Ortho Biotech to attend scientific meetings. G.H.J. has received honoraria from Celgene and Janssen-Cilag for speaking at educational meetings. J.A.C. has received honoraria and travel support from SAN (Science Agency and Network) for presentations that included aspects of results from the MRC Myeloma IX trial. The Clinical Trials Research Unit, University of Leeds, Leeds, United Kingdom (W.M.G., A.J.S., and S.E.B.), and the Mid Yorkshire Hospitals National Health Service Trust, Wakefield, United Kingdom (A.J.A.), received support from Celgene and OrthoBiotech for investigator travel to meetings for this study. M.T.D. declares no competing financial interests.
Correspondence: Gareth Morgan, MD, Section of Haemato-Oncology, The Institute of Cancer Research, Brookes Lawley Building, 15 Cotswold Road, London, Surrey, SM2 5NG, United Kingdom; e-mail: gareth.morgan@icr.ac.uk.
References
Author notes
G.J.M. and F.E.D. contributed equally to this work.