Abstract
The role of hedgehog (Hh) signaling in B lymphopoiesis has remained unclear. We observed that the proliferation of pro-B cells in stromal cocultures was impaired by interruption of Hh signaling, prompting us to investigate whether the target of Hh antagonism was intrinsic or extrinsic to the B-lymphoid compartment. In the present study, using conditional deletion of the pathway activator gene Smo, we found that cell-autonomous Hh signaling is dispensable for B-cell development, B-lymphoid repopulation of the BM, and humoral immune function. In contrast, depletion of the Smo protein from stromal cells was associated with impaired generation of B-lymphoid cells from hematopoietic stem progenitor cells, whereas reciprocal removal of Smo from these cells had no effect on the production of B-cell progenitors. Depletion of Smo from stromal cells was associated with coordinate down-regulation of genes for which expression is associated with osteoblastoid identity and B-lymphopoietic activity. The results of the present study suggest that activity of the Hh pathway within stromal cells promotes B lymphopoiesis in a non–cell-autonomous fashion.
Introduction
The major pathway of B lymphopoiesis from hematopoietic stem progenitor cells has been described in some detail previously.1 The earliest identifiable B-cell progenitors, termed pro-B cells, arise initially in the fetal liver and subsequently in the BM, carrying partial D-to-JH rearrangements of both Ig alleles. Subsequent, productive VH-to-DJH joining triggers the transition to the pre-B stage, at which time the Ig μ heavy chain associates with surrogate light chains to form the pre-BCR. Signals emanating from the pre-BCR suppress further heavy-chain rearrangement, activate Ig light-chain rearrangement, promote proliferation, inhibit apoptosis, and increase sensitivity to IL-7. After productive Ig light-chain rearrangement, counterselection of self-reactive specificities, and receptor editing, nascent B cells leave the marrow for peripheral lymphoid tissues.
In the BM, B-cell development occurs in essential association with stromal cells.2 Some effects of stromal cells on B-cell development are mediated by direct contact, such as the interaction of c-Kit on pro-B cells with SCF on stromal cells.3 Other effects are mediated by soluble products of stromal cells, such as IL-7, which supports maturation beyond the pro-B stage.4 However, the contributions of the stromal environment to B-lymphoid differentiation remain poorly defined, as evident from the requirement for stromal cells in addition to soluble factors in systems that support B lymphopoiesis ex vivo.
The Hedgehog (Hh) signaling pathway serves several developmental functions, including establishment of tissue polarity, embryonic patterning, and stem cell maintenance.5 Hh signaling is initiated by a family of ligands that includes Sonic Hh (Shh), Indian Hh (Ihh), and Desert Hh (Dhh). In the absence of ligand, the receptor Patched (Ptch) inhibits the activator Smoothened (Smo); the binding of ligand relieves inhibition of Smo by Ptch, permitting downstream signaling to the transcription factors Gli1, Gli2, and Gli3.6
Whereas Hh signaling has been implicated in the expansion7 and differentiation8,9 of hematopoietic stem cells, recent studies have suggested that cell-autonomous Hh signaling is not required for hematopoiesis in the mouse.10,11 Interference with Hh signaling is, however, associated with defects in T-cell development12,13 and activation.14,15 Although evidence exists for an antiapoptotic role of Hh in normal and malignant B-cell maintenance,16,17 the effect of Hh signal ablation on B-cell development remains unclear. In the present study, we examined the dependence of B-lymphoid development on intrinsic and extrinsic Hh activity. Despite the differential expression of Hh signaling mediators during B-cell development and the dependence of pro-B cell maintenance ex vivo on Hh activity, conditional ablation of Smo in the B lineage had no detectable effect on subsequent B-cell development or function. In contrast, the generation of B-lineage cells from hematopoietic stem progenitor cells ex vivo was impaired significantly by the extinction of Hh signaling in stromal cells. Our results suggest that non–cell-autonomous Hh signaling in the stromal compartment functions in the lymphoid-myeloid cell-fate decision by promoting the differentiation of hematopoietic stem progenitor cells into B-lymphoid progenitors.
Methods
Animals
Mice carrying the Smowt/null18 or Smoflox/flox alleles19 on a C57BL/6 background were gifts of N. Watkins and C. Peacock (Johns Hopkins University, Baltimore, MD). Conditional deletion of Smo was accomplished by crossing Smowt/null and Smoflox/flox mice to obtain Smonull/flox animals, which were then crossed with mice expressing cre under control of the CD19 (CD19-cre; Taconic)20 or mb-1 (mb-1-cre; Elias Hobeika, Biological Signaling Studies, University of Freiburg, Freiburg, Germany)21 promoter. Genotyping was performed by PCR.18,19 C57BL/6J mice were used where indicated. Mice were housed in accordance with the policies of the Johns Hopkins University Institutional Animal Care and Use Committee.
Flow cytometry
Cell suspensions were stained with fluorescently labeled Abs for 30 minutes on ice in PBS containing 0.5% BSA and 2mM EDTA. The following Abs were used: anti-B220 (RA3-6B2), anti-CD43 (S7), anti-CD19 (1D3), anti-IgM (R6-60.2), anti-IgD (11.26C), and anti-CD11b (M1/70, all from BD Pharmingen). Data were collected using an LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo 9.5.1 software (TreeStar).
Maintenance of pro-B cells
BM cell suspensions from 6- to 8-week-old C57BL/6J mice were maintained with autologous stromal cells in RPMI 1640 medium supplemented with 10% FCS, 50 U/mL of penicillin/streptomycin, 1mM sodium pyruvate, 2mM l-glutamine, 50μM β-mercaptoethanol, 10mM HEPES, MEM nonessential amino acids, and 10 ng/mL of IL-7 (PeproTech) at 37°C in 5% CO2. After 5 days, greater than 95% of the nonadherent cells expressed the B220+CD43+ pro-B cell phenotype. Aliquots of these cells (0.5 × 106 cells/mL) were cultured with PA6 stromal cells (2 × 104 cells/2.5 cm2) in the presence of cyclopamine (LKT Laboratories) or 5 μg/mL of neutralizing Hh Ab 5e1 (Developmental Studies Hybridoma Bank). Recombinant Shh (R&D Systems) was added to some cultures at 5 μg/mL. Proliferation was assayed at 48 hours of culture, after 16 hours labeling with [3H] thymidine at 1 μCi/100 μL. DMSO and isotype-matched Ab (Jackson ImmunoResearch Laboratories) served as controls for cyclopamine and 5e1, respectively.
Cell sorting and separation
B-cell developmental subsets were purified as described previously.22 CD19+ BM and splenic B-lymphoid cells were purified to > 90% by magnetic Ab separation (Miltenyi Biotec). Lin−Sca-1+c-Kit+ (LSK) hematopoietic progenitors were purified (> 98%) from BM of 6- to 10-week-old mice by a magnetic Ab separation scheme using selection against the lineage-specific markers CD5, CD45R (B220), CD11b, Gr-1 (Ly-6G/C), 7-4 (Neuto), Ter-119, and CD19 (Miltenyi Biotec). LSK progenitors transduced with pMIG-cre were sorted on the basis of green fluorescent protein (GFP) expression to > 95% purity using a FACS Aria cell sorter (BD Biosciences).
PCR assays
For mRNA analysis, polyadenylated RNA was isolated from cell lysates by oligo-dT chromatography (QIAGEN). Template cDNA was synthesized by reverse transcription. Semiquantitative PCR was performed with serially diluted cDNA template as follows: 94°C for 1 minute; 30 cycles of 94°C for 1 minute, Tm 5°C for 30 seconds, 72°C for 1 min/kb; and a final extension for 10 minutes at 72°C. Quantitative real-time PCR was performed with SYBR Green detection using the 7300 Real Time PCR System (ABI). Expression levels were normalized to Actb.
Assay for DNA rearrangement
Genomic DNA was purified using the DNeasy Blood and Tissue kit (QIAGEN). Primer pair sets are defined in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Depletion of Smo from OP9 cells
Lentiviral plasmids encoding shRNA were constructed in pLKO.1-puro using oligonucleotides TRCN0000026288 (Smo-specific; Open Biosystems) or SHC002 (nontemplated control; Sigma-Aldrich). Virus was generated and OP9 cells were infected as described previously.23 At 48 hours after transduction, puromycin (2.5 μg/mL) was added and cultures were maintained under selection for 10 days.
Deletion of Smo from LSK progenitors
Smofl/fl mice were treated intraperitoneally with 150 mg/kg of 5-fluorouracil (5-FU) and LSK cells were purified at 5 days as described in “Cell sorting and separation.” LSK cells were infected with viral particles derived from pMIG24 that had been engineered to encode cre (pMIG-Cre). Infection was carried out at 485g for 90 minutes at 22°C in the presence of 8 μg/mL of polybrene.
Hematopoietic stem progenitor cell differentiation
B-lymphoid differentiation assays were performed as described previously,25 with modifications. LSK cells were seeded on OP9 cells in the presence of FLT3L, SCF, and IL-7. B-lymphoid differentiation was induced with sequential removal of FLT3L and SCF at days 3 and 6. Cells were subsequently maintained in the presence of IL-7 and replated on fresh OP9 layers every 3 days.
Microarray analysis
Triplicate RNA samples were purified from control (nontemplated; NT) and Smo-depleted (Smo-KD) OP9 cells using the RNeasy kit (QIAGEN). Probe preparation and hybridization to the Mouse Exon 1.0 ST GeneChip (Affymetrix) was carried out by the Johns Hopkins Deep Sequencing and Microarray Core. Expression measures were computed using the robust multiarray average protocol and analysis of differential expression was carried out using Partek Genomics Suite Version 6.5. Functional clustering was performed using the DAVID Bioinformatics Resource.26,27 All microarray data are available at the Gene Expression Omnibus database under accession number GSE34421.
Statistical analysis
Except where indicated, the means of 2 groups were compared using the 2-tailed unpaired Student t test. Significance of differential gene expression was estimated by 1-way ANOVA.
Partial BM ablation, immunization, maintenance of stromal cell lines, and stem cell differentiation
For details on these procedures, see supplemental Methods.
Results
Pro-B cell maintenance impaired by Hh antagonists
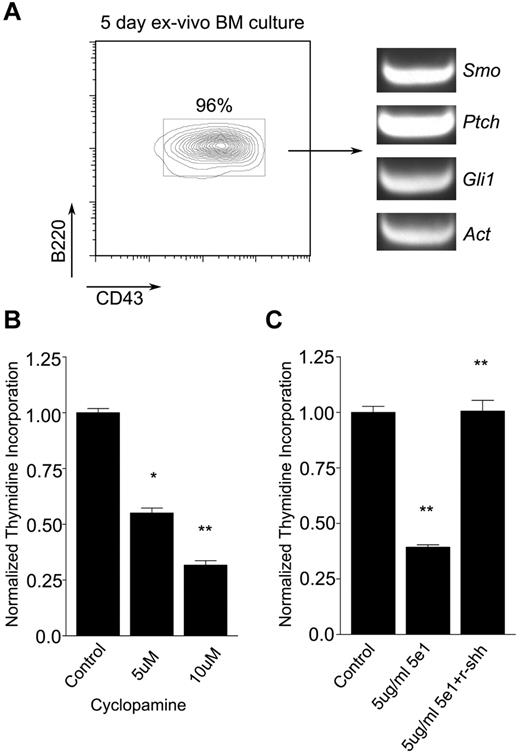
To determine whether Hh signals promote B-cell development, we first measured the effects of hedgehog antagonists on the maintenance of B-cell progenitors. Mouse BM cells were maintained ex vivo in the presence of autologous stromal cells and IL-7 for 5 days, by which time > 95% of nonadherent cells were B220+CD43+ pro-B cells (Figure 1A left). These cells were found to express Smo, Ptch, and Gli1, which is consistent with competence for Hh signaling (Figure 1A right). After treatment with increasing amounts of the Smo antagonist cyclopamine for 48 hours, pro-B cells showed a dose-dependent impairment of proliferation (Figure 1B). These findings were not attributable to nonspecific toxicity, because pro-B cell apoptosis was not sufficiently increased to explain the effect and no decrease in stromal cell number was observed (supplemental Figure 1). A similar effect on pro-B cell proliferation ex vivo was observed when Hh signaling was blocked with the anti-Hh neutralizing Ab 5e1 (Figure 1C), Moreover, the reduction in proliferative capacity seen in the presence of 5e1 was reversed by soluble recombinant Shh ligand (Figure 1C). These results suggested that Hh signaling promotes the maintenance of pro-B cells.
Hh pathway antagonists impair maintenance of pro-B cells ex vivo. (A) Expansion of pro-B cells ex vivo. Flow cytometric analysis of pro-B cell (B220+CD43+) expansion from murine BM cells maintained for 5 days in the presence of autologous stromal cells and IL-7 (left). Presence of Smo, Ptch, and Gli1 transcripts in pro-B cells maintained ex vivo as assayed by RT-PCR (right). (B-C) Effects of Hh antagonists on pro-B cell proliferation. Pro-B cells were maintained on PA6 stromal cells with IL-7 in the absence or presence of Hh antagonist for 48 hours and proliferation was measured by 3H thymidine incorporation (mean and SD, n = 3, *P < .01, **P < .005). (B) Cyclopamine was omitted (control) or added at increasing concentration as indicated. (C) Pro-B cells were treated with isotype control Ab (control), anti–Hh Ab 5e1, or Ab 5e1 supplemented with recombinant sonic Hh (r-shh) as indicated.
Hh pathway antagonists impair maintenance of pro-B cells ex vivo. (A) Expansion of pro-B cells ex vivo. Flow cytometric analysis of pro-B cell (B220+CD43+) expansion from murine BM cells maintained for 5 days in the presence of autologous stromal cells and IL-7 (left). Presence of Smo, Ptch, and Gli1 transcripts in pro-B cells maintained ex vivo as assayed by RT-PCR (right). (B-C) Effects of Hh antagonists on pro-B cell proliferation. Pro-B cells were maintained on PA6 stromal cells with IL-7 in the absence or presence of Hh antagonist for 48 hours and proliferation was measured by 3H thymidine incorporation (mean and SD, n = 3, *P < .01, **P < .005). (B) Cyclopamine was omitted (control) or added at increasing concentration as indicated. (C) Pro-B cells were treated with isotype control Ab (control), anti–Hh Ab 5e1, or Ab 5e1 supplemented with recombinant sonic Hh (r-shh) as indicated.
Differential expression of Hh signaling mediators during B-cell development
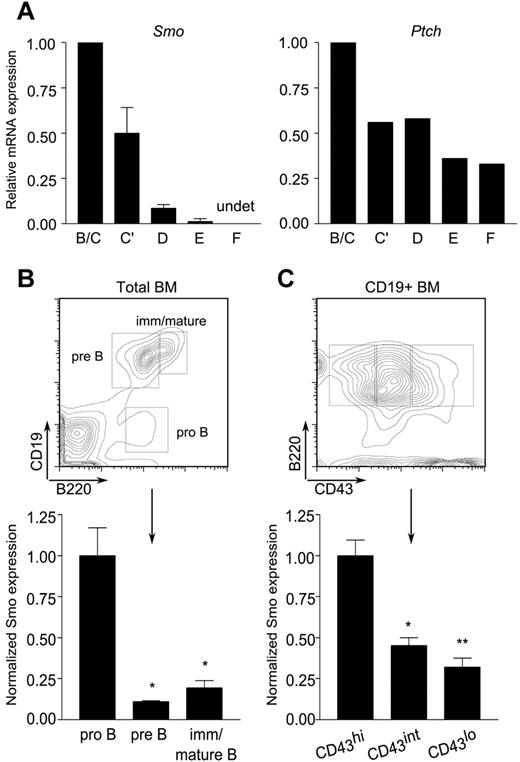
The impairment of pro-B cell maintenance ex vivo by Hh antagonists prompted us to investigate whether the target of antagonism was intrinsic or extrinsic to the B-lymphoid compartment. We began by assessing the presence of Hh signaling components in the B lineage. B-lymphoid developmental fractions B/C (B220+CD43+HSAintBP-1−/+), C′ (B220+CD43+HSAhiBP-1+), D (B220+CD43−IgM−IgD−), E (B220+CD43−IgM+IgD−), and F (B220+CD43−IgM+IgD+) were purified from mouse BM by FACS. Expression of Smo and Ptch was assayed by quantitative, real-time PCR. Both were abundant in developmental fractions B/C and C′, which span the pro-B and early, proliferating pre-B cell stages (Figure 2A). A decrease in the amounts of Smo and Ptch transcripts was observed in the more mature fractions. Smo expression was reduced in small pre-B cells (fraction D) and was nearly undetectable in newly formed (fraction E) and mature recirculating (fraction F) B cells (Figure 2A left). Expression of Ptch, the expression of which is positively regulated by Smo, showed a similar trend, although the range of expression was narrower than that of Smo (Figure 2A right).
Differential expression of Hh signaling components during B-cell development. (A) Reduced accumulation Smo and Ptch transcripts as a function of B-cell ontogeny. Quantitative RT-PCR of Smo (left) and Ptch (right) transcripts in B-lymphoid developmental fractions B/C (B220+CD43+HSAintBP-1−/+), C′ (B220+CD43+HSAhiBP-1+), D (B220+CD43−IgM−IgD−), E (B220+CD43−IgM+IgD−), and F (B220+CD43−IgM+IgD+). Data for each developmental fraction were normalized to an internal Actb (β-actin) control and then to the Actb-normalized value for developmental fraction B/C. Smo expression was determined in 2 independent samples of each developmental fraction; Ptch expression was determined in one of the sample sets used for Smo. (B-C) Surface expression of Smo is attenuated at the pro-B to pre-B transition. BM cells were stained for Smo and selected B-cell developmental markers and then analyzed by flow cytometry. (B top panel) Identification of pro-B (B220+CD19−), pre-B (B220+CD19+), and immature/mature B (B220hiCD19+) subsets on a representative plot of CD19 fluorescence against B220. (B bottom panel) Surface expression of Smo on developing B-lymphoid progenitors (n = 3, *P < .01, **P = .005). (C top panel) Identification of pro-B (B220+CD19+CD43hi), transitional pro-B (B220+CD19+CD43int), and pre-B cell (B220+CD19+CD43lo) subsets. (C bottom panel) Expression of Smo as cells traverse the pro-B to pre-B transition (n = 3, *P < .01, **P = .005). Expression of Smo was normalized to that of the B220+CD19+CD43hi subset.
Differential expression of Hh signaling components during B-cell development. (A) Reduced accumulation Smo and Ptch transcripts as a function of B-cell ontogeny. Quantitative RT-PCR of Smo (left) and Ptch (right) transcripts in B-lymphoid developmental fractions B/C (B220+CD43+HSAintBP-1−/+), C′ (B220+CD43+HSAhiBP-1+), D (B220+CD43−IgM−IgD−), E (B220+CD43−IgM+IgD−), and F (B220+CD43−IgM+IgD+). Data for each developmental fraction were normalized to an internal Actb (β-actin) control and then to the Actb-normalized value for developmental fraction B/C. Smo expression was determined in 2 independent samples of each developmental fraction; Ptch expression was determined in one of the sample sets used for Smo. (B-C) Surface expression of Smo is attenuated at the pro-B to pre-B transition. BM cells were stained for Smo and selected B-cell developmental markers and then analyzed by flow cytometry. (B top panel) Identification of pro-B (B220+CD19−), pre-B (B220+CD19+), and immature/mature B (B220hiCD19+) subsets on a representative plot of CD19 fluorescence against B220. (B bottom panel) Surface expression of Smo on developing B-lymphoid progenitors (n = 3, *P < .01, **P = .005). (C top panel) Identification of pro-B (B220+CD19+CD43hi), transitional pro-B (B220+CD19+CD43int), and pre-B cell (B220+CD19+CD43lo) subsets. (C bottom panel) Expression of Smo as cells traverse the pro-B to pre-B transition (n = 3, *P < .01, **P = .005). Expression of Smo was normalized to that of the B220+CD19+CD43hi subset.
We next examined surface expression of Smo on developing B cells. In accordance with the distribution of Smo transcripts, surface Smo expression was highest in early pro-B cells (B220+CD19−) and declined during maturation (Figure 2B). The pro-B to pre-B transition is marked by down-regulation of CD43. This decrease is accompanied by a corresponding decrease in the surface expression of Smo (Figure 2C). The presence of Hh signaling components in early B-cell progenitors prompted us to investigate whether there is a cell-autonomous requirement for Hh signaling in B-cell development.
Normal B-cell development in the absence of Hh signaling
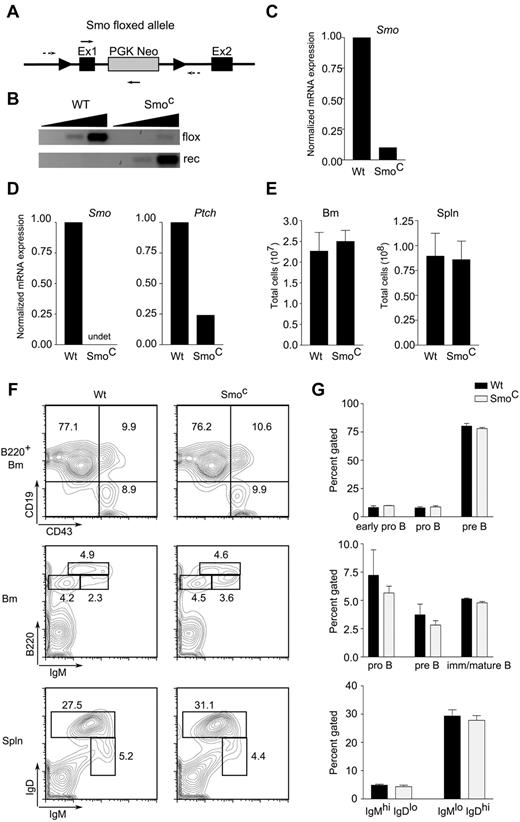
Hh signaling was ablated in the B lineage by conditional deletion of Smo. In initial experiments, expression of the cre recombinase, which performs deletion, was driven by the B-lineage–specific CD19 promoter (CD19-cre). Compound heterozygous mice carrying a floxed conditional Smo allele and a conventional null Smo allele (Smofl/null)19 were crossed to CD19-cre transgenic mice (supplemental Figure 2). In CD19-cre transgenic, Smofl/null animals, conditional deletion of Smo had no apparent effect on B-lymphoid development (supplemental Figure 2). Because the CD19 promoter is not active at the earliest stages of B-cell development, these mice cannot exhibit any requirement for Hh signaling in pro-B cells. We therefore generated a second line of Smofl/null mice (Figure 3A) in which cre expression is driven by the B-lymphoid–specific mb-1 promoter, the activity of which begins at the pro-B cell stage. Indeed, splenic B cells or ex vivo pro-B cells from mb-1-cre, Smofl/null animals, which we will refer to as SmoC mice, showed approximately 90% excision of the targeted allele (Figure 3B and data not shown). B-lineage cells from the BM (Figure 3C) or spleen (Figure 3D) showed a 10-fold or greater reduction in Smo transcripts; in splenic B cells, this was accompanied by a reduction in Ptch transcripts, which are regulated by Hh (Figure 3D).
Intrinsic Hh signaling is dispensable for B-cell development. (A) Diagram of targeted Smoflox allele. Exons 1 (Ex1) and 2 (Ex2) of Smo and the phosphoglycerokinase-Neo cassette (PGK Neo) are indicated. Arrowheads indicate loxP sites; dotted arrows, primers for amplification of unexcised, floxed allele; solid arrows, primers for detection of recombined allele. (B) Detection of Smo floxed (flox) and recombined (rec) alleles in CD19+ splenic B cells from wild-type or SmoC mice. Amplification was performed on 10-fold serial dilutions of genomic DNA template. (C-D) Quantitative RT-PCR of Smo and Ptch transcripts in CD19+ BM (C) or naive splenic B cells (D) from WT or SmoC mice. (E) Cellularity of BM (left) and spleen (right) in wild-type or SmoC mice. (F) Examination of B-lymphoid developmental subsets. BM (top and middle panels) and spleen (spln; bottom panels) from 6- to 8-week-old wild-type or SmoC mice were analyzed by flow cytometry. Early pro-B (B220+CD19−CD43+IgM−), pro-B (B220+CD19+CD43+IgM−), pre-B (B220+CD19+CD43−IgM+), and immature/mature B cell (B220highCD19+CD43−IgM+) subsets are indicated on representative BM plots. B220+IgMhiIgD−/lo and B220+IgMloIgDhi populations, enriched for immature/transitional B cells and mature follicular B cells, respectively, are identified on splenic plots. (G) Percentage representation of B-lymphoid subsets in wild-type or SmoC mice as defined in panel F (n = 3, mean and SD).
Intrinsic Hh signaling is dispensable for B-cell development. (A) Diagram of targeted Smoflox allele. Exons 1 (Ex1) and 2 (Ex2) of Smo and the phosphoglycerokinase-Neo cassette (PGK Neo) are indicated. Arrowheads indicate loxP sites; dotted arrows, primers for amplification of unexcised, floxed allele; solid arrows, primers for detection of recombined allele. (B) Detection of Smo floxed (flox) and recombined (rec) alleles in CD19+ splenic B cells from wild-type or SmoC mice. Amplification was performed on 10-fold serial dilutions of genomic DNA template. (C-D) Quantitative RT-PCR of Smo and Ptch transcripts in CD19+ BM (C) or naive splenic B cells (D) from WT or SmoC mice. (E) Cellularity of BM (left) and spleen (right) in wild-type or SmoC mice. (F) Examination of B-lymphoid developmental subsets. BM (top and middle panels) and spleen (spln; bottom panels) from 6- to 8-week-old wild-type or SmoC mice were analyzed by flow cytometry. Early pro-B (B220+CD19−CD43+IgM−), pro-B (B220+CD19+CD43+IgM−), pre-B (B220+CD19+CD43−IgM+), and immature/mature B cell (B220highCD19+CD43−IgM+) subsets are indicated on representative BM plots. B220+IgMhiIgD−/lo and B220+IgMloIgDhi populations, enriched for immature/transitional B cells and mature follicular B cells, respectively, are identified on splenic plots. (G) Percentage representation of B-lymphoid subsets in wild-type or SmoC mice as defined in panel F (n = 3, mean and SD).
Despite efficient deletion of Smo in B progenitors, interruption of Hh signaling was not accompanied by diminished cellularity of BM or spleen (Figure 3E). Examination of developmental subsets in BM failed to uncover an effect of Smo deletion on the sizes or proportions of the early pro-B (B220+CD19−CD43+IgM−), pro-B (B220+CD19+CD43+IgM−), pre-B (B220+CD19+CD43−IgM+), and immature/mature B cell (B220hiCD19+CD43−IgM+) populations (Figure 3F-G top and middle panels). Likewise, no alteration was seen in the splenic IgMhiIgDlo or IgMloIgDhi populations that are enriched for immature/transitional B cells and mature follicular B cells, respectively (Figure 3F-G bottom panels). These observations were consistent with the interpretation that B-lymphoid-autonomous Hh signaling is dispensable for B-cell development.
Smo-deficient B-lymphoid cells exhibit normal BM repopulation after ablation
Although inactivation of Smo in the B lineage had no effect on the steady-state cellularity of B-cell developmental subsets, it remained possible that a role for endogenous Hh signaling might be unmasked during BM repopulation after chemical ablation. We examined BM repopulation by B-progenitor subsets after the administration of 5-FU. A kinetic analysis of B-progenitor subsets and BM cellularity in wild-type mice revealed that steady-state representation of these subsets is attained within 14 days of 5-FU administration (supplemental Figure 3). We next administered a single dose of 5-FU to SmoC or wild-type mice and compared B-lymphoid repopulation of BM during the approach to steady-state (supplemental Figure 3). Repopulation proceeded with similar kinetics and subset cellularity in mutant and wild-type mice, indicating that B-lymphoid-autonomous Hh signaling is dispensable for the expansion of B-cell progenitors and repopulation of the BM.
Humoral immunity unaffected by ablation of Smo in the B lineage
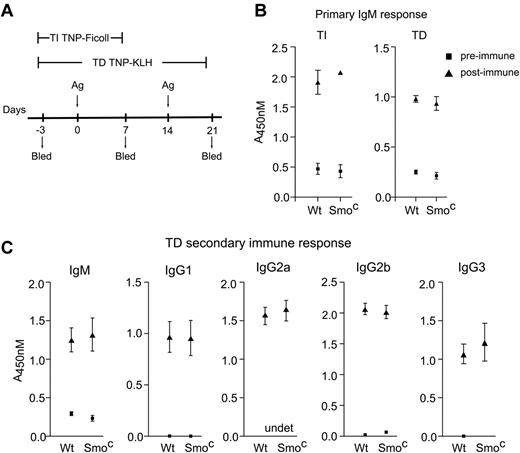
We observed a modest decrease in proliferation of Smo-deficient B cells in response to IgM cross-linking, although no such decrease was seen after treatment with lipopolysaccharide (supplemental Figure 4). The effect of Smo deficiency on proliferation was not, however, accompanied by impairment of humoral responsiveness. One week after immunization with TNP-Ficoll, a T-independent antigen (Figure 4A), serum IgM responses were similar in wild-type and mb-1-cre, Smofl/null mice (Figure 4B TI). Likewise, primary IgM (Figure 4B TD) and secondary IgM, IgG1, IgG2a, IgG2b, and IgG3 (Figure 4C) humoral responses to the T-dependent antigen TNP-KLH were unperturbed in mb-1-cre, Smofl/null mice. Therefore, the activity of Smo in B cells is apparently dispensable for primary or secondary humoral responses, including those involving class switching.
B-lineage-intrinsic Smo activity is dispensable for primary and secondary humoral responses. (A) T-independent (TI) and T-dependent (TD) immunization protocols. Wild-type and SmoC mice were immunized at time points labeled Ag with TNP-Ficoll or TNP-KLH to elicit TI and TD immune responses, respectively. Pre-immune (day −3) and immune (days 7 and 21) sera were collected as indicated. (B) Primary TI and TD IgM response. Wild-type or SmoC mice were immunized with TNP-Ficoll or TNP-KLH as in panel A, and IgM responses to TNP were measured by ELISA (n = 3, mean and SD). (C) Secondary responses to TD immunization. wild-type or SmoC mice were immunized with TNP-KLH as in panel A. Secondary IgM, IgG1, IgG2a, IgG2b, and IgG3 responses to TNP were measured by ELISA (n = 5, mean and SEM). Serum dilution titrations are provided in supplemental Figure 4.
B-lineage-intrinsic Smo activity is dispensable for primary and secondary humoral responses. (A) T-independent (TI) and T-dependent (TD) immunization protocols. Wild-type and SmoC mice were immunized at time points labeled Ag with TNP-Ficoll or TNP-KLH to elicit TI and TD immune responses, respectively. Pre-immune (day −3) and immune (days 7 and 21) sera were collected as indicated. (B) Primary TI and TD IgM response. Wild-type or SmoC mice were immunized with TNP-Ficoll or TNP-KLH as in panel A, and IgM responses to TNP were measured by ELISA (n = 3, mean and SD). (C) Secondary responses to TD immunization. wild-type or SmoC mice were immunized with TNP-KLH as in panel A. Secondary IgM, IgG1, IgG2a, IgG2b, and IgG3 responses to TNP were measured by ELISA (n = 5, mean and SEM). Serum dilution titrations are provided in supplemental Figure 4.
Disruption of Hh signaling in stromal cells impairs generation of B-lymphoid cells from hematopoietic stem cells
Whereas the proliferation of B-cell progenitors ex vivo was impaired by interruption of Hh signaling, cell-autonomous Hh activity was dispensable for B-cell development and function. We therefore investigated whether Hh signaling in stromal cells could account for the dependence of B-lymphoid maintenance on Hh. We began by assessing expression of Hh signaling mediators in primary, BM-derived stromal cells and 3 stromal cell lines capable of supporting lymphopoiesis ex vivo: OP9,28 PA6,29 and S17.30 All expressed transcripts encoding Ptch, Smo, and Gli1, as well as the Hh ligands Ihh and Dhh (Figure 5A and supplemental Figure 9A). The expression of Hh signal transducers and ligands is consistent with the possibility that these cells are capable of paracrine and autocrine signaling.
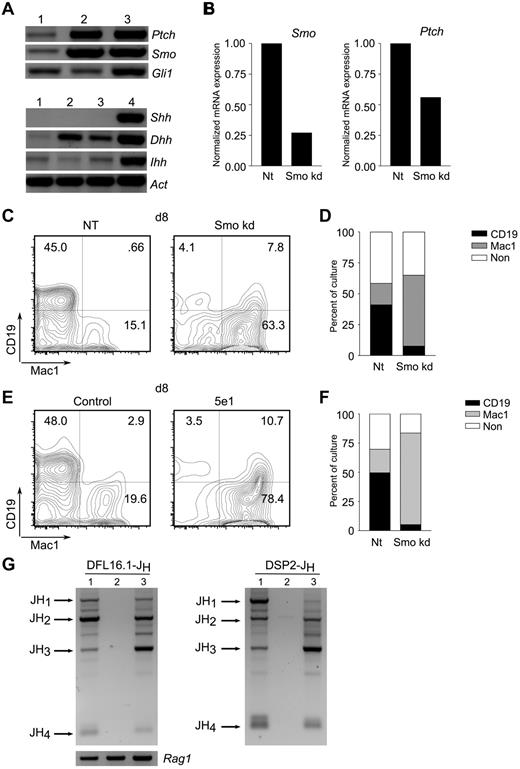
Hh pathway activity in stromal cells promotes B lymphopoiesis. (A) Expression of Hh pathway components in stromal cells. Detection of transcripts encoding Hh signaling components (top) and ligands (bottom) in primary BM-derived stromal cells (lane 1), stromal cell lines PA6 (lane 2), or OP9 (lane 3), and mouse brain (lane 4). (B) Characterization of Smo-depleted OP9 derivatives. OP9 cells were transduced with lentivirus particles expressing Smo-specific (Smokd) or control (NT) shRNA. Smo (left) and Ptch (right) transcripts were quantitated by RT-PCR. (C-D) Impaired B lymphopoiesis from LSK progenitors maintained on Smo-depleted OP9 cells. Purified LSK progenitors were maintained on control (NT) or Smo-deficient (Smokd) OP9 cells under conditions that promote B lymphopoiesis. Lymphoid (CD19+Mac1−) and myeloid (CD19-Mac1+) differentiation were assessed by flow cytometry. (C) Representative plots of LSK cultures at 8 days on control (NT) or Smo-deficient (Smo kd) OP9 cells. (D) Percentages of lymphoid (CD19+Mac1−), myeloid (CD19−Mac1+), and nonlineage (CD19−Mac1−, CD19+Mac1+) progeny (n = 3, P < .01). (E-F) Impaired B lymphopoiesis in the presence of neutralizing Ab to Hh. LSK progenitors were cultured on OP9 cells in the presence of anti–Hh Ab 5e1 or an isotype control under B-lymphopoietic conditions. B-lymphoid and myeloid progeny were detected by flow cytometry. (E) Representative plots of control and Ab-treated (5e1) LSK cultures at 8 days. (F) Percentages of B-lymphoid and myeloid cells (n = 3, P < .005). A kinetic analysis of LSK differentiation from days 3-15 of culture is presented in the supplemental materials. (G) Undetectable DJH rearrangement in 5e1-treated LSK-OP9 cultures. DFL16-JH (left) and DSP2-JH (right) rearrangements were assayed in control (lane 1) and 5e1-treated (lane 2) LSK-OP9 cultures at 12 days or in spleen (lane 3). Expected positions of rearrangements to JH1-4 are indicated. Amounts of template DNA were monitored by amplification of Rag1 (bottom left).
Hh pathway activity in stromal cells promotes B lymphopoiesis. (A) Expression of Hh pathway components in stromal cells. Detection of transcripts encoding Hh signaling components (top) and ligands (bottom) in primary BM-derived stromal cells (lane 1), stromal cell lines PA6 (lane 2), or OP9 (lane 3), and mouse brain (lane 4). (B) Characterization of Smo-depleted OP9 derivatives. OP9 cells were transduced with lentivirus particles expressing Smo-specific (Smokd) or control (NT) shRNA. Smo (left) and Ptch (right) transcripts were quantitated by RT-PCR. (C-D) Impaired B lymphopoiesis from LSK progenitors maintained on Smo-depleted OP9 cells. Purified LSK progenitors were maintained on control (NT) or Smo-deficient (Smokd) OP9 cells under conditions that promote B lymphopoiesis. Lymphoid (CD19+Mac1−) and myeloid (CD19-Mac1+) differentiation were assessed by flow cytometry. (C) Representative plots of LSK cultures at 8 days on control (NT) or Smo-deficient (Smo kd) OP9 cells. (D) Percentages of lymphoid (CD19+Mac1−), myeloid (CD19−Mac1+), and nonlineage (CD19−Mac1−, CD19+Mac1+) progeny (n = 3, P < .01). (E-F) Impaired B lymphopoiesis in the presence of neutralizing Ab to Hh. LSK progenitors were cultured on OP9 cells in the presence of anti–Hh Ab 5e1 or an isotype control under B-lymphopoietic conditions. B-lymphoid and myeloid progeny were detected by flow cytometry. (E) Representative plots of control and Ab-treated (5e1) LSK cultures at 8 days. (F) Percentages of B-lymphoid and myeloid cells (n = 3, P < .005). A kinetic analysis of LSK differentiation from days 3-15 of culture is presented in the supplemental materials. (G) Undetectable DJH rearrangement in 5e1-treated LSK-OP9 cultures. DFL16-JH (left) and DSP2-JH (right) rearrangements were assayed in control (lane 1) and 5e1-treated (lane 2) LSK-OP9 cultures at 12 days or in spleen (lane 3). Expected positions of rearrangements to JH1-4 are indicated. Amounts of template DNA were monitored by amplification of Rag1 (bottom left).
We proceeded to test the effect of Smo depletion in stromal cells on lymphoid differentiation. The murine stromal cell line OP9 supports lymphoid and myeloid differentiation from LSK hematopoietic progenitors and, as we have shown, expresses Hh signal mediators and ligands. OP9 cells were depleted of Smo transcripts by stable infection with lentiviruses expressing a Smo-specific shRNA or a scrambled, NT control. Infection with the Smo-specific virus resulted in a reduction of Smo mRNA to approximately 25% of control, and this was accompanied by reduced expression of the Hh transcriptional target Ptch (Figure 5B). Control and Smo-depleted OP9 cells were tested for their ability to support B-lymphoid differentiation from LSK progenitors under conditions permissive for B lymphopoiesis.31 OP9 cells infected with the control lentivirus supported robust B lymphopoiesis: by 8 days of culture, nearly half of all LSK-derived cells expressed the B-lymphoid marker CD19 (Figure 5C-D). In contrast, the yield of CD19+Mac-1− lymphocytes was reduced by more than 10-fold in cultures containing Smo-depleted OP9 cells (Figure 5C-D). Conversely, depletion of Smo from the OP9 compartment was associated with an increase in the yield of myeloid cells, as assessed by expression of Mac-1 (Figure 5C-D). This skewing toward myeloid differentiation was apparent by 3 days of culture, persisted for at least 12-15 days, and was reflected in absolute cell numbers and in percentage representations (supplemental Figure 6). We observed the emergence at later times of a CD19loMac1+ population; as demonstrated in the experiment of Figure 5G, these are not likely to represent B-lymphoid–lineage cells.
Interference with B-lymphoid differentiation and a concomitant increase in the yield of myeloid cells was also observed in OP9-LSK cocultures subjected to Hh ligand blockade by the 5e1 Ab (Figure 5E-F). Indeed, the time course over which CD19+Mac-1− and CD19−Mac-1+ populations appeared in cultures treated with the 5e1 Ab was similar to the kinetics observed for cultures containing Smo-depleted OP9 cells (supplemental Figure 7). We considered the possibility that the decrease in CD19+ cells seen after 5e1 Ab treatment or Smo-depletion might have resulted from a loss of CD19 expression in a population that had undergone partial B-lymphoid differentiation. Because differentiation to the pro-B-cell stage is accompanied by irreversible D-to-JH joining at the IgH locus, any cells that had attained this early stage of B-lymphoid development would remain marked by DJH joints regardless of subsequent changes in phenotype. We therefore assayed DJH rearrangements involving the DFL16 and DSP2 families in control or 5e1-treated LSK-OP9 cultures at 12 days. Whereas DJH joints were readily detected in control cultures, DJH recombination products were not observed in the presence of the 5e1 Ab (Figure 5G and supplemental Figure 8). These observations indicate that differentiation to the pro-B cell stage is largely or completely inhibited by 5e1 and suggest that no other cells present in this system, including the CD19loMac1+ population, are derived from cells that had attained the pro-B cell stage. The 5e1 Ab also inhibited B lymphopoiesis in cocultures of LSK with S17 stromal cells, indicating that the dependence of B lymphopoiesis on stromal Hh signaling is not a particular property of the OP9 system (supplemental Figure 9B-C).
B-lymphoid differentiation promoted by Hh signaling in stromal cells
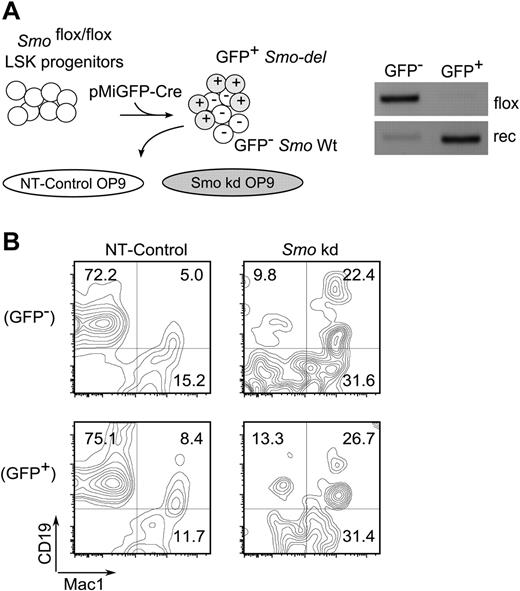
The results presented thus far suggested that B lymphopoiesis is promoted by a non–cell-autonomous Hh pathway activity in stromal cells. This was tested by reciprocal removal of Smo from LSK hematopoietic progenitors or from OP9 stromal cells. LSK progenitors were prepared from Smofl/fl mice and Smo excision was accomplished by infection with a lentivirus encoding cre and GFP (Figure 6A left). The GFP+ transductants exhibited robust deletion of Smo relative to GFP− cells (Figure 6A right). OP9 cells transduced with a control virus supported the generation of CD19+Mac-1− B-lymphoid cells from GFP+ (Smo-deleted) and GFP− (Smo-undeleted) LSK progenitors with similar efficiency (Figure 6B NT-Control). Depletion of Smo from OP9 cells was associated with impaired generation of B-lymphoid cells from Smo-deleted and Smo-undeleted LSK progenitors (Figure 6B Smo kd). The reductions in the yield of B-lymphoid cells were accompanied by increases in the proportion of Mac-1+ cells. These results are consistent with the interpretation that B lymphopoiesis, at least under the conditions of this ex vivo assay, is promoted by non–cell-autonomous Hh pathway activity in the stromal compartment.
Non–cell-autonomous contribution of Hh signaling to B lymphopoiesis. (A) Reciprocal removal of Smo from LSK-OP9 cultures. Left, experimental design. LSK progenitors from Smofl/fl mice were infected with lentiviral particles encoding cre and GFP (pMIG-Cre). Infected cells were cocultured under B-lymphopoietic conditions with control (NT) or Smo-deficient (Smo kd) OP9 cells. Right, assay for Smo excision in pMIG-Cre-transduced LSK progeny. GFP+ (infected) and GFP− (uninfected) LSK progenitors were purified by FACS and assayed for Smo excision as described in Figure 3. (B) Identification of B-lymphoid (CD19+Mac1−) and myeloid (CD19−Mac1+) progeny in LSK-OP9 cultures at 12 days. NT, control OP9 cells; Smo kd, Smo-depleted OP9 cells. Transduced and untransduced LSK progeny were resolved by gating on GFP.
Non–cell-autonomous contribution of Hh signaling to B lymphopoiesis. (A) Reciprocal removal of Smo from LSK-OP9 cultures. Left, experimental design. LSK progenitors from Smofl/fl mice were infected with lentiviral particles encoding cre and GFP (pMIG-Cre). Infected cells were cocultured under B-lymphopoietic conditions with control (NT) or Smo-deficient (Smo kd) OP9 cells. Right, assay for Smo excision in pMIG-Cre-transduced LSK progeny. GFP+ (infected) and GFP− (uninfected) LSK progenitors were purified by FACS and assayed for Smo excision as described in Figure 3. (B) Identification of B-lymphoid (CD19+Mac1−) and myeloid (CD19−Mac1+) progeny in LSK-OP9 cultures at 12 days. NT, control OP9 cells; Smo kd, Smo-depleted OP9 cells. Transduced and untransduced LSK progeny were resolved by gating on GFP.
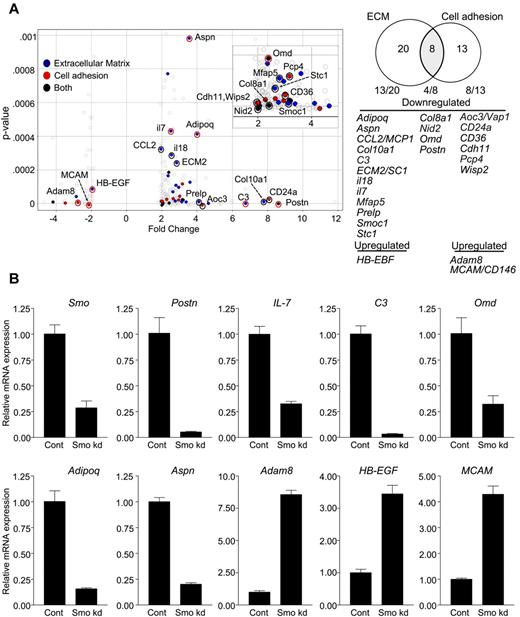
An economical interpretation of our results is that Hh signaling in stromal cells sustains phenotypic characteristics that promote B lymphopoiesis. To investigate this, we compared Smo-deficient and control OP9 cells with respect to genome-wide transcription. Significant (P < .001) differential expression of 2-fold or greater was observed for 264 markers (supplemental Table 1). Of these, 78% (206 of 264) were down-regulated in Smo-depleted OP9 cells. Functional annotation clustering revealed enrichment for transcripts encoding extracellular proteins, including extracellular matrix (ECM) components, cell adhesion molecules, cytokines, and other soluble factors (Table 1). Of the 41 differentially expressed genes that encode cell adhesion or ECM proteins, 25 specify products associated with osteoblastoid differentiation or hematopoiesis; 22 of these 25 genes are down-regulated in the absence of Hh signaling (Figure 7A). The loss of transcripts associated with osteoblastoid differentiation was of interest, because osteoblastoid stroma may support B-lymphoid differentiation in vivo.32
Functional clustering of differentially expressed genes in Smo-depleted OP9
| Category term* . | Feature† . | Count‡ . | Enrichment score§ . |
|---|---|---|---|
| Glycoprotein | Glycosylation, signal peptide, extracellular region | 116 | 10.25 |
| Pattern recognition | Polysaccharide, heparin, pattern binding | 14 | 4.45 |
| Extracellular matrix | Extracellular region and matrix | 28 | 3.72 |
| Cell adhesion | Biological, cell, and cell-cell adhesion | 21 | 3.16 |
| Oxidation reduction | Oxidation reduction and oxidoreductase | 22 | 3.08 |
| Category term* . | Feature† . | Count‡ . | Enrichment score§ . |
|---|---|---|---|
| Glycoprotein | Glycosylation, signal peptide, extracellular region | 116 | 10.25 |
| Pattern recognition | Polysaccharide, heparin, pattern binding | 14 | 4.45 |
| Extracellular matrix | Extracellular region and matrix | 28 | 3.72 |
| Cell adhesion | Biological, cell, and cell-cell adhesion | 21 | 3.16 |
| Oxidation reduction | Oxidation reduction and oxidoreductase | 22 | 3.08 |
Altered expression of factors promoting B lymphopoiesis and osteoblastoid differentiation in Smo-depleted OP9 cells. (A) Volcano plot (left) and Venn diagram (right) of differentially expressed and functionally clustered transcripts in Smo-depleted OP9 cells. In the volcano plot, statistical significance (determined by 1-way ANOVA) is graphed as a function of the fold change (control/Smo-depleted). Transcripts with known roles in hematopoiesis, osteoblastoid differentiation, or both are designated on the Volcano plot and identified in the Venn diagram. Transcripts encoding ECM proteins or cell adhesion proteins are designated by blue and red dots, respectively. Black dots represent transcripts encoding proteins that fall into both functional categories. Red circles designate transcripts with changes in expression verified by quantitative RT-PCR. Transcripts corresponding to each functional set are listed. (B) Validation of array results. Indicated transcripts in control or Smo-depleted (Smo kd) cells were quantified by RT-PCR and amounts were normalized to Actb. Normalized values for Smo-depleted cells are expressed relative to the corresponding values for control cells (mean and SD, n = 3).
Altered expression of factors promoting B lymphopoiesis and osteoblastoid differentiation in Smo-depleted OP9 cells. (A) Volcano plot (left) and Venn diagram (right) of differentially expressed and functionally clustered transcripts in Smo-depleted OP9 cells. In the volcano plot, statistical significance (determined by 1-way ANOVA) is graphed as a function of the fold change (control/Smo-depleted). Transcripts with known roles in hematopoiesis, osteoblastoid differentiation, or both are designated on the Volcano plot and identified in the Venn diagram. Transcripts encoding ECM proteins or cell adhesion proteins are designated by blue and red dots, respectively. Black dots represent transcripts encoding proteins that fall into both functional categories. Red circles designate transcripts with changes in expression verified by quantitative RT-PCR. Transcripts corresponding to each functional set are listed. (B) Validation of array results. Indicated transcripts in control or Smo-depleted (Smo kd) cells were quantified by RT-PCR and amounts were normalized to Actb. Normalized values for Smo-depleted cells are expressed relative to the corresponding values for control cells (mean and SD, n = 3).
Using quantitative PCR, we confirmed decreased accumulation in Smo-depleted OP9 cells of transcripts for periostin (Postn), the expression of which in OP9 cells is essential for B lymphopoiesis33 ; IL-7, a cytokine essential for B lymphopoiesis in adult mice that was present in the ex vivo differentiation systems used herein; adiponectin (Adipoq), asporin (Aspn), and osteomodulin (Omd), which are associated with osteoblastic differentiation34,35 ; and complement component 3 (C3), which is produced by osteoblasts in response to vitamin D36 (Figure 7B). We similarly confirmed increased accumulation in Smo-deficient OP9 cells of transcripts for heparin-binding epithelial growth factor (Hb-EGF), which blocks osteogenic differentiation of mesenchymal stem cells37 ; the metalloprotease adam8; and melanoma cell adhesion molecule (MCAM/CD146), which is expressed on mesenchymal stem cells but not on differentiated osteoblasts38 (Figure 7B). Reduced expression of transcripts for periostin and IL-7 was also observed in Smo-depleted S17 cells (supplemental Figure 9D). These results are consistent with the interpretation that Hh pathway activity in OP9 cells helps to maintain osteoblastoid identity and a B-lymphopoietic phenotype.
Discussion
In the BM, lymphopoiesis occurs in an intimate relationship with nonhematopoietic cells, which supply essential soluble and cell-associated factors. Several of these act on lymphoid progenitor cells to promote cell-autonomous regulatory programs that underlie differentiation. Less well understood are the non–cell-autonomous programs that operate within stromal cells to confer lymphopoietic activity. Ligand-dependent Hh stromal signaling has been shown to support survival of B-cell malignancies, and immunohistochemical analysis has documented expression of Hh ligand in BM stroma,16 but the contributions of non–cell-autonomous Hh activity to normal B-lymphoid development have been largely unexplored. The results presented herein indicate that Hh pathway activity in stromal cells can act non–cell-autonomously to promote B lymphopoiesis.
This interpretation rests on 4 lines of evidence: (1) interruption of Hh signaling impairs maintenance of B-lymphoid progenitors in coculture with stromal cells, (2) conditional ablation of Smo in early B-cell progenitors has no apparent effect on B-cell development or function, (3) depletion of Smo from stromal cells impairs differentiation of hematopoietic progenitors toward the B lineage, and (4) depletion of Smo from hematopoietic progenitors has no effect on their ability to give rise to B-lymphoid cells. The debilitating effect of Smo depletion on the support of B lymphopoiesis by stromal cells is accompanied by the reduced expression of several factors that promote B-cell development or are associated with osteoblastoid differentiation, suggesting that Hh signaling helps to maintain the B-lymphopoietic capacity of stromal cells.
The finding of the ability of cyclopamine to impair proliferation of pro-B cells ex vivo was consistent with a requirement for Hh signaling. In our assay, the antiproliferative effect of cyclopamine was apparently unaccompanied by cytotoxicity, because we observed only a small increase in apoptosis within the pro-B cell population and no decrease in the number of viable stromal cells. Moreover, treatment with the Hh-blocking Ab 5e1 was associated with a similar defect in pro-B cell maintenance, and this effect was reversed by the addition of recombinant Hh. These observations strongly suggested that Hh pathway activity in one or more cellular components of the ex vivo system was required for the maintenance of B-cell progenitors.
Expression of Hh pathway components in B-cell progenitors and the sharp down-regulation of Smo at the pro-B to pre-B transition were reminiscent of the pattern reported for Hh function in developing T cells.12 Surprisingly, therefore, conditional deletion of Smo had no apparent effect on B-cell development or on the kinetics of B-lymphoid repopulation after partial BM ablation. Moreover, mb-1-cre Smofl/null mice mounted normal humoral responses to T-independent and T-dependent antigens. These results strongly suggest that cell-autonomous Hh activity is dispensable for B-cell development and humoral immune responsiveness. Several qualifications deserve mention. First, although mb-1-cre expression begins in common lymphoid progenitors and is maintained throughout B-cell development,21 it remains possible that Hh signaling is required at an earlier developmental stage and is dispensable thereafter. However, this seems unlikely because stem cell progenitors conditionally deleted for Smo appear to retain hematopoietic potential.10,11 Second, despite the efficient deletion of Smo observed in B-lineage cells from mb-1-cre Smofl/null mice, the infrequent B-cell progenitors that retain Smo may be able to populate the B-lymphoid compartments. If this were the case, however, we would have expected a delay in the repopulation of B-lymphoid compartments in mb-1-cre Smofl/null animals, but this was normal. Third, it remains formally possible that Smo-independent mechanisms involving downstream Hh pathway components contribute to B-lymphoid development.39
After depletion of Smo, the ability of OP9 or S17 stromal cells to support B-lymphoid differentiation was found to be impaired in the present study. In OP9, the accompanying changes in gene expression showed highly significant enrichment of several overlapping functional categories, including ECM components and cell adhesion molecules. Nearly all of these were down-regulated after depletion of Smo and most directly promote B lymphopoiesis or are associated with an osteoblastoid phenotype. The relatively few ECM or cell-adhesion transcripts that were up-regulated after Smo depletion include HB-EGF, which antagonizes osteogenic differentiation,37 and MCAM, a marker for osteogenic progenitors that is lost after osteoblastoid differentiation.38 The erosion of osteoblastoid transcriptional features on Smo depletion is of interest because stromal cells of osteogenic lineage have been shown to modulate B lymphopoiesis in vivo, and depletion of osteoblastoid cells is associated with diminished accumulation of B-lymphoid progenitors.32 Moreover, histochemical analysis of Ptch+/LacZ mice has provided evidence that Hh signaling is active and modulated during osteoblastoid development.40
Morphogenic signals can induce transcriptional programs that confer the specialized properties of a particular stromal niche. Production of Hh by pancreatic cancer cells, for example, acts on neighboring stroma to activate genes that promote tumor growth.41 As another example, Wnt induces a transcriptional program in fibroblasts that confers the ability to promote stem-cell expansion42 ; in contrast, enforced expression of Wnt in stromal cells can interfere with their ability to support lymphopoiesis.43 Results presented herein are consistent with a mechanism in which Hh ligands, acting on stromal cells, maintain a transcriptional program that promotes B lymphopoiesis. The debilitating effect of Smo depletion on B lymphopoiesis may reflect a limitation in more than one factor. Of the transcripts reduced in Smo-deficient cells, several encode proteins that stimulate B lymphopoiesis. Transcripts encoding periostin (Postn), an ECM protein required by OP9 cells to support B lymphopoiesis,33 were reduced by 20-fold, whereas transcripts for the B-lymphopoietic factor IL-7 were reduced by nearly 4-fold. These reductions may be related, because depletion of periostin from OP9 cells is accompanied by reduced expression of IL-7.33 Because exogenous IL-7 was present in the culture systems used herein, it is unlikely that the effects of Smo depletion are exerted through decreased stromal expression of IL-7; rather, one or more additional factors is likely to be limiting. In addition to periostin, candidates include SC1/ECM2, which promotes B lymphopoiesis44 and the expression of which is reduced after Smo depletion.
Several studies have examined the effects of constitutive Hh pathway activation on hematopoietic stem cell function and lymphopoiesis. An increase in the number of LSK progenitors was observed in mice heterozygous for the Smo inhibitor Ptch,7 as well as in mice subjected to ubiquitous conditional ablation of Ptch.9 In the latter animals, the frequency of common lymphoid progenitors was decreased and lymphoid development was impaired.9 Enforced activation of Hh signaling in lymphoid lineages, however, had no effect on T- or B-cell development, whereas constitutive pathway activation in nonhematopoietic compartments was associated with increased numbers of LSK progenitors and decreased production of lymphoid cells.45 Because in these experiments Hh signaling was constitutively activated in all nonhematopoietic cells, comparison to the effects of Smo ablation is difficult, because any apparent discrepancy may be attributed to differences in the cellular populations in which Hh signaling was altered. A second possibility is that optimal B lymphopoiesis is tuned to a particular range of Hh signal strengths, with both overactivity and underactivity exerting deleterious effects. This view is supported by consideration of Wnt, which can promote hematopoiesis with strict dosage dependence.46
Consideration of non–cell-autonomous functions may resolve some inconsistent observations concerning the effects of Smo ablation on hematopoiesis. Conditional ablation of Smo by vav-cre was associated with impaired hematopoiesis, suggesting that Hh signaling is essential for this process,47 whereas deletion of Smo by Mx1-cre was compatible with normal hematopoiesis.10,11 One distinction between these experiments is the timing of excision by cre: vav-cre is active from embryonic development, whereas Mx1-cre is inducible and temporally restricted. Another difference lies in the tissue specificity of cre expression: vav-cre is active in endothelial and hematopoietic cells, whereas the activity of Mx1-cre varies among cell types.48 A third difference may be efficiency, because similar discrepancies between vav-cre and Mx1-cre mice with respect to ablation of β-catenin have been observed, with vav-cre supporting greater inactivation.49 Differences in the cellular distribution of cre have suggested that the impairment of hematopoiesis in vav-cre mice reflects a non–cell-autonomous requirement for Hh signaling.50 In this regard, the results of the present study may contribute to a resolution of current controversies concerning the role of Hh signaling in hematopoiesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dominic Dordai (Department of Molecular Biology and Genetics, Johns Hopkins University) for expert technical assistance.
This work was supported by the National Institutes of Health (grants CA16519 to S.D. and AI026782 to R.R.H.) and by the Maryland Stem Cell Research Fund (grant to S.D.). C.L.C. was supported in part by a National Institute of Allergy and Infectious Diseases training grant (T32AI007247).
National Institutes of Health
Authorship
Contribution: C.L.C. designed and executed the experiments, interpreted the results, and wrote the manuscript; R.R.H. purified the B-progenitor subsets; R.R.H. and M.R. provided reagents and analyzed the data; and S.D. designed the experiments, interpreted the results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephen Desiderio, 733 N Broadway, Baltimore, MD 21205; e-mail: sdesider@jhmi.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal