Abstract
Much remains unknown about the signals that induce early mesoderm to initiate hematopoietic differentiation. Here, we show that endoglin (Eng), a receptor for the TGFβ superfamily, identifies all cells with hematopoietic fate in the early embryo. These arise in an Eng+Flk1+ mesodermal precursor population at embryonic day 7.5 (E7.5), a cell fraction also endowed with endothelial potential. In Eng-knockout embryos, hematopoietic colony activity and numbers of CD71+Ter119+ erythroid progenitors were severely reduced. This coincided with severely reduced expression of embryonic globin and key bone morphogenic protein (BMP) target genes, including the hematopoietic regulators Scl, Gata1, Gata2, and Msx-1. To interrogate molecular pathways active in the earliest hematopoietic progenitors, we applied transcriptional profiling to sorted cells from E7.5 embryos. Eng+Flk-1+ progenitors coexpressed TGFβ and BMP receptors and target genes. Furthermore, Eng+Flk-1+ cells presented high levels of phospho-SMAD1/5, indicating active TGFβ and/or BMP signaling. Remarkably, under hematopoietic serum-free culture conditions, hematopoietic outgrowth of Eng-expressing cells was dependent on the TGFβ superfamily ligands BMP4, BMP2, or TGF-β1. These data demonstrate that the E+F+ fraction at E7.5 represents mesodermal cells competent to respond to TGFβ1, BMP4, or BMP2, shaping their hematopoietic development, and that Eng acts as a critical regulator in this process by modulating TGF/BMP signaling.
Introduction
During mouse development, hematopoiesis occurs at temporally and spatially distinct anatomic sites with the first appearance of hematopoietic cells observed in the blood island (BI) of the extraembryonic yolk sac (YS) at embryonic day 7 (E7.0).1 This first wave of primitive hematopoiesis produces primitive erythrocytes, megakaryocytes,2 and macrophages, and is followed by the generation of definitive hematopoietic precursors in the YS at approximately E8.25 days post coitum (dpc).1 The emergence of hematopoietic stem cells (HSCs) capable of repopulating adult mice is first observed at E10.5 in the aorta-gonad-mesonephros (AGM) region, and later on in other hematopoietic sites, such as YS, placenta, and fetal liver.3-5 HSCs have been proposed to originate from a common precursor for the hematopoietic and endothelial lineages, the hemangioblast, which was initially described using the in vitro embryonic stem cell (ESC) differentiation system,6 and was later confirmed in the mouse7 and zebrafish8 systems. In the murine embryo, this precursor has been identified to be enriched in the primitive streak (PS), and expresses fetal liver kinase 1 (Flk-1 or VEGFR2) in addition to brachyury. Subsequently, these cells migrate to the extraembryonic region, where they give rise to hematopoietic and endothelial cells of the BIs.7 Endothelial versus hematopoietic fate is thought to be specified en route to the extraembryonic destination because individual BIs are often polyclonal.9
To date, little is known regarding the molecular and cellular mechanisms involved in the origin and early development of the hematopoietic lineage. It has been reported that signals from extraembryonic ectoderm and visceral endoderm, including fibroblast growth factor 8 (FGF8), WNT3, and hedgehog, are crucial for proper mesoderm patterning, and the latter two are also involved in the specification of the hematopoietic program.10,11 Members of the TGFβ superfamily have been shown to play an essential role during vascular development and hematopoiesis. Ligands for the TGFβ family act through a receptor complex present in the cell surface, which upon phosphorylation causes the activation of distinct SMAD proteins. Embryonic lethality and impaired vasculature is a characteristic of mice lacking one of several TGFβ molecules, including TGFβ1 (ligand), TβRII, activin receptor-like kinase 1 (ALK1), and ALK5 (both type I receptors).12-15 From these, TGFβ1 and TβRII deficiency have been associated with impaired YS hematopoiesis.12,15 Bone morphogenetic proteins (BMPs) are also implicated in mesoderm induction and blood specification. Knockouts for BMP4,16 BMP2,17 and their common receptors ALK318 and BMPRII19 are embryonically lethal and cause reduced mesoderm formation. In particular, BMP4 is necessary for the commitment of mesodermal cells to the hematopoietic lineage, a process mediated by Gata1, Gata2, Scl, and Lmo2.20 Accordingly, embryos lacking BMP4 display impaired primitive hematopoiesis.16
Endoglin (Eng) is a type III receptor for the TGFβ superfamily, meaning that it functions as an accessory molecule associated with a type I/type II receptor complex21 that may affect the mode of signaling. Eng has been mostly studied in endothelial cells, where it plays an important role in TGFβ-dependent responses by balancing activating and inhibitory signals.22 Interestingly, Eng has also been detected in the long-term repopulating HSC in the adult bone marrow,23 as well as in HSCs and hematopoietic progenitor cells from the AGM at E11.5 and in fetal liver.24-26 Mice lacking Eng (Eng−/−) die around E10.5 due primarily to cardiovascular defects.27,28 9.5 dpc Eng−/− embryos display anemia of the YS, and this was assumed to be an indirect result of insufficient blood flow. Our previous studies using in vitro differentiating Eng−/− ESCs suggested an alternate hypothesis, that Eng is directly involved in the specification of the hematopoietic lineage. Deletion of Eng resulted in profound reduction in the frequency of hemangioblast and primitive erythroid progenitors (EryPs) from ESCs.29
The objective of the present study was to elucidate the role of Eng in vivo on the specification and development of the hematopoietic lineage. Our results show for the first time that Eng expression specifically identifies the first hematopoietic progenitors during embryonic development and that this receptor is required for proper interpretation of TGFβ/BMP signals, providing evidence for a key function for Eng as a regulator of TGFβ/BMP signaling at the origin of hematopoiesis.
Methods
Mouse models
All animals were handled in strict accordance with good animal practice as defined by the relevant national and/or local animal welfare bodies, and all animal work was approved by the University of Minnesota Institutional Animal Care and Use Committee. Eng-deficient embryos were generated by timed mating of Eng+/− heterozygotes; the morning of a positive plug was considered 0.5 dpc. Genotyping was performed as described previously.27 Time-pregnant wild-type (WT) CD1 mice were purchased from Charles River Laboratories.
Embryo dissection
Embryos were removed from decidua and the Reichert membrane and dissected in PBS supplemented with 10% FBS. At E7.5 dpc, embryos were staged according to morphologic criteria, and E7.25-E7.75 embryos were used for experiments. All Eng−/− embryos analyzed presented the same somite pair counting as the littermate controls (18-23 somite pairs).
Flow cytometry and cell sorting
E7.5 whole embryos (or E9.5 YS) were dissociated in trypsin 0.25% for 1 minute (3 minutes for YS) at 37°C, and analyzed by flow cytometry using the mAbs described in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). E7.5 cells were sorted based on Flk-1 and/or Eng expression on a FACSAria cell sorter (BD Biosciences) after the addition of propidium iodide (Pharmingen) to exclude dead cells. Data were analyzed using FlowJo Version 7.5.5 software (TreeStar).
Hematopoietic progenitor assay
To determine the myeloerythroid potential of each population, sorted cells were plated directly in hematopoietic medium for colony activity (M3434; StemCell Technologies). Plated cells were cultured in a humidified low-oxygen incubator (5% O2) at 37°C. Ery-P and definitive hematopoietic colonies were scored 6 and 10 days after plating, respectively. Alternatively, sorted cells were cocultured on OP9 stromal cells that had been preplated in 6-well plates (or 96-well plates for single-cell sorting) containing α-MEM medium (Gibco-BRL) supplemented with 10% FBS (Gemini), murine SCF (mSCF; 100 ng/mL), mIL3 (1.17 ng/mL), mG-CSF (100 ng/mL), mVEGF (10 ng/mL), human erythropoietin (hEpo; 2U/mL), human angiopoietin-1 (hAng-1; 100 ng/mL), 5 × 10−5M 2-mercaptoethanol, and 1% penicillin/streptomycin. Plated cells were cultured in a humidified incubator at 37°C in an environment of 5% CO2.
Endothelial cell culture and characterization
To determine the endothelial potential of the Eng+Flk-1+ cell fraction, sorted cells were cocultured with OP9 stromal cells in 6 well-plates (or 96-well plates for single-cell sorting) containing α-MEM medium (Gibco-BRL) supplemented with 10% FBS (Gemini), mSCF (100 ng/mL), mIL3 (1.17 ng/mL), mVEGF (10 ng/mL), hEpo (2U/mL), hAng-1 (100 ng/mL), basic FGF (bFGF; 10 ng/mL), 5 × 10−5 M 2-mercaptoethanol, and 1% penicillin/streptomycin. Plated cells were cultured in a low-oxygen incubator (5% O2) at 37°C for 7-10 days, and then fixed with 4% paraformaldehyde for 15 minutes at room temperature. Fixed cells were incubated with blocking buffer containing 5% normal donkey serum (Jackson ImmunoResearch Laboratories) and 0.1% Triton X-100 (Sigma-Aldrich) for 30 minutes at room temperature. Endothelial cells were labeled using primary Abs against CD31 (1:50), or vascular endothelial (VE)–cadherin (1:50) overnight at 4°C (supplemental Table 1), followed by incubation with Alexa Fluor 488–conjugated anti–rat Ab (1:200) and DAPI (1μg/mL; supplemental Table 1) for 1 hour at room temperature. Images were acquired by confocal microscopy (Zeiss LM510). Alternatively, endothelial/hematopoietic cocultures were trypsinized for 3 minutes at 37°C, and analyzed by FACS for hematopoietic (c-Kit, CD41, and CD45) and endothelial markers (VE-cadherin [VE-Cad] and Flk-1).
Liquid hematopoietic cell expansion using TGFβ1 or BMP2/4
Eng+ and Eng− cells were sorted from E7.5 embryos and cultured in 12 well-plates (1500-5000 cells/well) in serum-free hematopoietic medium containing StemSpan SFEM (StemCell Technologies), mSCF (100 ng/mL), mIL3 (100 ng/mL), and Flt3 ligand (mFlt3L 100 ng/mL), in the presence or absence of BMP2, BMP4, or TGFβ1 at different concentrations (0.1-25 ng/mL) in a low-oxygen incubator (5% O2) at 37°C. For cell growth, cells were followed for up to 2 weeks in culture using an inverted microscope (Zeiss Axiovert 40C). For characterization of hematopoietic potential, cell outgrowths were analyzed by cytologic examination and colony-forming activity after 5 and 4 days in culture, respectively. For cytologic analysis, cell outgrowths were centrifuged 300g for 5 minutes in 50 μL of PBS containing 4% FBS, and subjected to cytospinning (∼ 100g RPM for 5 minutes). Slides were stained using the Protocol Hema3 stain set (Fisher Diagnostics), and analyzed in a Zeiss Axio Imager M1 Upright Microscope (63× plan-apochromat oil objective).
Histologic analysis
Dissected embryos were immediately fixed in 2% paraformaldehyde for 20 minutes, incubated in 5% and 15% sucrose solutions until the embryos decanted, and frozen in 7.5% gelatin. Cryosections were incubated with primary Abs, described in supplemental Table 1, overnight at 4°C. Reactions were detected after 1 hour of incubation with the appropriate secondary Abs (supplemental Table 1). Staining was visualized by laser confocal scanning (Zeiss LM510).
Molecular analysis
For microarray experiments, E7.5 pooled embryos (25 litters, approximately 300 embryos) were dissected and 3000 cells were sorted in triplicate for E−F−, E−F+, E+F+, and E+F− fractions. RNAs were amplified using SuperAmp amplification (Miltenyi Biotec) and Cy3-labeled cDNAs hybridized to Agilent Whole Mouse Genome Oligo Microarray 4×44K. Microarray results were analyzed with GeneSpring GX software. Quantitative PCR (qPCR) validation was performed using TaqMan probes (Applied Biosystems). For E9.5 YS transcription analysis of Eng−/−, Eng+/−, and Eng+/+ embryos, isolated YS were submitted to RNA extraction using TRIzol reagent (Invitrogen). qPCR was performed as described previously. The microarray data are available at the Gene Expression Omnibus under accession number GSE37515.
Statistical analysis
Differences between Eng−/−, Eng+/−, and Eng+/+ E9.5 samples were assessed by ANOVA. Student t test (1-tailed) was used for hematopoietic progenitors at E7.5. For the microarray experiments, an unpaired t test was used after applying a > 1.5-fold change in gene expression.
Results
Eng marks the PS and early sites of hematopoiesis
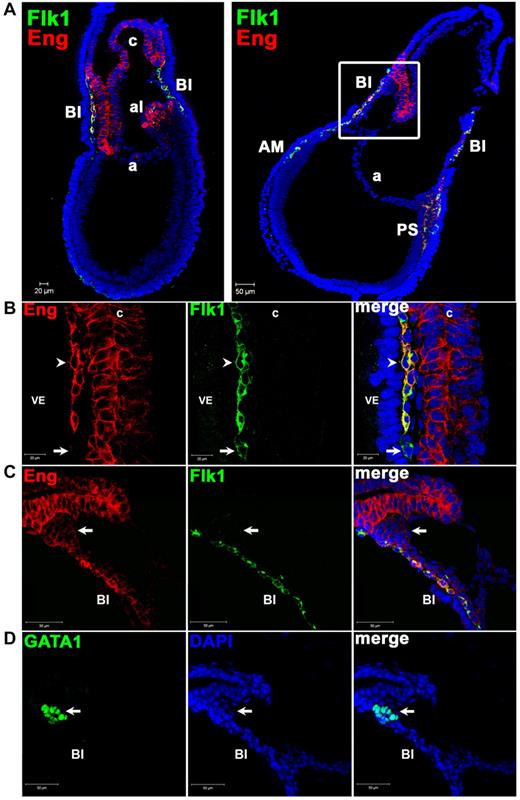
We began by examining the localization of Eng+ cells in E7.5 embryos, the developmental time point at which the first hematopoietic cells are generated. E7.5 embryos obtained from CD1 WT mice were analyzed by confocal microscopy for the expression of Eng, along with Flk-1 and GATA1, markers of early mesoderm and hematopoiesis, respectively. Distinct cell populations were found at this stage (Figure 1A-D). Eng+Flk-1+ (E+F+) cells were detected in the PS, in mesodermal cells of the BI, and some allantoic cells (Figure 1A). All of the chorion and most of the allantois were characterized by the presence of Eng and the absence of Flk-1 (Figure 1A-B). Dim expression of Eng was also detected in EryPs, characterized by coexpression with GATA1 (Figure 1C-D) and the absence of Flk-1 (Figure 1C). Eng−Flk-1+ (E−F+) cells were rare, and may represent mesodermal progenitors (Figure 1B arrow).
In situ identification of Eng+ cells in WT E7.5 embryos. (A) Confocal images of whole E7.5 embryos associated with close-up images shown in panels B through D. Immunostaining shows expression for Eng (red) and Flk-1 (green) and their merge (yellow-orange; 10× objective). DAPI is shown in blue. Eng is strongly expressed in the allantois (al) and the chorion (c). Some allantoic cells also express Flk-1. In addition to the extraembryonic region, Eng+Flk-1+ cells are also detected in the PS and anterior mesoderm (AM). (B) High magnification of E7.5 extraembryonic region shown in panel A (left) confirms coexpression (arrowhead) of Eng (red) and Flk-1 (green) in the developing BI next to the visceral endoderm (VE). Rare Eng−Flk-1+ cells are also observed in the BI region (arrow; 63× objective). (C-D) Serial cryosections of E7.5 embryos (panels C and D depict the square area shown in panel A right) stained with Abs to Eng and Flk-1 (C) or GATA1 (D). Extraembryonic mesoderm expresses Eng and Flk-1 (C), whereas primitive erythrocytes, characterized by the expression of GATA1 in the serial section (arrow in panel D) are EnglowFlk-1− cells (arrow in panel C).
In situ identification of Eng+ cells in WT E7.5 embryos. (A) Confocal images of whole E7.5 embryos associated with close-up images shown in panels B through D. Immunostaining shows expression for Eng (red) and Flk-1 (green) and their merge (yellow-orange; 10× objective). DAPI is shown in blue. Eng is strongly expressed in the allantois (al) and the chorion (c). Some allantoic cells also express Flk-1. In addition to the extraembryonic region, Eng+Flk-1+ cells are also detected in the PS and anterior mesoderm (AM). (B) High magnification of E7.5 extraembryonic region shown in panel A (left) confirms coexpression (arrowhead) of Eng (red) and Flk-1 (green) in the developing BI next to the visceral endoderm (VE). Rare Eng−Flk-1+ cells are also observed in the BI region (arrow; 63× objective). (C-D) Serial cryosections of E7.5 embryos (panels C and D depict the square area shown in panel A right) stained with Abs to Eng and Flk-1 (C) or GATA1 (D). Extraembryonic mesoderm expresses Eng and Flk-1 (C), whereas primitive erythrocytes, characterized by the expression of GATA1 in the serial section (arrow in panel D) are EnglowFlk-1− cells (arrow in panel C).
Hematopoietic activity is restricted to the Eng+ cell population
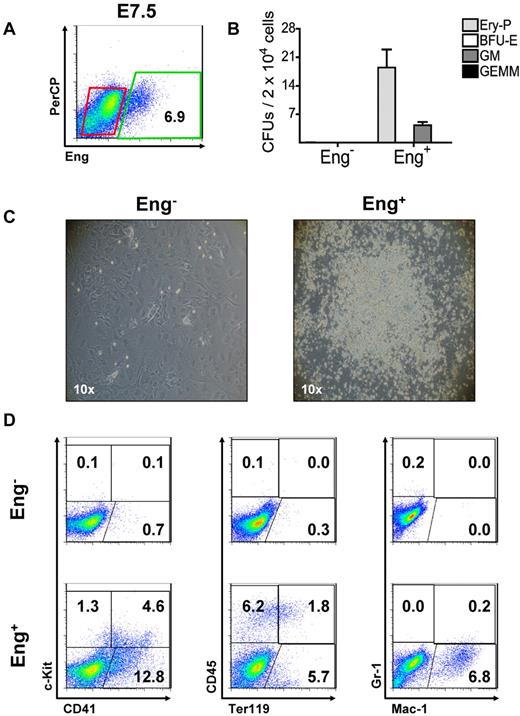
To determine whether the earliest hematopoietic precursors express Eng, we sorted Eng+ and Eng− cells from E7.5 embryos (Figure 2A) and evaluated their hematopoietic potential. Equal numbers of Eng+ and Eng− cells were plated directly into methylcellulose-based medium containing hematopoietic cytokines. Remarkably, only Eng+ cells gave rise to hematopoietic colonies (Figure 2B). Similar results were obtained when these 2 fractions were cocultured on OP9 stromal cells in the presence of hematopoietic cytokines (Figure 2C-D). After 5 days in culture, typical hematopoietic cell colonies were detected only in the Eng+ fraction (Figure 2C). Expression of hematopoietic specific markers by FACS (Figure 2D) and the presence of significant numbers of hematopoietic colony-forming cells (CFCs; supplemental Figure 1A-B) confirmed the hematopoietic nature of these outgrowths. Conversely, Eng− cells were unable to generate hematopoietic cells under these culture conditions (Figure 2C-D and supplemental Figure 1A). This was the case even when a 10-fold excess of cells was plated either in methylcellulose or OP9 cocultures (data not shown). To estimate the frequency of hematopoietic precursors at E7.5, we performed clonal analysis of Eng+ and Eng− cells (supplemental Figure 1C-E). Single cells were sorted and deposited in 96-well plates covered by an OP9 layer (supplemental Figure 1C). Of 384 Eng+ single-sorted cells, 4 were able to form hematopoietic colonies under these conditions (supplemental Figure 1D-E). No hematopoietic growth was observed in Eng−-sorted cells (0/384 wells). These results further indicate that hematopoietic activity is restricted to Eng+ cells at the origin of hematopoiesis.
Hematopoietic progenitors are restricted to the Eng+ fraction. (A) Flow cytometric analyses of WT E7.5 embryos. Eng expression is shown on the x-axis. Percentage represents the fraction of cells that express Eng. Gates represent Eng− (red) and Eng+ (green) fractions that were sorted for further in vitro characterization (B-D). The y-axis represents PerCP, which provides a measure of autofluorescence. (B) Eng−- and Eng+-sorted cell fractions (A) were assessed for their hematopoietic activity (CFUs). Hematopoietic colonies were detected exclusively in the Eng+ fraction. Eng− cells failed to produce any colonies. Error bars indicate the SEM from 3 independent experiments. (C) Emergence of robust hematopoietic outgrowths after the coculture of Eng+ (right panel) cells sorted from E7.5 embryos on OP9 cells for 5 days. Hematopoietic growth was virtually absent in Eng− cocultures (left panel). Photographs are representative of at least 3 independent experiments. (D) FACS characterization of Eng+- and Eng−-derived OP9 cocultures after a 5-day in vitro expansion. Representative profiles are shown for c-Kit and CD41 (left panels), CD45 and Ter119 (middle panels), and Gr-1 and Mac-1 (right panels). Fluorescence intensity for c-Kit, CD45, or Gr-1 is indicated on the y-axis and CD41, Ter119, or Mac-1 on the x-axis. Plots are representative of at least 3 independent experiments.
Hematopoietic progenitors are restricted to the Eng+ fraction. (A) Flow cytometric analyses of WT E7.5 embryos. Eng expression is shown on the x-axis. Percentage represents the fraction of cells that express Eng. Gates represent Eng− (red) and Eng+ (green) fractions that were sorted for further in vitro characterization (B-D). The y-axis represents PerCP, which provides a measure of autofluorescence. (B) Eng−- and Eng+-sorted cell fractions (A) were assessed for their hematopoietic activity (CFUs). Hematopoietic colonies were detected exclusively in the Eng+ fraction. Eng− cells failed to produce any colonies. Error bars indicate the SEM from 3 independent experiments. (C) Emergence of robust hematopoietic outgrowths after the coculture of Eng+ (right panel) cells sorted from E7.5 embryos on OP9 cells for 5 days. Hematopoietic growth was virtually absent in Eng− cocultures (left panel). Photographs are representative of at least 3 independent experiments. (D) FACS characterization of Eng+- and Eng−-derived OP9 cocultures after a 5-day in vitro expansion. Representative profiles are shown for c-Kit and CD41 (left panels), CD45 and Ter119 (middle panels), and Gr-1 and Mac-1 (right panels). Fluorescence intensity for c-Kit, CD45, or Gr-1 is indicated on the y-axis and CD41, Ter119, or Mac-1 on the x-axis. Plots are representative of at least 3 independent experiments.
Eng+Flk-1+ precursors are endowed with hematopoietic and endothelial potential at E7.5
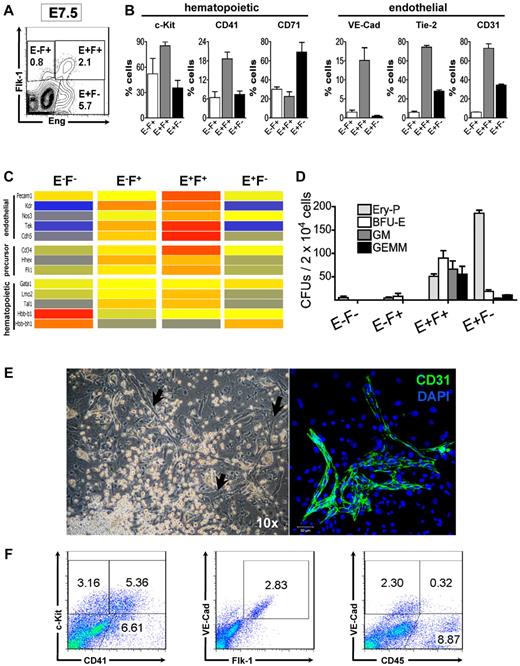
We also performed FACS profiling to better characterize the nature of Eng+ cells in E7.5 embryos. As indicated by confocal microscopy (Figure 1), distinct cell fractions were identified based on the expression of Eng and Flk-1: E+F+, E+F−, E−F+, E−F− (Figure 3A). These fractions were then further evaluated for the expression of VE-Cad, CD31, and Tie-2, surface markers associated with early mesoderm and the endothelial lineage,30 as well as CD41 and c-Kit, which together label early hematopoietic progenitors,31,32 and CD71, a marker of EryPs.33 The E+F+ cell population expressed both early hematopoietic and endothelial cell markers, whereas the E+F− cell fraction expressed higher levels of CD71 (2-fold compared with E+F+ and E−F+; Figure 3B).
Eng+Flk-1+ cells are endowed with hematopoietic and endothelial potential. (A) Flow cytometric analyses of WT E7.5 embryos for Eng (x-axis) and Flk-1 (y-axis). Gates represent Eng−Flk+ (E−F+), Eng+Flk+ (E+F+), and Eng+Flk− (E+F−) fractions that were sorted for further in vitro characterization (B-D). Numbers represent the percentage of gated cells that express a given surface antigen. (B) E−F+ (white bars), E+F+ (gray bars), and E+F− (black bars) cell fractions gated in panel A were further analyzed for the expression of hematopoietic and endothelial markers. Histograms represent the mean and error bars represent the SEM from at least 2 independent experiments. (C) Transcriptional analyses of E7.5 embryos based on Eng and Flk-1 expression. Clustering of genes found to be distinctively expressed among the E−F−, E−F+, E+F+, and E+F− fractions. Colors show the range of expression from blue (low expression) to red (high expression). Genes associated with endothelial, hematopoietic, and more primitive precursors were found to be highly expressed in the E+F+ fraction. (D) Frequency of hematopoietic progenitors in E−F−, E−F+, E+F+, and E+F− cells sorted from E7.5 embryos (representative gates are shown in panel A). Error bars indicate the SEM from 3 independent experiments. (E) Hematopoietic (round cells, left panel) and endothelial cells (arrows, left panel) emerged after the coculture of E+F+ cells onto OP9 stromal cells in the presence of hematopoietic and endothelial cytokines (10× objective). Staining for CD31 (green, right panel) confirmed the endothelial nature of these elongated cells. DAPI identifies nuclei (blue). Confocal micrograph represents results from 3 independent experiments (Zeiss LM510; 10× objective). (F) FACS characterization of cells showed in panel E after a 7-day in vitro expansion confirm the presence of hematopoietic (c-Kit+CD41+ and CD45+VE-Cad−) and endothelial (VE-Cad+Flk-1+ and VE-Cad+CD45−) cells in these cultures. Representative profiles are shown for c-Kit and CD41 (left panel), VE-Cad and Flk-1 (middle panel), and VE-Cad and CD45 (right panel).
Eng+Flk-1+ cells are endowed with hematopoietic and endothelial potential. (A) Flow cytometric analyses of WT E7.5 embryos for Eng (x-axis) and Flk-1 (y-axis). Gates represent Eng−Flk+ (E−F+), Eng+Flk+ (E+F+), and Eng+Flk− (E+F−) fractions that were sorted for further in vitro characterization (B-D). Numbers represent the percentage of gated cells that express a given surface antigen. (B) E−F+ (white bars), E+F+ (gray bars), and E+F− (black bars) cell fractions gated in panel A were further analyzed for the expression of hematopoietic and endothelial markers. Histograms represent the mean and error bars represent the SEM from at least 2 independent experiments. (C) Transcriptional analyses of E7.5 embryos based on Eng and Flk-1 expression. Clustering of genes found to be distinctively expressed among the E−F−, E−F+, E+F+, and E+F− fractions. Colors show the range of expression from blue (low expression) to red (high expression). Genes associated with endothelial, hematopoietic, and more primitive precursors were found to be highly expressed in the E+F+ fraction. (D) Frequency of hematopoietic progenitors in E−F−, E−F+, E+F+, and E+F− cells sorted from E7.5 embryos (representative gates are shown in panel A). Error bars indicate the SEM from 3 independent experiments. (E) Hematopoietic (round cells, left panel) and endothelial cells (arrows, left panel) emerged after the coculture of E+F+ cells onto OP9 stromal cells in the presence of hematopoietic and endothelial cytokines (10× objective). Staining for CD31 (green, right panel) confirmed the endothelial nature of these elongated cells. DAPI identifies nuclei (blue). Confocal micrograph represents results from 3 independent experiments (Zeiss LM510; 10× objective). (F) FACS characterization of cells showed in panel E after a 7-day in vitro expansion confirm the presence of hematopoietic (c-Kit+CD41+ and CD45+VE-Cad−) and endothelial (VE-Cad+Flk-1+ and VE-Cad+CD45−) cells in these cultures. Representative profiles are shown for c-Kit and CD41 (left panel), VE-Cad and Flk-1 (middle panel), and VE-Cad and CD45 (right panel).
We then performed transcriptional profiling of the E7.5 E−F−, E−F+, E+F+, and E+F− cell fractions to examine potential gene-expression signatures associated with their distinct potentials (Figure 3C and supplemental Figure 2A-B). Compared with E−F− cells, 1402 genes were found to be distinctively regulated in the E+F+ fraction, 2198 genes in the E−F+ fraction, and 1362 genes in the E+F− (1.5-fold, P < .05 cutoff; supplemental Figure 2A-B). Genes involved in hematopoietic and endothelial development were clustered together and were expressed at higher levels in E+F+ cells (Figure 3C). We also observed high expression of genes that had been associated with the hemangioblast precursor in the E+F+ fraction, such as Scl, Fli-1, Hhex, and Cd34.34,35 Embryonic globin (Hbb-bh1) and Gata1, genes involved in erythropoiesis, were found to be highly expressed in the E+F− fraction (Figure 3C and supplemental Figure 2C). This result is in agreement with the confocal analysis showing E+F− cells coexpressing GATA1 in the BIs of the YS (Figure 1C-D) and with the FACS staining indicating CD71 enrichment in the E+F− fraction (Figure 3B left). Conversely, levels of β-major (Hbb-b1) and embryonic globins were found to be elevated in the E−F− cell fraction (Figure 3C), indicating the presence of terminally differentiated erythrocytes in this population. These results were validated by qPCR analyses (supplemental Figure 2C).
We then examined the hematopoietic potential of these sorted fractions by assessing the frequency of CFCs. Most of the hematopoietic precursor activity was found in the E+F+ fraction, including EryP, blast-forming units-erythroid (BFU-E), granulocyte-macrophage (GM), and granulocyte-erythroid-monocyte-megakaryocyte (GEMM) colonies (Figure 3D and supplemental Figure 3A), whereas the highest frequency of EryP-CFCs was found in the E+F− fraction (Figure 3D). Consistently, E−F− and E−F+ cells rarely gave rise to hematopoietic colonies (Figure 3D) even when these cells were plated in excess (data not shown). The detection of definitive erythroid colonies (BFU-E) at E7.5 shown in the present study and not shown previously1 was probably because of the low-oxygen culture conditions (5%) used herein. Under normoxia, BFU-E colonies were not detected (data not shown). Cytospin analyses of individual colonies confirmed the definitive nature of colonies scored as BFU-E1 because cell contents within these colonies were much smaller for the most part and were enucleated, depending on the stage of maturation (supplemental Figure 3B). Furthermore, RT-PCR (data not shown) and qPCR (supplemental Figure 3C) analyses demonstrated that these E7.5 definitive erythroid colonies expressed levels of adult β-major globin similar to those obtained at E9.5 under both normoxic and hypoxic conditions (supplemental Figure 3C).
The endothelial potential of the E+F+ cell fraction was confirmed by culturing sorted cells on OP9 stroma in the presence of a cocktail of early hematopoietic and endothelial cytokines, including SCF, IL-3, Epo, VEGF, Ang-1, and bFGF. As can be observed in Figure 3E (left panel), under these culture conditions, a distinct outgrowth of hematopoietic and endothelial cells emerged. The endothelial nature of adherent cells was confirmed by immunostaining to CD31 (Figure 3E right panel) and VE-Cad (supplemental Figure 3C). FACS characterization further confirmed the presence of endothelial (VE-Cad+Flk-1+CD45−) and hematopoietic (c-Kit+CD41+ and CD45+) cells in these cultures (Figure 3F).
We then performed clonal analysis in 96-well plates, as described for Eng+ cells, which demonstrated that a subset of E+F+ single cells have the ability to give rise to both hematopoietic and endothelial cell lineages (supplemental Figure 3D), although at a low cloning efficiency. We found that 11.46 ± 1.88 of 380 E+F+ cells (frequency, 1:33.8 ± 6.0) were able to give rise to hematopoietic colonies under these conditions (data not shown). We stained 20 clonally derived hematopoietic outgrowths with CD31 and observed endothelial cell growth in 2 of these clones (supplemental Figure 3D).
These findings support the notion that, at E7.5, E+F+ cells represent a very early precursor population endowed with hematopoietic and endothelial potential. The E+F− cell fraction corresponds to a heterogeneous population composed of EryPs and allantoic and chorionic cells.
Eng is required for proper hematopoietic development
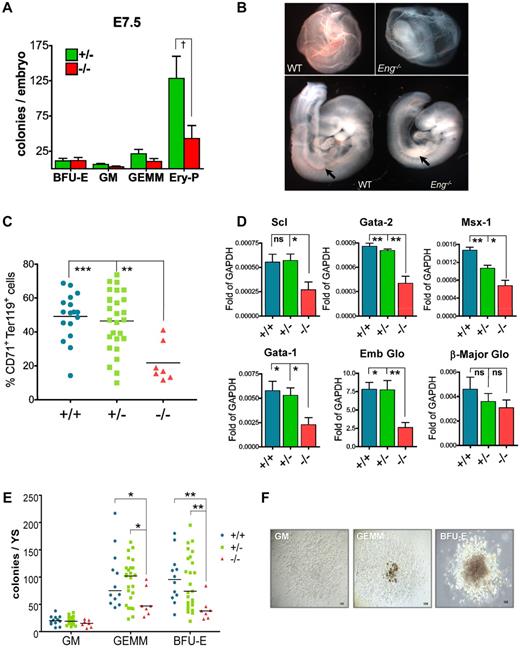
To determine the necessity of Eng at this early stage, we evaluated hematopoietic colony activity of E7.5 Eng-knockout embryos. A significant reduction was detected in the frequency of EryP colonies in Eng−/− embryos (Figure 4A). Accordingly, E9.5 Eng−/− embryos had a pale appearance indicative of anemia (Figure 4B), which was correlated with a dramatic reduction of CD71+Ter119+ erythroblasts in Eng−/− E9.5 YS (Figure 4C and supplemental Figure 4), confirming impaired primitive erythropoiesis in these embryos. In accordance with reduced hematopoietic activity, gene-expression analyses showed that Eng−/− E9.5 YS expressed reduced levels of Gata1, Gata2, Scl, Msx1, and embryonic globin compared with control littermates (Figure 4D). Interestingly, the first 4 are known target genes of BMP4.20 To determine whether the lack of Eng also affects definitive hematopoiesis, we quantified the hematopoietic progenitor activity of Eng−/− E9.5 YS. At this later time point, significantly reduced numbers of BFU-E and GEMM progenitors were also observed in Eng−/− YS (Figure 4E), whereas the committed CFU-GM lineage was unaffected (Figure 4E). No changes in colony morphology were observed (Figure 4F). These results confirm our hypothesis that Eng is required for proper hematopoietic development during embryogenesis.
Defective hematopoiesis in Eng−/− embryos. (A) Whole E7.5 embryos derived from Eng+/− intercross were plated in M3434 and cultured in a low-oxygen incubator. The number of EryPs was reduced drastically in Eng−/− embryos (43.00 ± 18.2; n = 3) compared with Eng+/− (128.3 ± 31.52; n = 3) embryos (P = .0395 by 1-tailed Student t test). Definitive hematopoietic colonies do not show significant differences at this early time point. (B) YS from WT E9.5 embryos show normal vasculature, full of erythrocytes, whereas YS from Eng−/− embryos present primitive vasculature containing very few RBCs. Severe anemia was also observed in the AGM region of Eng−/− embryos (arrows in bottom panels). (C) The frequency of CD71+Ter119+ cells (erythroblasts) is severely decreased in Eng−/− YS compared with Eng+/− or Eng+/+ YS. **P < .05 and ***P < .01 by ANOVA. (D) qPCR gene-expression analyses in E9.5 YS from WT (+/+; n = 9), heterozygotes (+/−; n = 18), and knockout embryos (−/−; n = 15). Transcripts are normalized to GAPDH. Bars represent average gene expression for each genotype. Error bars indicate the SEM for each genotype. *P < .05 and **P < .01 by ANOVA. (E) E9.5 YS cells from Eng+/− intercrosses were assayed for hematopoietic colony formation. The frequency of GEMM and BFU-E progenitors was decreased significantly in the YS of knockout embryos (−/−; n = 7) compared with heterozygous (+/−; n = 25) or WT (+/+; n = 12). Horizontal bar represents the mean. *P < .05 and **P < .01 by ANOVA. (F) Representative GM, GEMM, and BFU-E colonies are shown at similar magnification (10× objective).
Defective hematopoiesis in Eng−/− embryos. (A) Whole E7.5 embryos derived from Eng+/− intercross were plated in M3434 and cultured in a low-oxygen incubator. The number of EryPs was reduced drastically in Eng−/− embryos (43.00 ± 18.2; n = 3) compared with Eng+/− (128.3 ± 31.52; n = 3) embryos (P = .0395 by 1-tailed Student t test). Definitive hematopoietic colonies do not show significant differences at this early time point. (B) YS from WT E9.5 embryos show normal vasculature, full of erythrocytes, whereas YS from Eng−/− embryos present primitive vasculature containing very few RBCs. Severe anemia was also observed in the AGM region of Eng−/− embryos (arrows in bottom panels). (C) The frequency of CD71+Ter119+ cells (erythroblasts) is severely decreased in Eng−/− YS compared with Eng+/− or Eng+/+ YS. **P < .05 and ***P < .01 by ANOVA. (D) qPCR gene-expression analyses in E9.5 YS from WT (+/+; n = 9), heterozygotes (+/−; n = 18), and knockout embryos (−/−; n = 15). Transcripts are normalized to GAPDH. Bars represent average gene expression for each genotype. Error bars indicate the SEM for each genotype. *P < .05 and **P < .01 by ANOVA. (E) E9.5 YS cells from Eng+/− intercrosses were assayed for hematopoietic colony formation. The frequency of GEMM and BFU-E progenitors was decreased significantly in the YS of knockout embryos (−/−; n = 7) compared with heterozygous (+/−; n = 25) or WT (+/+; n = 12). Horizontal bar represents the mean. *P < .05 and **P < .01 by ANOVA. (F) Representative GM, GEMM, and BFU-E colonies are shown at similar magnification (10× objective).
Eng may act as a regulator of BMP2/4 and TGFβ1 signaling during early hematopoiesis
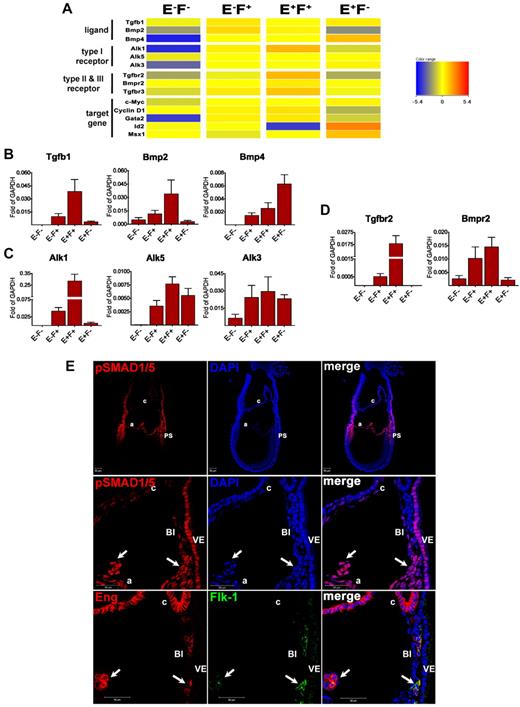
To investigate the possible molecular mechanism underlining the function of Eng on the emergence of hematopoietic progenitors, we examined the transcript levels for members of the TGFβ superfamily among these subpopulations. High levels of transcripts for Tgfβ1, TgfbrI (both Alk1 and Alk5), and TgfbrII were detected in the E+F+ fraction (Figure 5A-D), suggesting that an autocrine TGFβ1 pathway may be active in E+F+ cells. In addition, Bmp2, Alk3, BmprII (Figure 5A-D), components of the BMP pathway, and CyclinD1 and c-Myc, reported target genes for BMP2,36 were found to be highly expressed in this fraction (Figure 5A and supplemental Figure 2C), suggesting that the BMP2 pathway is also active in E+F+ cells.
TGFβ1/BMP signaling is active in Eng-expressing cells at E7.5. (A) Clustering of genes associated with the TGFβ superfamily among the E−F−, E−F+, E+F+, and E+F− fractions showing high expression of these TGFβ superfamily members in Eng-expressing cells. (B-D) Confirmatory qPCR analyses for selected TGFβ superfamily members, including ligands (B), type I receptors (C), and type II receptors (D). Transcripts are normalized to Gapdh. Error bars indicate the SEM from 3 independent biologic datasets. (E) E7.5 cryosectioned embryos were stained for pSMAD1/5 and analyzed by confocal microscopy. pSMAD1 was found to be highly expressed in the PS, and extraembryonic mesoderm (underlying visceral endoderm). Top panel shows low magnification of the whole embryo shown in the bottom panels (10× objective). We observed a gradient of staining for pSMAD1/5 (top panel), with the highest expression in the same areas where Eng and/or Flk-1 are present, as demonstrated in Figure 1. Middle and bottom panels show confocal images of E7.5 serial sections demonstrating abundant expression of phosphorylated SMAD1/5 (pSMAD1/5, arrows in middle panels) in extraembryonic E+F+ cells (arrows in bottom panels). Chorionic E+F− cells show dim expression of pSMAD1/5. Note that visceral endoderm cells also show strong staining for pSMAD1/5 (63× objective). A indicates amnion; c, chorion; and VE, visceral endoderm.
TGFβ1/BMP signaling is active in Eng-expressing cells at E7.5. (A) Clustering of genes associated with the TGFβ superfamily among the E−F−, E−F+, E+F+, and E+F− fractions showing high expression of these TGFβ superfamily members in Eng-expressing cells. (B-D) Confirmatory qPCR analyses for selected TGFβ superfamily members, including ligands (B), type I receptors (C), and type II receptors (D). Transcripts are normalized to Gapdh. Error bars indicate the SEM from 3 independent biologic datasets. (E) E7.5 cryosectioned embryos were stained for pSMAD1/5 and analyzed by confocal microscopy. pSMAD1 was found to be highly expressed in the PS, and extraembryonic mesoderm (underlying visceral endoderm). Top panel shows low magnification of the whole embryo shown in the bottom panels (10× objective). We observed a gradient of staining for pSMAD1/5 (top panel), with the highest expression in the same areas where Eng and/or Flk-1 are present, as demonstrated in Figure 1. Middle and bottom panels show confocal images of E7.5 serial sections demonstrating abundant expression of phosphorylated SMAD1/5 (pSMAD1/5, arrows in middle panels) in extraembryonic E+F+ cells (arrows in bottom panels). Chorionic E+F− cells show dim expression of pSMAD1/5. Note that visceral endoderm cells also show strong staining for pSMAD1/5 (63× objective). A indicates amnion; c, chorion; and VE, visceral endoderm.
Alk3 and BmprII were also detected in the E+F− fraction, along with Bmp4 (Figure 5A-D) and its downstream targets, Msx1, Id2, and Gata237,38 (Figure 5A and supplemental Figure 2C), suggesting that the BMP4 pathway is particularly active in the Eng-single-positive cell population. Activation of Id2 transcription can also be promoted on TGFβ1 stimulation through ALK1/SMAD1 signaling.39 However, because E+F− cells presented very limited expression of TgfbrII and Alk1, Id2 up-regulation probably results from BMP4 signaling and not TGFβ1. Accordingly, BMP4 secreted by E+F− cells may have a paracrine effect on E+F+ cells through the same receptor complex involved in BMP2 signaling, as suggested by the high expression of its target gene Gata238 in this cell fraction (Figure 5A and supplemental Figure 2C).
To further confirm whether the described pathways are active, we evaluated phosphorylation of SMAD1/5 together with Eng and Flk-1 in serial sections of WT embryos at E7.5. SMAD1 is phosphorylated on stimulation with BMPs and TGFβ1 (through the ALK3 and ALK1 receptors, respectively), acting as a downstream effector.40 We observed a gradient of pSMAD1/5 expression, with the highest signal present between the proximal embryo proper and the extraembryonic region (Figure 5E top panel and supplemental Figure 5). E+F+ cells present in the BI (Figure 5E bottom panel) also stained for pSMAD1/5 (Figure 5E middle panel), confirming that TGFβ1/BMP signaling is active in these cells. The PS, which contains E+F+ cells, also stained for pSMAD1/5 (Figure 5E top panel and supplemental Figure 5). In addition, high levels of pSMAD1/5 were observed throughout the allantois, which is composed of E+F+ and E+F− cells (supplemental Figure 5).
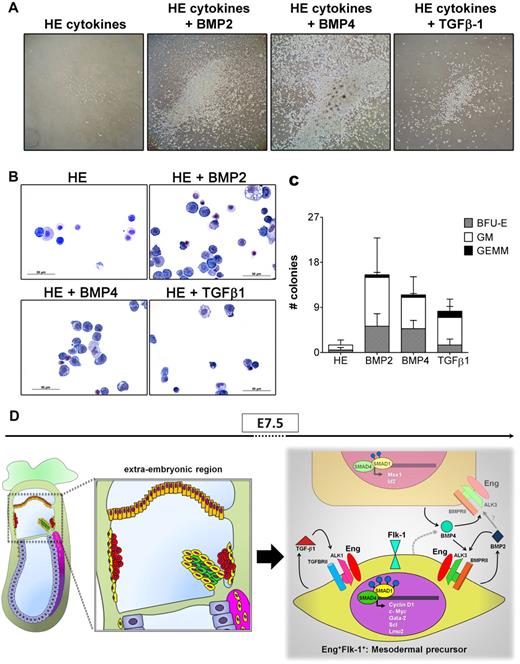
We also assessed whether the addition of BMP2, BMP4, or TGF-β1 would exert a biologic effect on ex vivo cultures of Eng-expressing cells obtained from E7.5 embryos. For these analyses, we plated the same cell number of sorted cells (Eng+ and Eng−) in multiple wells of 12-well plates in serum-free medium containing a cocktail of hematopoietic (HE) cytokines, as detailed in “Liquid hematopoietic cell expansion using TGFβ1 or BMP2/4.” We then added individual TGFβ ligands (BMP2, BMP4, or TGF-β1) to wells containing basic medium (HE cytokines) and evaluated their effect. Eng− cells failed to grow under all of the culture conditions assessed here (data not shown). Although Eng+ cells survived in control wells (Figure 6A left panel), they did not proliferate when cultured solely in the presence of basal medium (only HE cytokines). Conversely, the addition of BMP2, BMP4, or TGF-β1 to E7.5 Eng+ cell cultures resulted in significant proliferation, which was most prominent in response to BMP4 (Figure 6A). The hematopoietic nature of these outgrowths was confirmed by cytospin/Wright-Giemsa staining (Figure 6B) and colony assays, which revealed no significant differences between the 3 ligands in terms of colony type (Figure 6C). These findings suggest that the E+F+ fraction represents mesodermal cells that may respond to signals from TGFβ1, BMP4, and BMP2 (summarized in Figure 6D) to shape their commitment into hematopoietic progenitors and that Eng is a critical regulator in this process, acting by modulating TGF/BMP signaling through interaction with each receptor complex.
A model to depict the role of Eng during early hematopoiesis. (A) E7.5 Eng+-sorted cells were cultured in serum-free hematopoietic medium containing SCF, IL-3, and Flt3L (HE) in the presence or absence of BMP2 (10 ng/mL), BMP4 (25 ng/mL), or TGFβ1 (25 ng/mL). Representative images of hematopoietic outgrowths obtained from each culture condition (10-day cultures) are showed at similar magnification (10× objective). Expansion of hematopoietic cells occurred only when members of TGFβ superfamily were added to the basic medium containing HE cytokines, particularly BMP4. Eng+ cells survived but did not proliferate in the absence of BMP2/4 or TGFβ1. Representative data from at least 2 independent experiments are shown. (B) Cytologic examination of outgrowths obtained from Eng+ sorted cells, cultured for 5 days in HE medium in the presence or absence of BMP2, BMP4, or TGFβ1 demonstrates the hematopoietic nature of these cells. Representative cytospins of 2 independent experiments. (C) Number of hematopoietic progenitors in Eng+ cell cultures after 4 days of expansion in HE medium with or without BMP2, BMP4, and TGFβ1. Bars represent the average number of colonies from 4 independent experiments. Error bars represent the SEM for each type of colony. (D) Schematic representation of an E7.5 embryo (left panel) shows the localization of Eng+ cells during early development. E+F+ and E+F− cells are represented in yellow and orange, respectively. Red round cells represent primitive erythrocytes. During the formation of BIs at E7.5, E+F− cells (right panel, orange), possibly from the chorion, secrete BMP4, which through autocrine signaling may lead to activation of SMAD1 after binding to the Eng/ALK3/BMPRII receptor complex, with subsequent activation of the downstream genes Msx1 and Id2. BMP4 may also have a paracrine effect in E+F+ cells (right panel, yellow), early mesodermal progenitors present in the extraembryonic region that are endowed with hematopoietic and endothelial potential, because these cells express Eng/ALK3/BMPRII receptors and high levels of Gata2, Scl, and Lmo2, also target genes of BMP4. The finding that E+F+ cells display high levels of TGFβ1 and BMP2, as well as their respective receptor complexes Eng/ALK1/TGFBRII and Eng/ALK3/BMPRII, suggest a potential autocrine loop, because high levels of pSMAD1/5 (blue circles) and c-Myc and Cyclin D1 are detected in these cells. The important role played by Eng in early hematopoiesis may be the modulation of the activation of TGFβ1 and BMP2/4 signaling pathways. The gray dashed arrow indicates a possible secreted molecule and the gray solid arrow represents a potential signaling pathway.
A model to depict the role of Eng during early hematopoiesis. (A) E7.5 Eng+-sorted cells were cultured in serum-free hematopoietic medium containing SCF, IL-3, and Flt3L (HE) in the presence or absence of BMP2 (10 ng/mL), BMP4 (25 ng/mL), or TGFβ1 (25 ng/mL). Representative images of hematopoietic outgrowths obtained from each culture condition (10-day cultures) are showed at similar magnification (10× objective). Expansion of hematopoietic cells occurred only when members of TGFβ superfamily were added to the basic medium containing HE cytokines, particularly BMP4. Eng+ cells survived but did not proliferate in the absence of BMP2/4 or TGFβ1. Representative data from at least 2 independent experiments are shown. (B) Cytologic examination of outgrowths obtained from Eng+ sorted cells, cultured for 5 days in HE medium in the presence or absence of BMP2, BMP4, or TGFβ1 demonstrates the hematopoietic nature of these cells. Representative cytospins of 2 independent experiments. (C) Number of hematopoietic progenitors in Eng+ cell cultures after 4 days of expansion in HE medium with or without BMP2, BMP4, and TGFβ1. Bars represent the average number of colonies from 4 independent experiments. Error bars represent the SEM for each type of colony. (D) Schematic representation of an E7.5 embryo (left panel) shows the localization of Eng+ cells during early development. E+F+ and E+F− cells are represented in yellow and orange, respectively. Red round cells represent primitive erythrocytes. During the formation of BIs at E7.5, E+F− cells (right panel, orange), possibly from the chorion, secrete BMP4, which through autocrine signaling may lead to activation of SMAD1 after binding to the Eng/ALK3/BMPRII receptor complex, with subsequent activation of the downstream genes Msx1 and Id2. BMP4 may also have a paracrine effect in E+F+ cells (right panel, yellow), early mesodermal progenitors present in the extraembryonic region that are endowed with hematopoietic and endothelial potential, because these cells express Eng/ALK3/BMPRII receptors and high levels of Gata2, Scl, and Lmo2, also target genes of BMP4. The finding that E+F+ cells display high levels of TGFβ1 and BMP2, as well as their respective receptor complexes Eng/ALK1/TGFBRII and Eng/ALK3/BMPRII, suggest a potential autocrine loop, because high levels of pSMAD1/5 (blue circles) and c-Myc and Cyclin D1 are detected in these cells. The important role played by Eng in early hematopoiesis may be the modulation of the activation of TGFβ1 and BMP2/4 signaling pathways. The gray dashed arrow indicates a possible secreted molecule and the gray solid arrow represents a potential signaling pathway.
Discussion
Despite extensive research into the ontogeny of blood cells, much of the molecular mechanisms that control specification of hematopoietic cell fate from the mesoderm remain unknown. It has been demonstrated that Flk-1+ mesodermal cells migrate extraembryonically from the PS to give rise to the allantois, the mesodermal layer of the chorion, and the BIs of the YS.7 The latter contains the first hematopoietic and endothelial progenitors of the embryo. In the present study, we have shown that virtually all embryonic hematopoietic activity is restricted to the Eng+ population. At E7.5, hematopoietic progenitors are enriched in the E+F+ subset of cells and are characterized by a distinct hematopoietic and endothelial molecular signature, as evidenced by flow cytometry and transcriptional profiling of this fraction. These results are consistent with previous studies showing expression of endothelial markers on the earliest hematopoietic progenitors during embryogenesis.41-43 Some investigators have suggested that this indicates an endothelial origin for the hematopoietic cells of the YS44 ; however, budding of hematopoietic cells from a frank endothelium has only been observed clearly in the dorsal aorta of the AGM at E10.5.45,46 Our results favor the hypothesis of an extraembryonic mesodermal progenitor that is endowed with hematopoietic and endothelial potential at this early stage. When E+F+ cells isolated from E7.5 embryos were plated onto OP9 cocultures in the presence of hematopoietic and endothelial growth factors, cells for both hematopoietic and endothelial lineages could be detected. Our findings also indicate that the majority of the E+F+ cells are already committed to an hematopoietic or endothelial fate. At E7.5, Eng also labels primitive EryPs, as shown by coexpression with GATA1 (Figure 1C-D), whereas Flk-1 is absent in these cells. These results are supported by the colony assay and transcriptional analyses showing a high frequency of EryP progenitors and increased expression of Gata1 and embryonic globin in the E+F− fraction.
Interestingly, Eng expression was also found in the allantois and chorion. Chorion derives from trophectoderm and mesoderm, whereas the allantois is solely mesodermal in origin.11 By day 8.5, these 2 structures fuse to give rise to the placenta, which is now known to be a rich source of hematopoietic stem/progenitor cells in the embryo.47 The abundant expression of Eng in the allantois is consistent with a role in the regulation of its reported hematopoietic activity,48,49 and that of the placenta at later time points.5,47,50 This bears further investigation. Recent studies have shown that Eng marks hematopoietic stem/progenitor cells and HSC-producing endothelium from the AGM of E11.5 embryos.24,25 These finds suggest that modulation of TGFβ superfamily signals may be critical to HSC formation at this site, which also warrants further investigation.
In accordance with our findings in WT mice that point to an important role for Eng during early hematopoiesis, in the present study we show for the first time that deficiency in Eng leads to defective primitive hematopoiesis, as clearly evidenced by the reduced numbers of EryP progenitors at E7.5, the lower frequency of CD71+Ter119+ EryPs of E9.5 Eng−/− YS, the overall reduced definitive hematopoietic colony activity at E9.5, and down-regulation of Scl, Gata1, and embryonic globin.
To determine which signaling pathway Eng may control during early hematopoiesis, we investigated the expression of genes related to TGFβ superfamily signaling in E7.5-sorted cells. We found that type I and II receptors, as well as target genes of TGFβ1, BMP2, and BMP4 signaling, are highly expressed in E+F+ cells (Figure 5) at E7.5. The activity of these pathways was confirmed by the high levels of pSMAD1/5 detected at this early developmental stage, which coincided with Eng expression (Figure 5E). Proliferation of E7.5 Eng-expressing cells required a TGFβ superfamily ligand (Figure 6A).
Several studies have demonstrated the importance of BMP4 secreted by extraembryonic ectoderm during early embryogenesis. BMP4 is necessary for posterior PS formation16 and, together with its downstream effector SMAD1, is also required for allantois development.16 BMP4 plays an essential role in mesoderm specification toward the hematopoietic fate,51 a process that involves the BMP4/SMAD1-mediated up-regulation of hematopoietic genes such as Gata1, Gata2, Scl, and Lmo2.20 Similarly, BMP2 has been reported to be required for mesoderm formation52 and the development of the amnion, chorion, and allantois.17 Although there is no report linking TGFβ1 with mesodermal/blood specification, TGFβ1 was found to be highly expressed in the allantois and in the BI as early as E7.5.53 Moreover, embryos null for TGFβ1 and TβRII display impaired hematopoiesis,12,15 indicating that this signaling pathway is also important for early blood development. In accordance with this, a recent study by Baron et al has demonstrated that EryP progenitors are regulated by TGFβ1.54
Eng is mostly known for its interaction with TGFβ1 in endothelial cells, but it also binds to BMPs, in particular to BMP7 and BMP2, in combination with their respective receptor complex.21 Although there have been no previous data indicating an interaction between Eng and BMP4, our present findings provide strong evidence that Eng is important for BMP4 signaling, because this pathway was found to be active in Eng+ cells and BMP4 induced a proliferative response in these cells. Target genes for BMP4 were found to be down-regulated in Eng−/− YS, including Gata1, Scl, Gata2, and Msx1 (Figure 4D). This was corroborated by the significantly lower levels of pSMAD1/5/8 and downstream targets of BMP4 observed previously in differentiating, Eng-deficient ESCs.55
The results of the present study demonstrate that Eng is required for proper hematopoietic development and that virtually all YS hematopoiesis is restricted to the Eng+ cell fraction. This progenitor population responds to BMP4, BMP2, and TGFβ1. The presence of Eng is crucial for directing the TGFβ/BMP signal appropriately through SMAD1/5, which is necessary for the emergence of blood during embryonic development.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (grants R01 HL085840-01, U01 HL100407, and R01 HL085729) and the American Heart Association (Jon Holden DeHaan Foundation 0970499).
National Institutes of Health
Authorship
Contribution: L.B. designed and conducted the experiments, analyzed and interpreted the data, and wrote the manuscript; M.I. designed and conducted the experiments and analyzed the data; T.M., N.K.-N., and J.B. conducted the experiments; D.J.G. and M.K. interpreted the data; M.L. provided the mice and interpreted the data; and R.C.R.P. supervised the overall project, designed the experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rita C. R. Perlingeiro, PhD, Lillehei Heart Institute, University of Minnesota, 4-124 Nils Hasselmo Hall, 312 Church St SE, Minneapolis, MN 55455; e-mail: perli032@umn.edu.