Abstract
Multiple transcription factors regulate B-cell commitment, which is coordinated with myeloid-erythroid lineage differentiation. NF-κB has long been speculated to regulate early B-cell development; however, this issue remains controversial. IκB kinase-α (IKKα) is required for splenic B-cell maturation but not for BM B-cell development. In the present study, we unexpectedly found defective BM B-cell development and increased myeloid-erythroid lineages in kinase-dead IKKα (KA/KA) knock-in mice. Markedly increased cytosolic p100, an NF-κB2–inhibitory form, and reduced nuclear NF-κB p65, RelB, p50, and p52, and IKKα were observed in KA/KA splenic and BM B cells. Several B- and myeloid-erythroid–cell regulators, including Pax5, were deregulated in KA/KA BM B cells. Using fetal liver and BM congenic transplantations and deleting IKKα from early hematopoietic cells in mice, this defect was identified as being B cell–intrinsic and an early event during hematopoiesis. Reintroducing IKKα, Pax5, or combined NF-κB molecules promoted B-cell development but repressed myeloid-erythroid cell differentiation in KA/KA BM B cells. The results of the present study demonstrate that IKKα regulates B-lineage commitment via combined canonical and noncanonical NF-κB transcriptional activities to target Pax5 expression during hematopoiesis.
Introduction
A hierarchical transcriptional network regulates early B-cell development that is coordinated with the differentiation of myeloid and erythroid lineages from hematopoietic stem cells (HSCs) in the BM.1 NF-κB, a group of structurally related transcription factors, is required for B-cell maturation in the spleen.2,3 Although its role in BM B lymphopoiesis has long been speculated,4-7 this issue remains controversial.
The NF-κB family is composed of RelA (p65), RelB, c-Rel, NF-κB1 (p50 and precursor p105), and NF-κB2 (p52 and precursor p100).8 These molecules form homodimers or heterodimers that bind to consensus DNA elements in promoters and enhancers to regulate gene expression. The p65:p50 and RelB:p52 heterodimers preferentially lead to canonical and noncanonical NF-κB activation, respectively. NF-κB inhibitors of IκBs, p100, and p105 bind to NF-κB in the cytoplasm, inhibiting NF-κB transcriptional activity. The IκB kinase (IKK) complex, composed of 2 highly conserved kinases, IKKα and IKKβ, and a regulator subunit, IKKγ (NF-κB essential modifier; NEMO), phosphorylates IκBα, which leads to IκBα degradation. The released canonical NF-κB dimers then translocate to the nucleus, thereby regulating gene expression.9 Alternatively, NF-κB–inducing kinase (NIK) and IKKα phosphorylate the C-terminal region of p100 to induce p100 processing, which generates p52.3,10 The resulting RelB:p52 heterodimers then translocate to the nucleus. The noncanonical NF-κB pathway is triggered by a specific group of receptors present in certain types of cells.11 In addition, the increased p100 proteins can bind to cytosolic p65:p50 dimers,12,13 thereby functioning like an IκB. In addition, IKKα has been shown to phosphorylate NIK, which triggers NIK degradation.14 Therefore, IKKα may regulate the canonical NF-κB pathway through these alternative mechanisms.
In addition to the role of IKKα in embryonic skin development through an NF-κB–independent mechanism,15 genetic evidence has shown that IKKα is required for B-cell maturation and secondary lymphoid organ development largely through the noncanonical NF-κB pathway.3 Given the diverse biochemical activities of IKKα, whether IKKα links noncanonical and canonical NF-κB pathways under physiologic conditions remains to be demonstrated. A large number of genetic studies have shown that canonical and noncanonical NF-κB activities provide survival signals to maintain and expand the immature B-lymphocyte population, supporting B-cell maturation. The B-cell maturation process is primarily mediated by the BCR and the receptor for the B cell–activating factor of the TNF family (BAFF-R)–led signal-transduction cascades in the spleen.3,16,17 BCR and BAFF-R, however, are not expressed or not fully functional in early BM B cells.18 Most cells express TNF receptor 1 (TNFR1). p65, IKKγ, and IKKβ, but not IKKα, are required to protect BM lymphocytes from TNFα/TNFR1-mediated apoptosis.2,4,19 Although the lack of NF-κB activity causes lymphocyte death via the TNFR pathway, myeloid cells are dramatically increased in irradiated mice receiving p65−/−, p65−/−/p50−/−, or Ikkβ−/− fetal liver (FL) cells,4,19 implying that IKK/NF-κB may inhibit myeloid cell production in BM. In addition, combined deletions of NF-κB components, such as p50 and p52 or p50 and RelB, frequently show a synergic effect on B-cell development,20-22 suggesting that some targets of NF-κB may require both canonical and noncanonical NF-κB activities.
HSCs undergo several differentiation steps, including pre-pro-B, pro-B, and pre-B, to generate immature B cells in the BM. This process is tightly regulated by a set of transcription factors. Jimi et al6 and Feng et al7 showed that overexpressed IκBα impairs early B-cell development. p100−/− mice display a defect at the transition from the pro-B to pre-B stages, which may be related to elevated p52 levels.5 Although p50 or RelB single knockout does not impair BM B-cell development, p50 and RelB double knockouts cause a significant decrease in BM B cells and an increase in myeloid cells in mice.22 Furthermore, alymphoplasia (aly) mice with an autosomal recessive mutation in NIK develop a BM B cell–intrinsic defect.23 NF-κB is activated throughout all B-cell developmental stages.6,24 These results suggest that NF-κB participates in BM B lymphopoiesis. Conversely, using mb1-Cre, Derudder et al have demonstrated that deleting combined IKKα and IKKβ subunits or IKKγ does not impair BM B-cell development in mice, concluding that IKKα, IKKβ, and NF-κB are dispensable for early B-cell progenitors.24 A decade ago, Kaisho et al2 and Senftleben et al3 used adoptive-transfer experiments to identify a role for IKKα in splenic B-cell maturation. The splenic B-cell defect was only partially rescued by transgenic (Tg) Bcl2, which increases cell survival, implying that IKKα is able to imprint a B cell–autonomous program in FL stem cells.
During hematopoiesis, one of the mechanisms underlying early B-cell commitment is the simultaneous suppression of myeloid-erythroid differentiation.1 For example, the transcription factor Pax5 up-regulates the expression of genes encoding proteins that promote B-cell differentiation, but down-regulates the expression of genes encoding proteins that promote myeloid-erythroid cell differentiation.25 Therefore, reducing Pax5 and increasing the levels of C/EBPα (a transcription factor required for myeloid-lineage development25 ) can accelerate the conversion of mature B cells to pluripotent cells, and this is initiated by stem-cell genes.26 These data suggest that a defect in pluripotent cells may cause a switch in cell lineages.
In the present study, we surprisingly found a reduction in pre-pro-B, pro-B, and pre-B cells and an increase in myeloid-erythroid lineages and early progenitors in the BM of kinase-dead IKKα knock-in mice (IkkαK44A/K44A or KA/KA27 ). We also show how IKKα regulates early BM B-cell development via the combined noncanonical and canonical NF-κB pathways and NF-κB target genes and identify a new function for IKKα in early B-lymphocyte development during hematopoiesis.
Methods
Mice
All of the mice used in this study were cared for in accordance with the guidelines of our institution's animal care and use committee (protocols 08-074 and 08-075) and were housed in a specific pathogen-free animal facility at the National Cancer Institute. IkkαK44A/K44A (KA/KA), Ikkα−/−, and IkkαAA/AA mice with a BL6 or FVB background were described previously.27-29 Bcl2 Tg mice [002320, B6.Cg-Tg(BCL2)25Wehi/J] and VavCre mice (008610) were originally purchased from The Jackson Laboratory. Animal studies were approved by the National Cancer Institute's animal care and use committee.
Flow cytometric analysis
B220+ cells isolated from the spleens and BM of 6- to 8-week-old mice were enriched using CD45R MicroBeads (130-049-501; Miltenyi Biotec). Single-cell suspensions were stained and analyzed on the multicolor LSR II cell analyzer (BD Biosciences). FITC-, PE-Cy7-, PE-, APC-, Alexa Fluor 450–, and APC/Cy7-labeled Abs against B220 (RA3-6B2), BP-1 (63C), CD11b (M1/70), CD11c (N418), CD19 (MB19-1), CD34 (RAM 34), CD43 (eBioR2/60), c-Kit (2B8), IgM (II/41), IgD (11-26c, 11-26), TER119 (TER119), CD23 (B3B4), CD21/CD35 (4E3), CD93/AA4.1 (AA4.1), and PE-conjugated rat IgG2a (eBP2a) were purchased from eBiosciences. The anti-Sca1 (Ly-6A/E; D7) Ab was purchased from BD Biosciences. Analyses were performed with FlowJo Version 9.3.2 software (TreeStar).
Accession numbers
Original microarray data (accession number GSE30363) have been deposited at the National Center for Biotechnology Information Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/).
Results
Mice expressing kinase-dead IKKα exhibit a defect in BM and splenic B-lymphocyte development
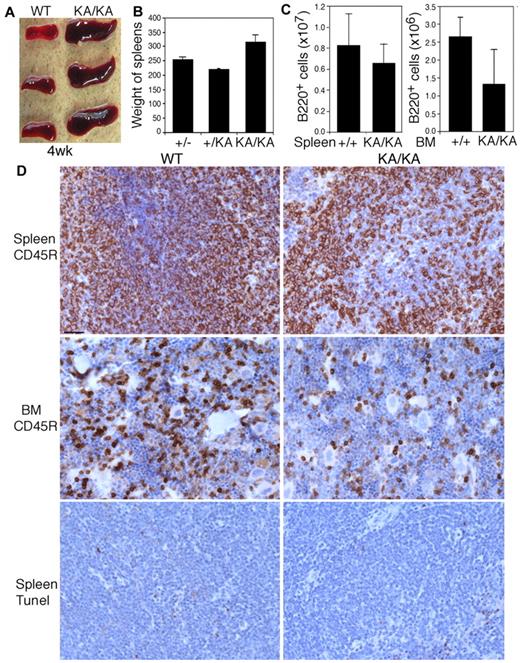
Kinase-dead IKKα knock-in (IkkαK44A/K44A or KA/KA27 ) mice developed enlarged spleens compared with wild-type (WT) mice by 4 weeks of age (Figure 1A). Unexpectedly, the total B-cell numbers were lower in KA/KA BM and spleens compared with WT (Figure 1B-C). Histopathologic and immunostaining analyses showed increased extramedullary hematopoiesis and disrupted germinal centers in KA/KA spleens (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The reduction of B cells in KA/KA spleens and BM was verified with the B-cell marker CD45R (B220); however, no significant increase in cell death was detected in KA/KA versus WT spleens (Figure 1D), suggesting that reduced KA/KA B cells may not be caused by increased apoptosis. Further characterization of splenic B cells revealed a significant reduction in KA/KA B220+, CD19+B220+, IgM+B220+, IgD+B220+, and marginal zone (CD21+CD23− gated on B220+) B cells (Figure 2A). The number of WT and KA/KA follicular B cells (CD21loCD23hi gated on B220+) was similar. We also found decreased transitional type 1 (T1), a block at T2, and decreased T3 B cells in KA/KA spleens compared with WT (supplemental Figure 1B). Therefore, KA/KA splenic B-cell development is impaired. Moreover, KA/KA mice lacked inguinal, cervical, and mesenteric lymph nodes, and Peyer patches (supplemental Figure 1C); therefore, IKKα kinase inactivation impairs the development of secondary lymphoid organs.
Reduction in the number of B cells from the spleens and BM of KA/KA mice compared with WT mice. (A-C) Spleen sizes (A), spleen weights (B), and the number of B220+ cells from the spleens and BM (C) of WT (+/+), +/KA, and KA/KA mice at 4 weeks of age. Each group contained 3 mice. The data are presented as means ± SD. (D) Comparison of B cells (CD45R) and cell death (TUNEL staining) in the spleens and BM of WT and KA/KA mice. Brown indicates immunohistochemical-stained cells with an anti-CD45R Ab or TUNEL assay; blue, nuclear countering staining. Scale bar indicates 50 μm.
Reduction in the number of B cells from the spleens and BM of KA/KA mice compared with WT mice. (A-C) Spleen sizes (A), spleen weights (B), and the number of B220+ cells from the spleens and BM (C) of WT (+/+), +/KA, and KA/KA mice at 4 weeks of age. Each group contained 3 mice. The data are presented as means ± SD. (D) Comparison of B cells (CD45R) and cell death (TUNEL staining) in the spleens and BM of WT and KA/KA mice. Brown indicates immunohistochemical-stained cells with an anti-CD45R Ab or TUNEL assay; blue, nuclear countering staining. Scale bar indicates 50 μm.
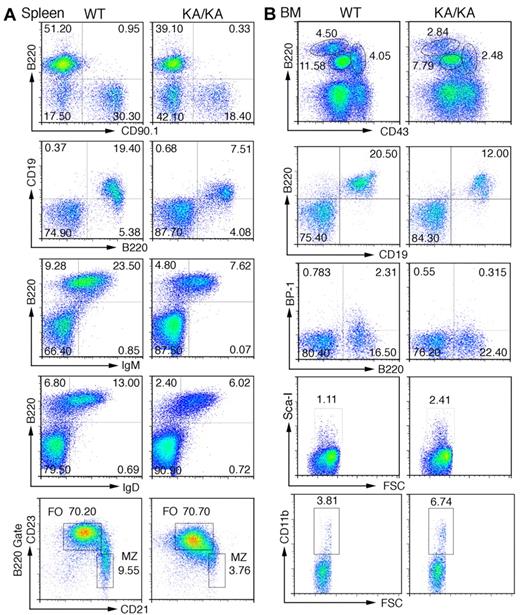
Defects in splenic and BM B-cell development in KA/KA mice. (A) Analysis of the total splenic B-cell population of WT and KA/KA mice at 6 weeks of age using flow cytometry with the indicated B-cell surface markers B220+CD90.1+, B220+CD19+, B220+IgM+, B220+IgD+, CD21+CD23− (B220+ gate), and CD21loCD23hi (B220+ gate). Numbers are the percentage of the cell population; MZ, marginal zone; FO, follicular. (B) Analysis of BM cells isolated from WT and KA/KA mice at 6 weeks of age using flow cytometry with B-cell and progenitor-cell markers. A combination of markers was used to define pre-pro-B cells (B220+CD43hiCD19−BP1−CD24loIgM−), pro-B cells (B220+CD43medBP-1+CD19+ IgM−), and pre-B cells (B220hiCD43−CD19+IgM−).
Defects in splenic and BM B-cell development in KA/KA mice. (A) Analysis of the total splenic B-cell population of WT and KA/KA mice at 6 weeks of age using flow cytometry with the indicated B-cell surface markers B220+CD90.1+, B220+CD19+, B220+IgM+, B220+IgD+, CD21+CD23− (B220+ gate), and CD21loCD23hi (B220+ gate). Numbers are the percentage of the cell population; MZ, marginal zone; FO, follicular. (B) Analysis of BM cells isolated from WT and KA/KA mice at 6 weeks of age using flow cytometry with B-cell and progenitor-cell markers. A combination of markers was used to define pre-pro-B cells (B220+CD43hiCD19−BP1−CD24loIgM−), pro-B cells (B220+CD43medBP-1+CD19+ IgM−), and pre-B cells (B220hiCD43−CD19+IgM−).
We further characterized the BM B-cell profiles and found a significant reduction in B220hiCD43−IgM− (pre-B), B220+CD43medIgM− (pro-B), and B220+CD43hiIgM− (pre-pro-B) populations in KA/KA BM compared with WT (Figure 2B top panel and supplemental Figure 2A). A CD19−CD24loBP1− gate was used to confirm reduced pre-pro-B cells (B220+CD43hi) in KA/KA BM (supplemental Figure 2B). KA/KA B220+CD19+ and B220+BP-1+CD19+ (pro-B) cells were reduced in KA/KA BM compared with WT (Figure 2Bii-iii). Reduced B220+IgM−, B220loIgM+, and B220hiIgM+ populations were observed in KA/KA BM compared with WT (supplemental Figure 2C). In contrast, hematopoietic progenitors Sca-I+ (Ly-6A/E, a Pax5-dependent gene25 ), CD34+ (data not shown), and CD11b cells were increased in KA/KA BM compared with WT (Figure 2B bottom panels). These results reaffirmed that IKKα inactivation impairs early B-cell development in the BM.
Intrinsic defects in early B-lymphocyte development in KA/KA mice
BM stroma influences lymphocyte development; however, it is unknown whether IKKα intrinsically regulates BM B-cell development. Therefore, we transplanted WT and KA/KA BM cells into irradiated mice and determined the properties of these cells in the BM of radiation chimeras. Genotypes of the donor BM and tail DNAs from reconstituted radiation chimeras were confirmed by PCR (Figure 3A). We found that the populations of B220hiCD43− (pre-B), B220+CD43med (pro-B), and B220+CD43hi (pre-pro-B) cells were reduced in the BM of irradiated WT mice receiving KA/KA BM compared with the BM of irradiated WT mice receiving WT BM (Figure 3B and supplemental Figure 2D), suggesting that WT stroma did not correct the defect of KA/KA BM and that KA/KA BM cells have an intrinsic defect in BM B lymphopoiesis. We also found that the number of pre-pro-B cells in the BM of irradiated KA/KA mice receiving KA/KA BM was further reduced compared with those in the BM of irradiated WT mice receiving KA/KA BM (Figure 3B). Pre-B cells increased and pre-pro-B cells slightly decreased in the BM of irradiated KA/KA mice receiving WT BM compared with the BM of irradiated WT mice receiving WT BM, suggesting that the KA/KA stroma has an impact on BM B-cell differentiation (supplemental Figure 2D).
Intrinsic B-cell defect in KA/KA BM. (A) Genotype of the tail DNA and BM B220+ cell DNA of WT and KA/KA recipient mice analyzed using PCR. (B) Statistical analysis of BM pre-pro-B, pro-B, and pre-B-cell populations of γ-irradiated recipient mice receiving BM from the donor mice as indicated. The data represent means ± SD calculated from 4 independent BM-transfer experiments. (C) Flow cytometric analysis of BM and splenic WT (CD45.1+) and KA/KA (CD45.1−) B cells in the BM and spleens of irradiated mice receiving mixed WT (CD45.1+) and KA/KA (CD45.1−) BM cells.
Intrinsic B-cell defect in KA/KA BM. (A) Genotype of the tail DNA and BM B220+ cell DNA of WT and KA/KA recipient mice analyzed using PCR. (B) Statistical analysis of BM pre-pro-B, pro-B, and pre-B-cell populations of γ-irradiated recipient mice receiving BM from the donor mice as indicated. The data represent means ± SD calculated from 4 independent BM-transfer experiments. (C) Flow cytometric analysis of BM and splenic WT (CD45.1+) and KA/KA (CD45.1−) B cells in the BM and spleens of irradiated mice receiving mixed WT (CD45.1+) and KA/KA (CD45.1−) BM cells.
To further verify whether IKKα inactivation has an intrinsic effect on B cells, we injected mixed normal BM cells (CD45.1+) and KA/KA BM cells (CD45.1−) into irradiated Rag1−/− mice. Analysis showed a reduction in KA/KA BM cells (CD45.1−) and in splenic CD19+/B220+ and IgD+/IgM+ B cells and an increase in CD45.1− BM erythroid cells compared with CD45.1+ BM B and splenic B cells in irradiated recipients (Figure 3C). The results also showed a slight reduction in CD45.1+ BM B cells compared with WT BM transplanted cells (Figure 3C), suggesting that KA/KA BM cells (CD45.1−) may influence WT BM cells (CD45.1+) in a mixture of CD45.1+ and CD45.1− BM cells, which is consistent with the results shown in Figure 3B. These results indicate that IKKα inactivation induces an intrinsic defect in BM B-cell development.
We also examined BM B-cell profiles in IkkαAA/AA mice in which 2 serine sites at amino acids 178 and 180 were replaced by alanine within the IKKα kinase domain28 and found a slight reduction in early B cells (supplemental Figure 2E), suggesting that AA/AA mutations do not affect early BM B-cell development significantly.
IKKα inactivation-induced B-cell defect is an early event during hematopoiesis
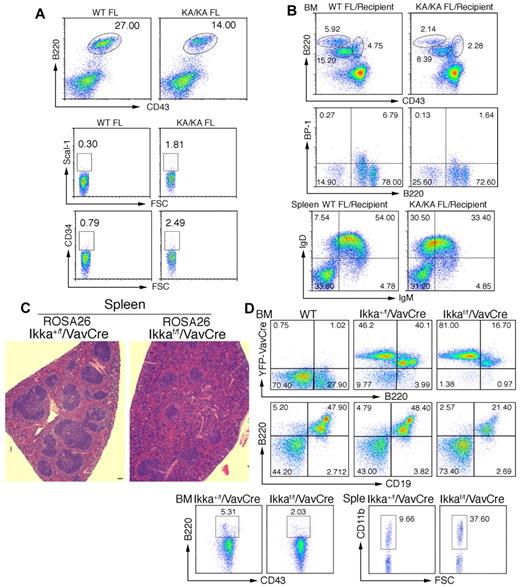
To determine whether IKKα regulates B-cell development at an early stage, we examined FL B220+ cells in WT and KA/KA E13.5 embryos and found reduced KA/KA FL B220+CD43+ cells and increased KA/KA FL progenitors with CD34 and Scal-I markers compared with WT FL cells (Figure 4A). The KA/KA FL cells consistently generated reduced pre-pro-B, pro-B, and pre-B cells in the BM and reduced mature B cells in the spleens of irradiated Rag−/− mice compared with WT FL cells (Figure 4B). The Ikkα−/− FL cells of E12.5 embryos showed reduced B220+CD43+ cells (supplemental Figure 3A) and generated reduced B220+CD19+ cells in the BM and reduced mature B cells in the spleens of irradiated Rag−/− chimeras compared with WT FL cells (supplemental Figure 3B-C). These results imply that the IKKα inactivation-induced defect in B-cell differentiation occurs at an early stage.
IKKα inactivation-mediated B-cell defect is an early event. (A) Flow cytometric analysis of B-cell and progenitor profiles in the FL of embryonic day 13.5 WT and KA/KA mouse embryos. (B) Analyses of BM and splenic B-cell profiles of irradiated Rag−/− mice (Recipients) receiving WT and KA/KA FL (embryonic day 13.5) cells using flow cytometry with B220+CD43+, B220+BP-1+, and IgD+IgM+ markers. (C) Histopathology of H&E-stained spleens from Ikkα+/f/VavCre/ROSA26 and Ikkαf/f/VavCre/ROSA26 mice. Scale bar indicates 50 μm. (D) Analyses of the BM and splenic (Sple) B-cell profiles of Ikkα+/f/VavCre/ROSA26 and Ikkαf/f/VavCre/ROSA26 mice using flow cytometry with B220+, B220+CD43+, B220+CD19+, and CD11b+ markers.
IKKα inactivation-mediated B-cell defect is an early event. (A) Flow cytometric analysis of B-cell and progenitor profiles in the FL of embryonic day 13.5 WT and KA/KA mouse embryos. (B) Analyses of BM and splenic B-cell profiles of irradiated Rag−/− mice (Recipients) receiving WT and KA/KA FL (embryonic day 13.5) cells using flow cytometry with B220+CD43+, B220+BP-1+, and IgD+IgM+ markers. (C) Histopathology of H&E-stained spleens from Ikkα+/f/VavCre/ROSA26 and Ikkαf/f/VavCre/ROSA26 mice. Scale bar indicates 50 μm. (D) Analyses of the BM and splenic (Sple) B-cell profiles of Ikkα+/f/VavCre/ROSA26 and Ikkαf/f/VavCre/ROSA26 mice using flow cytometry with B220+, B220+CD43+, B220+CD19+, and CD11b+ markers.
We further investigated whether IKKα deletion in HSCs impairs B-cell differentiation using HSC-specific VavCre30 in Ikkαf/f mice and a yellow fluorescent protein (in ROSA26 mice from The Jackson Laboratory) to indicate Cre activity. Disrupted architecture of the spleen was seen in Ikkaf/f/VavCre/ROSA26 mice (Figure 4C), suggesting splenic B-cell defects. Yellow fluorescent protein-positive BM B220+ and B220+CD19+ cells were decreased, BM B220+CD43hi (pre-pro-B) cells gated on the CD19−CD24loBP1+ population were decreased, and splenic CD11b cells were increased in Ikkaf/f/VavCre/ROSA26 mice compared with Ikka+/f/VavCre/ROSA26 mice (Figure 4D). These results indicate that IKKα plays an important role in early B-cell development during hematopoiesis. In addition, B cell–specific Tg-Bcl2 mainly increased the number of B cells, but did not alter the differentiation of intrinsic multilineages as observed in KA/KA mice (supplemental Figure 4A and data not shown), further supporting the idea that apoptosis is not a major cause of reduced BM B cells in KA/KA mice.
The noncanonical and canonical NF-κB pathways are impaired in the BM and splenic B lymphocytes of KA/KA mice
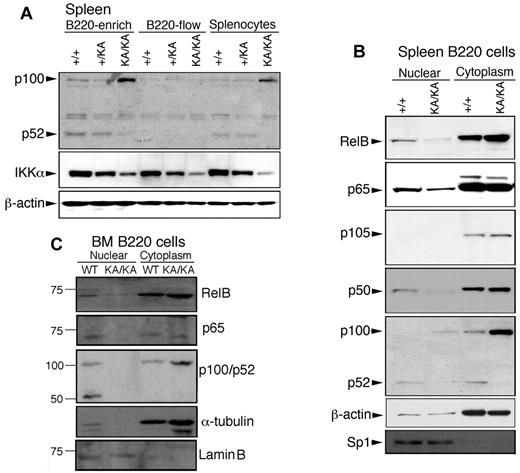
To determine whether IKKα inactivation affects the noncanonical or canonical NF-κB pathway, we first compared p100 and p52 levels in splenic and BM B220+ cells from KA/KA and WT mice and found substantially increased p100 levels in B220+ (B220-enriched) cells and splenocytes (total spleen) and reduced p52 in KA/KA mice compared with WT (Figure 5A). The processing of p100 to p52 was not detected in non-B cells (B220-flow), showing that p100 regulation is B-cell specific. Regardless of cell type, the endogenous IKKα levels were reduced in KA/KA mice (Figure 5A). We further examined NF-κB activities in WT and KA/KA B cells and found that KA/KA splenic B220+ cells contained reduced nuclear RelB, p65, p50, and p52, and increased cytosolic p100 compared with WT (Figure 5B). In addition, KA/KA BM B220+ cells contained reduced nuclear RelB, p65, and p52 and increased cytosolic p100 levels compared with WT BM (Figure 5C). Interestingly, the nuclear IKKα level was reduced in KA/KA BM B220+ cells (supplemental Figure 4B). These results indicate that IKKα inactivation impairs both noncanonical and canonical NF-κB pathways in splenic and BM B220+ cells.
Reduced activity of canonical and noncanonical NF-κB components in the splenic and BM B cells of KA/KA mice. (A) Western blot showing the indicated protein levels in splenic cells. B220-enrich indicates column-purified B220+ cells; B220-flow, non-B cells that passed through the column; splenocytes, total cell lysate from whole spleen; β-actin, protein-loading control. (B-C) p65 (RelA), RelB, p100/52, and p105/p50 expression levels in nuclear and cytoplasmic fractions of spleen and BM B220+ cells of WT (+/+) and KA/KA mice. Lamin B and sp1 were used as controls for the purity of nuclear extracts.
Reduced activity of canonical and noncanonical NF-κB components in the splenic and BM B cells of KA/KA mice. (A) Western blot showing the indicated protein levels in splenic cells. B220-enrich indicates column-purified B220+ cells; B220-flow, non-B cells that passed through the column; splenocytes, total cell lysate from whole spleen; β-actin, protein-loading control. (B-C) p65 (RelA), RelB, p100/52, and p105/p50 expression levels in nuclear and cytoplasmic fractions of spleen and BM B220+ cells of WT (+/+) and KA/KA mice. Lamin B and sp1 were used as controls for the purity of nuclear extracts.
IKKα inactivation down-regulates the expression of genes required for B-cell development and up-regulates the expression of genes relevant to myeloid-erythroid-lineage cell development
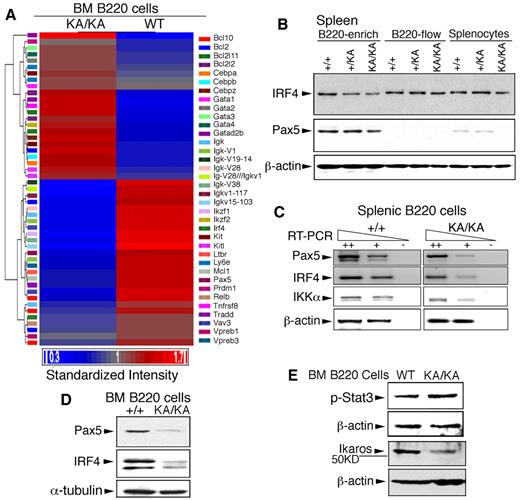
To identify the targets of IKKα inactivation in BM B-cell development, we used complementary DNA microarray to study the global gene-expression profiles of WT and KA/KA BM B220+ cells. This analysis showed that genes relevant to B-cell development were down-regulated, whereas genes relevant to myeloid-erythroid lineages were up-regulated, in KA/KA compared with WT BM B220+ cells (supplemental Table 1). Those genes found to be both highly relevant to B-cell and immune system development and significant in their expression pattern (supplemental Table 2) were used to generate a heat map of supervised hierarchical clustering (Figure 6A). Pax5, IFN regulatory factor 4 (Irf4), and Ikaros (Ikzf1),25,31,32 which affect progenitors and regulate early B cell–lineage commitment, were down-regulated and gata1 (a transcription factor required for erythropoiesis33 ) was up-regulated in KA/KA BM B cells compared with WT (Figure 6A). The microarray analysis supports a role for IKKα in early BM B-cell development and hematopoiesis.
IKKα inactivation deregulates the expression of genes involved in BM B-cell development. (A) Heat map and hierarchical cluster analysis expression of 36 genes (supplemental Table 2) in WT and KA/KA BM B220+ cells, as identified using microarray analysis. Red and blue indicate up-regulated and down-regulated genes, respectively. Standardized intensity is indicated by the fold values (Log2) of gene expression in WT and KA/KA BM B cells. (B) Western blot showing IRF4 and Pax5 levels in B220+-enriched cells, B220-flow, and splenocyte lysates (see Figure 5A). β-actin, protein-loading control (same as in Figure 5A). (C) Expression of Pax5, IRF4, and IKKα analyzed with RT-PCR on serially diluted total RNA isolated from the BM B220+ cells of WT and KA/KA mice. ++, +, cDNA dilution; -, negative control; β-actin, PCR control. (D) IRF4 and Pax5 levels in the BM B220+ cells of WT (+/+) and KA/KA mice detected with Western blot. α-tubulin, protein-loading control. (E) Stat3 activity (p-Stat3) and Ikaros level in the BM B220+ cells of WT and KA/KA mice. β-actin, protein-loading control.
IKKα inactivation deregulates the expression of genes involved in BM B-cell development. (A) Heat map and hierarchical cluster analysis expression of 36 genes (supplemental Table 2) in WT and KA/KA BM B220+ cells, as identified using microarray analysis. Red and blue indicate up-regulated and down-regulated genes, respectively. Standardized intensity is indicated by the fold values (Log2) of gene expression in WT and KA/KA BM B cells. (B) Western blot showing IRF4 and Pax5 levels in B220+-enriched cells, B220-flow, and splenocyte lysates (see Figure 5A). β-actin, protein-loading control (same as in Figure 5A). (C) Expression of Pax5, IRF4, and IKKα analyzed with RT-PCR on serially diluted total RNA isolated from the BM B220+ cells of WT and KA/KA mice. ++, +, cDNA dilution; -, negative control; β-actin, PCR control. (D) IRF4 and Pax5 levels in the BM B220+ cells of WT (+/+) and KA/KA mice detected with Western blot. α-tubulin, protein-loading control. (E) Stat3 activity (p-Stat3) and Ikaros level in the BM B220+ cells of WT and KA/KA mice. β-actin, protein-loading control.
Because Pax5 and IRF4 are important for B cells,34 we further verified the levels of Pax5 and IRF4 expression in splenic and BM B220+ cells. Western blotting and RT-PCR showed reduced Pax5, IRF4, and IKKα proteins and reduced RNA in KA/KA splenic B220+ cells (Figures 5A and 6B-C). Furthermore, similar down-regulation of the Pax5 and IRF4 proteins was seen after analysis of BM B cells from KA/KA versus WT mice (Figure 6D). Validation of our microarray results detected reduced Ikaros protein and increased Stat3 activity in KA/KA BM B220+ cells compared with WT (Figure 6E). These results suggest that deregulated Pax5, IRF4, Ikaros, and IKKα may contribute to abnormal hematopoietic development in KA/KA mice.
IKKα and combined noncanonical and canonical NF-κB regulate the expression of Pax5 and IRF4 in BM B cells during hematopoiesis
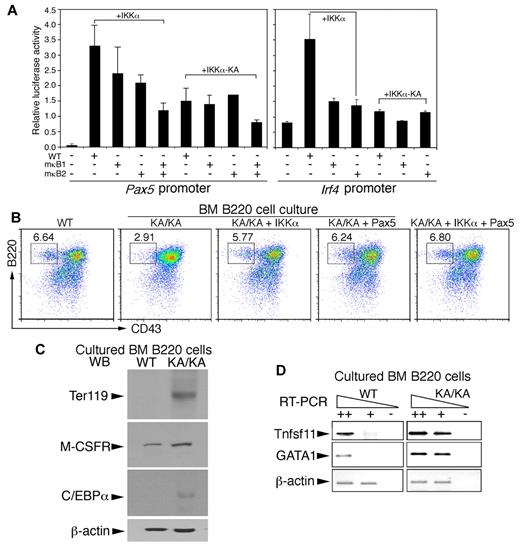
DNA-sequence analysis revealed evolutionarily conserved NF-κB consensus-binding sites (κB1 bound by canonical NF-κB dimers and κB2 bound by noncanonical NF-κB dimers9 ) in Pax5 and Irf4 promoters (supplemental Figure 5A-B). We then mutated the κB1 and κB2 elements to generate mutant Pax5 promoters (mκB1, mκB2, and mκB1mκB2) and mutant Irf4 promoters (mκB1 and mκB2) in luciferase reporter vectors. We further tested the activities of these Pax5 and Irf4 promoters in a Raji B-cell line coexpressing IKKα or kinase-inactive IKKα (IKKα-KA). Luciferase analyses showed that IKKα expression activated both Pax5 and Irf4 promoters (Figure 7A both graphs lane 2); mκB1 moderately diminished Pax5 promoter activation and reduced Irf4 activation 3-fold (Figure 7A both graphs lane 3); mκB2 diminished the activity of both Pax5 and Irf4 promoters (Figure 7A both graphs lane 4); and mκB1mκB2 severely disrupted Pax5 promoter activation (Figure 7A lane 5). IKKα-KA expression eliminated the induced transcription of the Pax5 and Irf4 promoters (Figure 7A). In addition, ectopic expression of IKKα, but not IKKα-KA, induced Pax5 (supplemental Figure 6A) and IRF4 (data not shown) expression. These results suggest that IKKα regulates Pax5 and Irf4 transcription through the combined noncanonical and canonical NF-κB activities.
IKKα and combined noncanonical and canonical NF-κB regulate Pax5 and Irf4 promoter activities and BM B-cell differentiation. (A) The activities of Pax5, Irf4, mutant Pax5, and mutant Irf4 promoters containing mutations within κB1 (mκB1) or κB2 (mκB2) in pGL3b luciferase reporter vectors were examined in Raji B cells using the luciferase reporter assay. +IKKα indicates coexpression of IKKα and promoter plasmids; +IKKα-KA, coexpression of IKKα-KA and promoter plasmids. (B) Flow cytometric analyses of B220+ BM B cells in RPMI 1640 medium supplemented with 10% FBS for 3 days after reintroduction of IKKα, IKKα-KA, Pax5, and IKKα+Pax5 plasmids into BM B cells using AMAXA nucleofection. (C) Levels of Ter119, M-CSFR, and C/EBPα in cultured WT and KA/KA BM B cells were detected with Western blotting. β-actin, protein-loading control. (D) RT-PCR showing expression levels of Tnfsf11 and GAGA1 in cultured WT and KA/KA BM B cells. ++, +, cDNA dilution; -, negative control; β-actin, PCR control.
IKKα and combined noncanonical and canonical NF-κB regulate Pax5 and Irf4 promoter activities and BM B-cell differentiation. (A) The activities of Pax5, Irf4, mutant Pax5, and mutant Irf4 promoters containing mutations within κB1 (mκB1) or κB2 (mκB2) in pGL3b luciferase reporter vectors were examined in Raji B cells using the luciferase reporter assay. +IKKα indicates coexpression of IKKα and promoter plasmids; +IKKα-KA, coexpression of IKKα-KA and promoter plasmids. (B) Flow cytometric analyses of B220+ BM B cells in RPMI 1640 medium supplemented with 10% FBS for 3 days after reintroduction of IKKα, IKKα-KA, Pax5, and IKKα+Pax5 plasmids into BM B cells using AMAXA nucleofection. (C) Levels of Ter119, M-CSFR, and C/EBPα in cultured WT and KA/KA BM B cells were detected with Western blotting. β-actin, protein-loading control. (D) RT-PCR showing expression levels of Tnfsf11 and GAGA1 in cultured WT and KA/KA BM B cells. ++, +, cDNA dilution; -, negative control; β-actin, PCR control.
To determine the effect of IKKα, Pax5, IRF4, and NF-κB on BM B-cell development, we reexpressed IKKα, IKKα-KA, NF-κB, Pax5, and IRF4 in freshly isolated KA/KA BM B220+ cells using AMAXA nucleofection and then cultured these cells. As shown in Figure 7B, decreased B220+CD43+ cells were detected in KA/KA cells compared with WT cells. Reexpressing IKKα or Pax5, alone or combined, elevated the number of KA/KA B220+CD43+ cells (Figure 7B), whereas reexpressed IKKα-KA and IRF4 did not (supplemental Figure 6B). Furthermore, if IKKα regulated Pax5 expression through NF-κB, then reintroduced NF-κB should promote KA/KA B-cell differentiation. Our results demonstrated that reexpressed p65/p50/RelB and p65/p52/RelB partially rescued B-cell numbers, whereas reexpressed p65 or p52/RelB did not produce a similar rescue (supplemental Figure 6C). These results suggest that IKKα-mediated B-cell development requires noncanonical and canonical NF-κB activities to up-regulate Pax5 expression.
Although KA/KA B220+ cell numbers were reduced in culture compared with WT (Figure 7B and supplemental Figure 6C), we found that cells positive for the Ter119 (expressed on erythrocytes and erythroid precursors), CD11b, and CD11c markers were increased, but CD19 cells were reduced in KA/KA BM B220+ cell cultures (supplemental Figure 6D), suggesting that the reduced B-lineage commitment of KA/KA BM cells might result in increased erythroid-myeloid lineage differentiation. Such a notion was supported by Western blot and RT-PCR, which showed that cultured KA/KA BM B cells expressed higher levels of Ter119, M-CSFR, C/EBPα, Tnfsf11, and GATA-1 (megakaryocytic/erythroid lineage) compared with cultured WT cells (Figure 7C-D). Pax5 is known to repress M-CSFR, C/EBPα, Tnfsf11, and GATA1 expression.25,35 Pax5 down-regulation may therefore elevate the expression of GATA1, Tnfsf11, TER119, C/EBPα, and M-CSFR, which in turn contributes to KA/KA BM B-cell differentiation into the myeloid-erythroid lineages.
Discussion
In the present study, we found that IKKα inactivation induced a BM B-cell defect, increased myeloid-erythroid lineages, and impaired both the noncanonical and canonical NF-κB pathways in BM and splenic B cells. We further revealed that IKKα regulated early BM B-cell development via the 2 NF-κB pathways to target transcription of Pax5, a critical B-cell regulator. These results provide novel evidence that the IKKα/NF-κB–mediated signaling cascade regulates B-cell commitment in the BM. IKKα therefore emerges as a crucial player in hematopoiesis.
IKKα and NF-κB signaling pathways in B cells
A major inhibitory mechanism underlying the inactivation of NF-κB by IκBs, p100, or p105 is to retain NF-κB dimers in the cytoplasm. Inactivated NIK or IKKα impairs NF-κB2 p100 processing, resulting in increased p100. Therefore, the increased p100 binds and retains RelB in the cytoplasm. In the present study, we found very high cytosolic p100 levels in KA/KA splenic and BM B cells compared with WT, but similar p100 levels in WT and KA/KA non-B cells. However, endogenous IKKα levels were reduced in the B cells and non-B cells of KA/KA mice (Figure 5A). These results indicate that IKKα inactivation specifically impairs the noncanonical NF-κB pathway in B cells. Although we expected to see reduced nuclear p52 and RelB levels in KA/KA B cells, surprisingly, the nuclear p65 and p50 levels were also reduced in KA/KA B cells compared with WT. These results suggest that IKKα inactivation impairs both noncanonical and canonical NF-κB pathways. Novack et al12 have shown that NIK deficiency induces a marked increase in p100 levels in BM cells after stimulation of the receptor activator of NF-κB ligand (RANKL). The increased p100 binds to p65 and p50, thereby blocking p65:p50 nuclear translocation, although canonical NF-κB activity remains intact. Basak et al have reported a similar result in IKKα-deficient cells.13 Therefore, the elevated p100 protein induced by IKKα inactivation may retain RelB, p65, and p50 in the cytoplasm of KA/KA B cells, as seen with NIK deficiency. In addition, the NIK level does not increase in KA/KA cells (data not shown), which supports the argument that reduced nuclear p65 and p50 may be caused by increased p100 in KA/KA B cells. It is documented that canonical NF-κB signaling is required for marginal zone B-cell development.36 KA/KA marginal zone B cells were significantly reduced compared with WT, indicating that down-regulated canonical NF-κB activity may contribute to these B-cell defects.
Canonical NF-κB activity is required to protect BM B cells from TNFR signaling. Although the canonical NF-κB activity was reduced in KA/KA B cells, we did not observe dramatically increased death of these cells. It is possible that the remaining basal NF-κB activity may be sufficient to protect the KA/KA B cells because IKKβ and IKKγ are present. In addition, we found increased Stat3 levels in KA/KA B cells,37 which may contribute to BM B-cell survival.
IKKα-, NF-κB–, and B cell–specific target genes
Five NF-κB members, RelA, RelB, c-Rel, p50, and p52, form homodimers or heterodimers when binding to putative κB1 (canonical) or κB2 (noncanonical) DNA sequences on the promoters of the target genes. Gene-knockout studies have revealed most of the physiologic functions of NF-κB. If some NF-κB targets contain both κB1 and κB2 sites that contribute to promoter activity, double NF-κB knockouts may be insufficient to reveal the full effect on these targets. Knockout of the Pax5 transcription factor blocks B-cell differentiation at the pro-B stage.25 In the present study, we found that IKKα inactivation down-regulated the expression of multiple genes, including Pax5 and IRF4, which are relevant to B-cell and HSC development. Furthermore, both κB1 and κB2 putative transcription-binding sites are present in Pax5 and Irf4 promoters and are required for the activities of these promoters (Figure 7A). In particular, we found that a single mutation of κB1 or κB2 caused only a minor reduction in Pax5 promoter transcriptional activity, but that combined κB1 and κB2 mutations caused a significant reduction. Reintroducing 3 NF-κB components, not 2, promoted KA/KA BM B-cell development. These results suggest that inactivating 1 or 2 NF-κB components may not cause Pax5 down-regulation in vivo. These results may explain why KA/KA BM B cells lacking noncanonical and canonical NF-κB activities display defects in B progenitors. IKKα inactivation did not completely block the Pax5 and IRF4 levels, suggesting that IKKα and NF-κB may not be the only regulators of Pax5 and IRF4 expression. Furthermore, reexpressed Pax5, but not IRF4, rescued the KA/KA BM B-cell defect. IRF4 down-regulation may contribute to the splenic B-cell defect in KA/KA mice. Moreover, we found reduced nuclear IKKα levels in KA/KA BM B cells compared with WT. IKKα can bind to chromatin, regulating the expression of many genes.38,39 Therefore, IKKα down-regulation or inactivation may affect many molecular events through diverse avenues.
Pax5 promotes B-cell differentiation and simultaneously represses myeloid-erythroid lineage development.25 In the present study, we found that cultured KA/KA BM B cells developed more myeloid-erythroid cells and expressed elevated C/EBPα, GATA1, M-CSFR, Tnfsf11, Ter119, and Ly-6A/E levels.25 It is known that Pax5 represses the expression of these genes. We further showed that reexpressed IKKα reduced myeloid-erythroid cells in the cultured KA/KA BM cells. These results support the idea that Pax5 is a downstream target of IKKα and NF-κB in early BM B-cell development. In addition, VavCre-induced IKKα deletion impaired BM and splenic B-cell development and increased myeloid cells in Ikkαf/f/VavCre mice, suggesting that IKKα inactivation interrupts the coordinated regulation of B lineage and myeloid-erythroid lineages in progenitors during hematopoiesis.
IKKα, B-cell development, and lymphoid organ development
The disagreement between our results and Derudder's results on BM B cells is likely because of the timing of IKKα deletion.24 We found that progenitors were altered in KA/KA BM and FL cells compared with WT. Guo et al5 have shown an association between altered progenitors and B-cell defects in the BM of p100−/− mice. In general, Cre-induced physiologic function from deleting genes occurs later than the expression of the promoters that drive Cre because cells take time to remove all of the targeted proteins. Conversely, transgenes show profound effects when their promoters are activated in vivo.38 Therefore, mb1-IκBα Tg mice show a defect in BM B cells, but mb1Cre-induced gene deletion may not be rapid enough to demonstrate the function of the targeted genes.6,24 Interestingly, Derudder et al showed that the B-cell defect associated with mb1Cre-induced IKKα and IKKβ deletion is completely rescued by Tg-Bcl224 ; however, Kaisho et al2 and our group have consistently demonstrated that Tg-Bcl2 only partially rescues B-cell defects in radiation chimera receiving Ikkα−/− FL cells, supporting the notion that IKKα regulates the activities of progenitors during hematopoiesis.
Furthermore, the aly mutation disrupts the interaction between NIK and IKKα.14 Inactivated IKKα or NIK can increase p100 levels, which further attenuates canonical NF-κB activity. KA/KA mice and aly/aly mice do not develop lymphoid nodes and Peyer patches, but do display hematopoietic defects.23 Although IkkαAA mice do not develop Peyer patches, they still develop small lymphoid nodes.28 Mercurio et al40 have suggested that mutations at 178 and 180 within the ATP activation loop of IKKα maintain a low level of autophosphorylated IKKα, but that a mutation at the ATP-binding site completely abolishes this autophosphorylation. In addition, IkkαAA mice possess a normal endogenous IKKα level,3 but IKKα expression is down-regulated in KA/KA mice (Figure 5A). These different mutations may contribute to the different phenotypes observed in KA/KA and IkkαAA mice. Conversely, both KA/KA mice (data not shown) and IkkαAA mice28 develop a similar defect in mammary gland development. Therefore, IKKα functions differently in different organs.
Many parameters may affect phenotypes in different experiments and in different genetically modified mice. The interaction of B cells with other lineages or stroma has an impact on B-cell development. In addition, the effect of IKKα KA/KA and IKKα null mutations may not be the same for certain physiologic functions. Indeed, in the present study, we observed that B220+CD43+ cells were lower in KA/KA FL cells than in Ikkα−/− FL cells, and that the BM B-cell profile patterns generated by KA/KA FL and Ikkα−/− FL were not completely identical in radiation chimeras. A decade ago, 2 studies reported defects in splenic B cells, but not BM B cells, in Ikkα−/− FL-transfer experiments.2,3 These disagreements require further investigation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr John Hiscott (Sir Mortimer B. Davis Jewish General Hospital, Montreal, QC) and Dr Kazuhiko Igarashi (Tohoku University, Tohoku, Japan) for providing the human IRF-4 reporter construct and Pax5 expression vector; and Dr Ulrich Siebenlist and Dr Stephen Anderson (National Institutes of Health, Bethesda, MD) for commenting on this manuscript.
This work was supported by the National Cancer Institute, National Institutes of Health, Bethesda, MD (ZIA BC 011212 and ZIA BC 011391 to Y.H).
National Institutes of Health
Authorship
Contribution: M.Y.B. and Y.H. designed, performed, and analyzed the experiments and wrote the manuscript; J.W.-B., F.Z., Z.C., and S.L. designed, performed, and analyzed the experiments; and D.C.G. and M.K. provided materials and discussed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yinling Hu, PhD, Cancer and Inflammation Program, Center for Cancer Research, Frederick National Laboratory for Cancer Research, Bldg 567, Rm 252, Frederick, MD 21701; e-mail: huy2@mail.nih.gov.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal