Abstract
FoxP3+ regulatory T cells (Tregs) suppress GVHD while preserving graft-versus-tumor effects, making them an attractive target for GVHD therapy. The donor-derived Treg pool can potentially be derived from the expansion of preexisting natural Tregs (nTregs) or from de novo generation of inducible Tregs (iTregs) from donor Tconvs in the transplantation recipient. Using an MHC-mismatched model of acute GVHD, in the present study we found that the Treg pool was comprised equally of donor-derived nTregs and iTregs. Experiments using various combinations of T cells from wild-type and FoxP3-deficient mice suggested that both preexisting donor nTregs and the generation of iTregs in the recipient mice contribute to protection against GVHD. Surprisingly, CD8+FoxP3+ T cells represented approximately 70% of the iTreg pool. These CD8+FoxP3+ T cells shared phenotypic markers with their CD4+ counterparts and displayed suppressive activity, suggesting that they were bona fide iTregs. Both CD4+ and CD8+ Tregs appeared to be protective against GVHD-induced lethality and required IL-2 and TGFβ receptor expression for their generation. These data illustrate the complex makeup of the donor-derived FoxP3+ Treg pool in allogeneic recipients and their potential role in protection against GVHD.
Introduction
Regulatory T cells (Tregs) are a subset of T cells crucial for protecting the host from an overactive immune response.1-5 A mouse strain known as Scurfy6 and patients with immune dysregulation, polyendocrinopathy, enteropathy, and X-linked (IPEX) syndrome lack Tregs because they harbor a mutation in the transcription factor FoxP3,7-9 which is essential for Treg development and function. As a result, a T cell–dependent systemic fatal autoimmune syndrome ensues in both Scurfy and IPEX syndrome patients.
The suppressive activity of Tregs is not limited to self-reactive T cells, because they can also inhibit allotypic responses.10 This finding has yielded significant interest from a transplantation perspective, because Tregs could potentially suppress both transplantation rejection and a detrimental side effect of BM transplantation (BMT), GVHD. BMT is often necessary in conjunction with treatment of hematologic malignancies, because normal hematopoietic cells are killed during high-dose chemotherapy or radiation therapy, leading to BM failure and inadequate hematopoiesis. The stem cells obtained from an allogeneic donor also contain a population of mature, donor-derived T cells. Because a fraction of these T cells can recognize allogeneic MHC (major mismatch) or allogeneic peptides presented by self-MHC (minor mismatch) directly, this causes a systemic T cell–mediated attack against the allogeneic recipient, leading to GVHD.11 Although GVHD can be reduced by the use of autologous or MHC-matched donors, there are benefits to using allogeneic BMT as therapy. Most importantly, alloreactive donor T cells display a graft-versus-tumor effect, killing residual malignant cells that may have escaped therapy.12 The use of Tregs in BMT settings has gathered considerable interest because of its potential to inhibit allotypic conventional T-cell (Tconv) responses causing GVHD while preserving their graft-versus-tumor effects.13-15
One challenge that has hindered the therapeutic use of Tregs is the difficulty of obtaining sufficient Treg numbers to infuse into the recipient. An alternative approach to adoptive transfer of Tregs is to administer a drug that preferentially increases Tregs over conventional T cells (Tconvs) in the recipient. Whereas earlier studies demonstrated the efficacy of co-adoptive transfer of Tregs with Tconvs in protection against GVHD,13-17 more recent studies have focused on investigating strategies to increase Tregs in vivo for GVHD therapy.18-22 Matters are further complicated by the presence of 2 distinct populations of Tregs. FoxP3+ Tregs can be largely divided into naturally arising Tregs (nTregs), which develop in the thymus, and inducible Tregs (iTregs), which are converted from Tconvs in the periphery.1,2,23,24 Because the molecular requirements and signal transduction pathways in the proliferation and generation of these Treg subsets differ,23 it is crucial to establish which Treg subset(s) plays a role in protection against disease. To date, it is still unclear whether the donor-derived Treg pool generated during GVHD is composed of nTregs or iTregs and how iTregs are generated during GVHD.
In the present study, we investigated the contribution of the different FoxP3+ Treg subsets in a mouse model of MHC-mismatched GVHD. We show that the Treg pool during GVHD is comprised equally of donor-derived nTregs and iTregs that were generated in the recipient from donor Tconvs. Using T cells from FoxP3-deficient mice, we found that both donor-derived nTregs and the generation of iTregs in the recipient mice contributed to protection against GVHD-induced weight loss. Looking further into the iTreg pool, we found, surprisingly, that CD8+FoxP3+ T cells represented approximately 70% of all iTregs. These CD8+ Tregs shared phenotypic markers with their CD4+ counterparts and displayed suppressive activity. Both CD4+ and CD8+ iTregs were important in protection against GVHD and required IL-2 receptor (IL-2R) and TGFβ receptor (TGFβR) expression for their optimal generation in vivo. Our data suggest that iTregs contribute to protection against GVHD and are generated by an IL-2 and TGFβ-dependent mechanism. Therefore, in addition to nTreg expansion, strategies aimed at increasing the conversion of donor-derived Tconvs to iTregs might be beneficial for the treatment of GVHD.
Methods
Mice
C57BL/6 (B6), B6.SJL (CD45.1 congenic), DBA2, B6D2F1, and loxp-flanked TGFβR2 (TGFβR2F/F)25 mice were purchased from the National Cancer Institute. FoxP3 green fluorescent protein knock-in (FoxP3.GFP KI), B6.PL (Thy1.1 congenic), CD25−/−, and RAG−/− mice were purchased from The Jackson Laboratory. B6D2F1 (CD45.1/CD45.2 heterozygous) and CD45.1+ FoxP3.GFP KI mice were created by crossing B6.SJL mice to DBA2 mice and FoxP3.GFP KI mice, respectively. TGFβR2F/F were crossed to Cre recombinase-human estrogen receptor (CreT2)26 transgenic mice and the ROSA-enhanced yellow fluorescent protein (ROSA-YFP) reporter,27 which were gifts from Drs E. Brown (University of Pennsylvania) and F. Constantini (Columbia University), respectively. Mice were housed in pathogen-free conditions and treated in strict compliance with institutional animal care and use committee regulations of the University of Pennsylvania, which approved the protocol for this study.
Flow cytometry, cell sorting, and data analysis
Abs for flow cytometry were purchased from BD Pharmingen, BioLegend, or eBiosciences. Aqua fluorescent live-dead stain and CFSE were purchased from Invitrogen. Cells were stained with live-dead stain before surface Ab staining or intracellular staining for IFNγ, IL-2, FoxP3, and/or CTLA4. For cell sorting, T cells were enriched with CD4 and CD8α magnetic beads (Miltenyi Biotec) before staining with Abs against CD4, CD8α, CD25, CD45.1, CD45.2, and CD45Rb. FoxP3+ cells were sorted by GFP fluorescence using the FoxP3.GFP KI mouse. Flow cytometry of cells and cell sorting were performed with the FACSCanto and FACSAria (BD Biosciences), respectively. Data were analyzed with FlowJo Version 8.8.7 software (TreeStar). Statistical analysis was performed by t test, paired t test, ANOVA, or log-rank test using Excel 2004 software (Microsoft) as appropriate.
GVHD induction with T cells from Scurfy/WT mixed BM chimeras
Mixed BM chimeras were made by injecting BM from CD45.1+ Scurfy or FoxP3.GFP KI and B6 mice at a 2:1 ratio into irradiated B6 mice (1000 cGy). Eight weeks later, GFP+ and GFP− CD45.1+ T cells were FACS sorted from the spleens of the BM chimeras and used as a source of effector T cells for GVHD induction. Eight- to 12-week-old female B6D2F1 mice were irradiated with a total of 1000 cGy in 2 equal doses separated by 12 hours. Three to 6 hours after the final irradiation, the mice were intravenously injected with 0.5 × 106 FACS-sorted Tconvs (GFP− wild-type [WT] or Scurfy) with or without 0.1 × 106 FACS-sorted Tregs (GFP+ WT) combined with 2 × 106 RAG−/− splenocytes and 3 × 106 T cell–depleted BM. The injection of RAG−/− splenocytes was necessary for GVHD induction and optimal donor-derived T-cell expansion (A. Satake, unpublished observations, July 29, 2010). In some experiments, irradiated B6D2F1 mice were injected with 0.6 × 106 FACS-sorted CD4+ Tconvs (GFP− WT or Scurfy) mixed with 0.4 × 106 FACS-sorted CD8+ Tconvs (GFP− WT or Scurfy) combined with 2 × 106 RAG−/− splenocytes and 3 × 106 T cell–depleted BM. Mortality and weight were monitored over time. Mice were euthanized when their weight loss was > 29% of their initial body weight.
Phenotypic and functional analysis of Tregs during GVHD
Irradiated B6D2F1 was intravenously injected with 3 × 106 T cell–depleted BM cells and 5 × 106 splenocytes from CD45.1+ B6 or FoxP3.GFP KI mice. Donor cells from the spleen, inguinal lymph nodes (LNs), and mesenteric LNs were phenotypically analyzed by flow cytometry at the indicated time points after transplantation. To measure cytokine production, splenocytes were cultured in the presence of phorbol 12-myristate 13-acetate (PMA; 50 ng/mL), ionomycin (1 μg/mL), and Brefeldin A (10μM) for 6 hours before staining with Abs. In experiments using IL-2 immune complexes Brefeldin A (ICs), IL-2 ICs were prepared by mixing 5 μg of anti–IL-2 Ab (clone JES6-1D; Bio-X-Cell) and 1 μg of murine IL-2 (eBiosciences) and incubating for 30 minutes on ice28 before injection on days 1, 2, 3, and 6 after transplantation. To determine the suppressive activity of CD4+ and CD8+ Tregs, CD45.1+CD8+GFP+, CD45.1+CD8+GFP−, or CD45.1+CD4+GFP+ cells were FACS sorted from the spleens of CD45.1+ FoxP3.GFP KI → B6D2F1 mice on day 8. CD4+GFP− cells (Tconvs) were FACS sorted from FoxP3.GFP KI mice as effectors, CFSE labeled, cocultured with the sorted CD4+ or CD8+ T cells at various Tconv:Treg ratios, and stimulated with anti-CD3 (0.01 μg/mL) and irradiated feeder splenocytes. CFSE dilution of Tconvs was analyzed 4 days after coculture and the percentage divided was calculated using FlowJo Version 8.8.7 software.
Characterization of Treg populations during GVHD
To determine the origin of the donor Tregs, 0.15 × 106 FACS-sorted GFP+ Tregs (CD4+ and CD8+) from FoxP3.GFP KI mice and 1.5 × 106 FACS-sorted CD4+ and CD8+ GFP− cells from CD45.1+ FoxP3.GFP mice were combined with 2 × 106 RAG−/− splenocytes and 3 × 106 T cell–depleted BM and injected into irradiated B6D2F1 mice. On day 8 after transplantation, secondary lymphoid organs were harvested and analyzed by flow cytometry. To determine the role of TGFβR in the induction of iTregs in vitro and in vivo, TGFβR2F/F/ROSA-YFP/CreT2 and control TGFβR2+/+/ROSA-YFP/CreT2 mice were treated orally with 250 μg/g body weight of tamoxifen for 5 days and rested for 5 days. YFP+CD4+ and YFP+CD8+ naive Tconvs (CD25−CD45RBhi) were FACS sorted from the spleens of these mice and used for in vitro and in vivo studies. For in vitro studies, the T cells were cultured with plate-immobilized anti-CD3 (2 μg/mL) and anti-CD28 (2 μg/mL) with or without human IL-2 (5 ng/mL; PeproTech) and/or human TGFβ (2 ng/mL; R&D Systems). Four days later, the T cells were analyzed for FoxP3 expression by flow cytometry. For in vivo studies, the T cells were combined at a 1:1 ratio with FACS-sorted CD4+ and CD8+ Tconvs (CD25−CD45RBhi) from Thy1.1+ mice (WT competitor). A total of 0.5 × 106 CD8+ T cells and 0.75 × 106 CD4+ T cells combined with 2 × 106 RAG−/− splenocytes and 3 × 106 T cell–depleted BM cells were injected into irradiated B6D2F1 mice. On day 8 after transplantation, secondary lymphoid organs were harvested and analyzed for expression of FoxP3 in CD4+ and CD8+ T cells. A Treg generation index was then calculated based on the percentage of FoxP3+ CD4+ or CD8+ T cells of WT competitor versus the TGFβR2+/+ or TGFβR2F/F (CD45.2+CD45.1−) origin. The following formula was used: Treg index = [(%FoxP3+ of TGFβR2+/+ or TGFβR2F/F)/(%FoxP3+ of Thy1.1+)]/average of [(%FoxP3+ of TGFβR2+/+)/(%FoxP3+ of Thy1.1+)]. When testing the role of CD25 in the generation of iTregs, a similar experiment was performed using Tconvs from WT B6, CD25−/−, and Thy1.1 mice. Tconvs from CD25−/− mice were FACS sorted by gating on CD45RBhiCD4+ T cells, which resulted in an approximately 10-fold reduction in preexisting CD4+ Tregs (< 0.5% of all CD4+ T cells).
Results
The generation of iTregs confers protection against GVHD
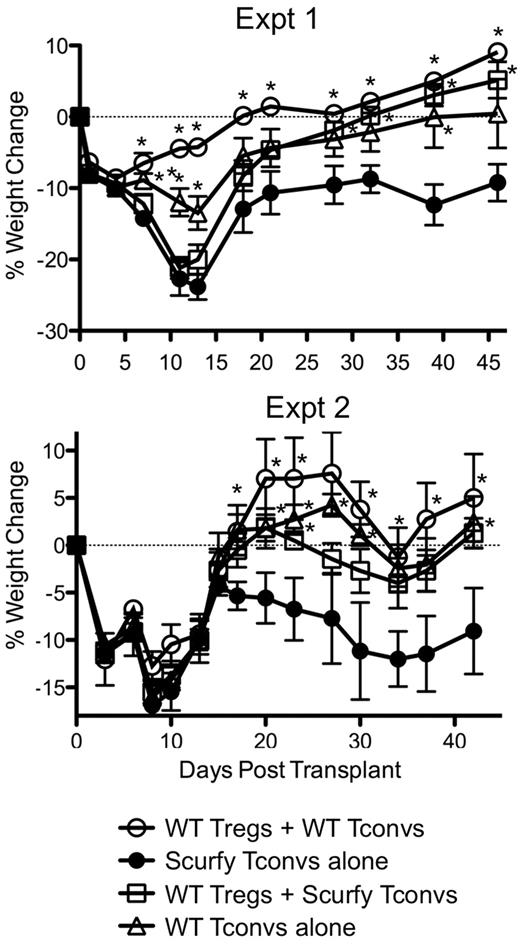
To determine the contribution of nTregs and iTregs to protection against GVHD, we compared the ability of Tconvs from WT and FoxP3-deficient Scurfy mice to cause GVHD-induced weight loss. Because Scurfy mice lack Tregs and contain hyperactivated Tconvs, mixed BM chimeras using CD45.1+ Scurfy (or CD45.1+ FoxP3.GFP KI mice as controls) and CD45.2+ WT BM were created to obtain unactivated Scurfy T cells. CD4+ and CD8+ T cells of Scurfy origin contained a similar fraction of CD44high cells, proliferated similarly and expressed slightly but not overtly higher levels of IFNγ production after stimulation with PMA/ionomycin compared with T cells of FoxP3.GFP KI origin (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). GVHD was then induced in irradiated B6D2F1 mice using the FACS-sorted T-cell populations mixed in the following manner: (1) WT GFP− Tconvs + GFP+ nTregs (has nTregs and also can generate iTregs), (2) WT GFP− Tconvs alone (has no nTregs but can generate iTregs), (3) Scurfy Tconvs + GFP+ nTregs (has nTregs but has no ability to generate iTregs), and (4) Scurfy Tconvs alone (has no nTregs and has no ability to generate iTregs). As expected, weight loss in mice receiving Scurfy Tconvs alone was significantly more pronounced compared with mice receiving WT Tconvs and nTregs (Figure 1), suggesting that GVHD is exacerbated in the absence of Tregs. The presence of either donor-derived nTregs or iTregs alone protected against GVHD-induced weight loss but was suboptimal compared with mice with both donor-derived nTregs and iTregs (Figure 1). Histopathologic analysis revealed GVHD-induced pathology in the skin and large intestine, but no appreciable differences were noted between the groups (data not shown). To further test whether there were inherent differences in the ability of Scurfy and WT Tconvs to induce GVHD, Tconvs from WT and Scurfy mice were mixed and injected into irradiated B6D2F1 mice. The weight loss observed in these mice was comparable with that in mice receiving WT Tonvs alone (data not shown), suggesting that the relative ability of Tconvs from Scurfy and WT mice to induce GVHD was similar. These data suggest that whereas donor-derived iTregs and nTregs contribute individually to protection against GVHD-induced weight loss, both subsets are needed for optimal protection. However, given that the Scurfy Tconvs were slightly more activated (higher IFNγ production) than WT Tconvs before injection, we cannot entirely exclude the possibility that the activation status of the Scurfy Tconvs also contributed to more severe GVHD in recipient mice receiving Scurfy Tconvs.
nTregs and iTregs contribute to protection against GVHD-induced weight loss. CD45.1+ FoxP3.GFP or Scurfy BM was mixed with CD45.2+ B6 BM at a 2:1 ratio and injected into irradiated B6 mice. CD45.1+ GFP+ and GFP− T cells were sorted from the mixed BM chimeras. The T cells were then used as effectors for GVHD induction by mixing the T cells in the following combinations: nTregs + iTregs (0.1 × 106 GFP+ Tregs + 0.5 × 106 GFP− Tconvs), nTregs alone (0.1 × 106 GFP+ Tregs + 0.5 × 106 Scurfy Tconvs), iTregs alone (0.5 × 106 GFP− Tconvs), and no Tregs (0.5 × 106 Scurfy Tconvs). Weight changes were monitored over time. Two independent experiments are shown. The results are represented as the percent mean weight change ± SEM (n = 10 mice/group for experiment I and n = 5 mice/group in experiment II). *P < .05 by ANOVA compared with the group receiving no Tregs (Scurfy Tconvs alone).
nTregs and iTregs contribute to protection against GVHD-induced weight loss. CD45.1+ FoxP3.GFP or Scurfy BM was mixed with CD45.2+ B6 BM at a 2:1 ratio and injected into irradiated B6 mice. CD45.1+ GFP+ and GFP− T cells were sorted from the mixed BM chimeras. The T cells were then used as effectors for GVHD induction by mixing the T cells in the following combinations: nTregs + iTregs (0.1 × 106 GFP+ Tregs + 0.5 × 106 GFP− Tconvs), nTregs alone (0.1 × 106 GFP+ Tregs + 0.5 × 106 Scurfy Tconvs), iTregs alone (0.5 × 106 GFP− Tconvs), and no Tregs (0.5 × 106 Scurfy Tconvs). Weight changes were monitored over time. Two independent experiments are shown. The results are represented as the percent mean weight change ± SEM (n = 10 mice/group for experiment I and n = 5 mice/group in experiment II). *P < .05 by ANOVA compared with the group receiving no Tregs (Scurfy Tconvs alone).
A large proportion of donor-derived FoxP3+ T cells are CD8+ during acute GVHD
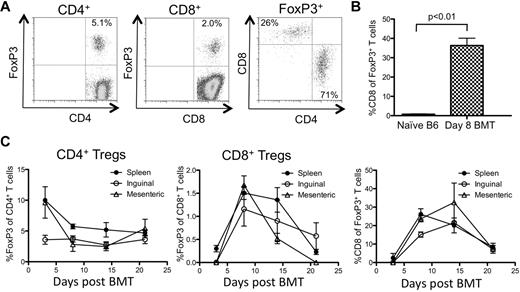
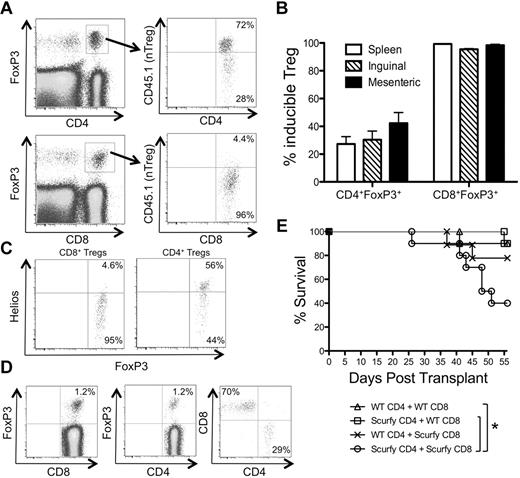
Given our data suggesting that nTregs and iTregs appear to contribute to GVHD disease protection, we next examined how the donor-derived Treg pool was established after BMT. Irradiated B6D2F1 mice were injected with BM and splenocytes from B6 mice and the appearance of Tregs in the secondary lymphoid organs was tracked at different times. Using this approach, the weight loss observed was similar to that seen in GVHD induced by FACS-sorted T cells (Figure 1 and supplemental Figure 2). On day 8 after transplantation, approximately 5% of the donor-derived CD4+ T cells in the spleen were FoxP3+ (Figure 2A), which represented an approximately 50% decrease in the fraction of Tregs contained in the initial inoculum (10%-12% of CD4+ T cells). Unexpectedly, CD8+FoxP3+ T cells (1%-3% of all CD8+ T cells) were detected in the spleen (Figure 2A,C) and in the LNs (Figure 2C). These CD8+FoxP3+ T cells represented approximately 30% of the total donor-derived FoxP3+ pool on day 8 after GVHD induction compared with < 1% of the FoxP3+ pool in nontransplanted mice (Figure 2A-B). Although the fraction of FoxP3+ T cells among CD4+ T cells and their absolute numbers remained fairly constant during the first 3 weeks of GVHD, the proportion and absolute numbers of CD8+FoxP3+ T cells and their contribution to the total Treg pool peaked between weeks 1 and 2 after GVHD induction and declined by week 3 (Figure 2C and supplemental Figure 3). These Tregs were most likely of donor splenic and not BM origin, because T cells of donor origin could not be detected in mice receiving T cell–depleted BM alone 2 weeks after BMT (data not shown). These data suggest that CD8+FoxP3+ T cells are a significant but transient contributor to the donor-derived FoxP3+ T-cell pool during acute GVHD.
CD8+ Tregs contribute significantly to the total Treg pool during GVHD. Irradiated CD45.2+ B6D2F1 mice (H-2bxd) were injected with splenocytes and T cell–depleted BM from CD45.1+ FoxP3.GFP mice (H-2b). (A) Representative FACS plots of splenocytes harvested on day 8 after BMT are shown. The plots are gated on donor (CD45.1+) CD4+ (left plot), CD8+ (middle plot), and FoxP3+ T cells (right plot). The numbers in the plots represent the fraction of cells within that quadrant. (B) The average contribution of CD8+FoxP3+ cells to the total FoxP3+ pool on day 8 after BMT (n = 3) was compared with naive unirradiated untransplanted B6 mice (n = 3). (C) The fraction of donor T cells that were FoxP3+ of CD4+ (left) or CD8+ (middle) and the fraction of donor CD8+ of total donor FoxP3+ T cells (right) were analyzed at various times after transplantation. One representative of 3 independent experiments is shown.
CD8+ Tregs contribute significantly to the total Treg pool during GVHD. Irradiated CD45.2+ B6D2F1 mice (H-2bxd) were injected with splenocytes and T cell–depleted BM from CD45.1+ FoxP3.GFP mice (H-2b). (A) Representative FACS plots of splenocytes harvested on day 8 after BMT are shown. The plots are gated on donor (CD45.1+) CD4+ (left plot), CD8+ (middle plot), and FoxP3+ T cells (right plot). The numbers in the plots represent the fraction of cells within that quadrant. (B) The average contribution of CD8+FoxP3+ cells to the total FoxP3+ pool on day 8 after BMT (n = 3) was compared with naive unirradiated untransplanted B6 mice (n = 3). (C) The fraction of donor T cells that were FoxP3+ of CD4+ (left) or CD8+ (middle) and the fraction of donor CD8+ of total donor FoxP3+ T cells (right) were analyzed at various times after transplantation. One representative of 3 independent experiments is shown.
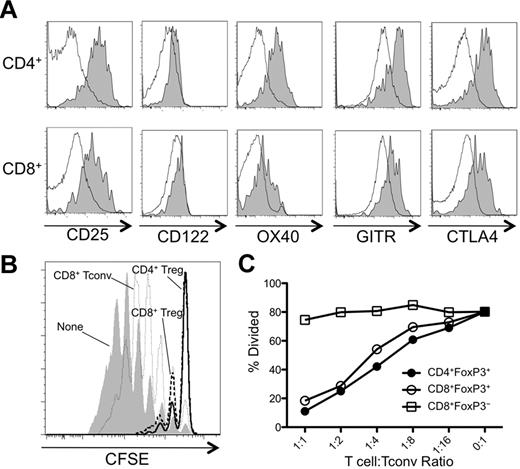
We next analyzed the day 8 GVHD CD8+FoxP3+ T cells for expression of surface markers commonly associated with CD4+ Tregs. Similar to CD4+ Tregs from day 8 BMT mice, CD8+FoxP3+ T cells expressed components of the high-affinity IL-2R (CD122 and CD25) and other coreceptors important for the function of CD4+ Tregs, including OX40, GITR, and CTLA4 (Figure 3A), at a higher level than seen with CD8+FoxP3− T cells. However, given that these markers are also associated with activated T cells, it is unclear whether these markers are expressed on CD8+FoxP3+ T cells in other conditions. We next tested the suppressive function of CD8+FoxP3+ T cells. Although slightly less efficient than CD4+ Tregs, CD8+FoxP3+ T cells attenuated the proliferation of Tconvs potently (Figure 3B and supplemental Figure 4). CD8+FoxP3− T cells did not suppress Tconv proliferation, suggesting that the inhibitory activity of CD8+ T cells was restricted to the FoxP3+ subset (Figure 3B).
CD8+Foxp3+ and CD4+Foxp3+ cells share phenotypic markers and exhibit potent suppressive function. Irradiated CD45.2+ B6D2F1 mice (H-2bxd) were injected with splenocytes and T cell–depleted BM from CD45.1+ FoxP3.GFP mice (H-2b). (A) On day 8 after transplantation, CD45.1+ CD4+ T cells (top panels) and CD8+ T cells (bottom panels) were analyzed for expression of CD25, CD122, OX40, GITR, and CTLA4. The shaded and open histograms represent FoxP3+ and FoxP3− cells, respectively. (B) CD4+ and CD8+ Tregs (GFP+) and CD8+ Tconvs (GFP−) were FACS sorted from spleens on day 8 after transplantation and cocultured with CFSE-labeled CD4+ Tconvs, irradiated splenocytes, and anti-CD3 Ab at the indicated T cell:Tconv ratios. A representative CFSE dilution plot by Tconvs is shown in cultures without Tregs or at a 1:1 T cell:Tconv ratio. (C) A corresponding percent divided value was calculated with FlowJo Version 8.8.7 software and plotted against all T cell:Tconv ratios tested. One representative of at least 2 independent experiments is shown.
CD8+Foxp3+ and CD4+Foxp3+ cells share phenotypic markers and exhibit potent suppressive function. Irradiated CD45.2+ B6D2F1 mice (H-2bxd) were injected with splenocytes and T cell–depleted BM from CD45.1+ FoxP3.GFP mice (H-2b). (A) On day 8 after transplantation, CD45.1+ CD4+ T cells (top panels) and CD8+ T cells (bottom panels) were analyzed for expression of CD25, CD122, OX40, GITR, and CTLA4. The shaded and open histograms represent FoxP3+ and FoxP3− cells, respectively. (B) CD4+ and CD8+ Tregs (GFP+) and CD8+ Tconvs (GFP−) were FACS sorted from spleens on day 8 after transplantation and cocultured with CFSE-labeled CD4+ Tconvs, irradiated splenocytes, and anti-CD3 Ab at the indicated T cell:Tconv ratios. A representative CFSE dilution plot by Tconvs is shown in cultures without Tregs or at a 1:1 T cell:Tconv ratio. (C) A corresponding percent divided value was calculated with FlowJo Version 8.8.7 software and plotted against all T cell:Tconv ratios tested. One representative of at least 2 independent experiments is shown.
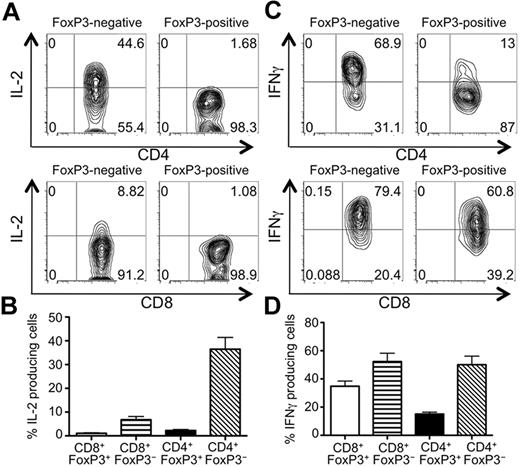
One hallmark of Tregs is their anergic phenotype, demonstrated by their inability to produce IL-2 and their failure to proliferate with TCR stimulation alone. Whereas CD4+ and CD8+ Tconvs produced IL-2, neither CD4+ nor CD8+ Tregs isolated from GVHD mice expressed IL-2 after stimulation with PMA/ionomycin (Figure 4A-B) or with anti-CD3/CD28 (data not shown). Moreover, CD8+ Tregs proliferated in IL-2 but did not survive in cultures when stimulated with anti-CD3 alone (data not shown). In addition to IL-2 production, we examined whether the Tregs arising during GVHD expressed the proinflammatory cytokine IFNγ. Both CD4+ and CD8+ Tregs expressed IFNγ, although relatively less compared with their FoxP3− counterparts, suggesting that these Tregs might have added effector function on top of their suppressive capability. These results demonstrate that the CD8+FoxP3+ T cells arising during GVHD display many characteristics of Tregs and are capable of suppressing T-cell proliferation.
FoxP3+ CD4+ and CD8+ T cells produce IFNγ but not IL-2 after restimulation in vitro. Irradiated CD45.2+ B6D2F1 mice (H-2bxd) were injected with splenocytes and T cell–depleted BM from CD45.1+ FoxP3.GFP mice (H-2b). On day 8 after transplantation, splenocytes were stimulated with PMA/ionomycin in the presence of BFA for 6 hours. The expression of IL-2 (A-B) and IFNγ (C-D) by CD45.1+ CD4+ (top panels) and CD8+ (bottom panels) FoxP3− and FoxP3+ T cells were analyzed by flow cytometry. One representative contour plot of 3-4 independent experiments and compiled data of all experiments represented as mean ± SEM of n = 15-18 mice are shown.
FoxP3+ CD4+ and CD8+ T cells produce IFNγ but not IL-2 after restimulation in vitro. Irradiated CD45.2+ B6D2F1 mice (H-2bxd) were injected with splenocytes and T cell–depleted BM from CD45.1+ FoxP3.GFP mice (H-2b). On day 8 after transplantation, splenocytes were stimulated with PMA/ionomycin in the presence of BFA for 6 hours. The expression of IL-2 (A-B) and IFNγ (C-D) by CD45.1+ CD4+ (top panels) and CD8+ (bottom panels) FoxP3− and FoxP3+ T cells were analyzed by flow cytometry. One representative contour plot of 3-4 independent experiments and compiled data of all experiments represented as mean ± SEM of n = 15-18 mice are shown.
Donor-derived CD4+ and CD8+ Tconvs convert to iTregs during GVHD
To determine the origin of the donor-derived CD4+ and CD8+ Tregs during GVHD, congenically marked T-cell populations from spleens of FoxP3.GFP mice were sorted into either preexisting Tregs (CD45.1+GFP+ T cells) or Tconvs (CD45.2+GFP− T cells). The T-cell populations were mixed at physiologic ratios with T cell–depleted BM, injected into irradiated B6D2F1 mice, and harvested on day 8. In both the spleens and LNs, almost all CD8+ Tregs were iTregs (Figure 5A-B). A smaller but substantial fraction (30%-40%) of CD4+ Tregs was also formed by peripheral conversion of FoxP3− CD4+ T cells (Figure 5A-B). Furthermore, CD8+ Tregs were Helioslo, whereas approximately 50% of CD4+ Tregs were Helioshi. Because Helios has been suggested to be expressed exclusively by nTregs and not iTregs,29 these findings are consistent with the notion that almost all CD8+ Tregs are iTregs. Very few CD8+ iTregs were found when syngeneic B6D2F1 splenocytes were injected into irradiated B6D2F1 mice or when B6 T cells were injected into lymphopenic RAG KO mice with or without irradiation (supplemental Figure 5), suggesting that TCR stimulation by allogeneic MHC is necessary for the generation of CD8+ Tregs in BMT mice. The generation of both CD4+ and CD8+ iTregs did not require the presence of preexisting donor-derived nTregs, because the adoptive transfer of FoxP3− T cells alone resulted in the appearance of FoxP3+ Tregs (Figure 5D). In this setting, in which all Tregs were derived from donor-derived FoxP3− T cells, the majority (approximately 70%) of the Tregs expressed CD8 (Figure 5D).
CD8+ Tregs represent a large fraction of iTregs that are generated in the recipient during GVHD. (A) GFP+ Tregs (CD4+ and CD8+) were sorted from CD45.1+ FoxP3.GFP mice (H-2b) and mixed with GFP− Tconvs (CD4+ and CD8+) sorted from CD45.2+ FoxP3.GFP mice (H-2b). The T cells were combined with RAG−/− splenocytes (H-2b) and T cell–depleted BM (H-2b) and injected into irradiated CD45.1/CD45.2 heterozygous B6D2F1 mice (H-2bxd). (A) Representative plot examining CD45.1 positivity of donor-derived GFP+ Tregs (host cells are also gated out by CD45.1/CD45.2 double positivity) is shown for CD4+ (top panels) and CD8+ (bottom panels) Tregs in the spleen on day 8 after transplantation. (B) The percentage of CD4+ and CD8+ Tregs derived from Tconvs (CD45.1−) was determined in the spleen and inguinal and mesenteric LNs of mice (n = 3) on day 8 after transplantation. Results are expressed as mean ± SEM. (C) Irradiated CD45.2+ B6D2F1 (H-2bxd) mice were injected with splenocytes and T cell–depleted BM from CD45.1+ FoxP3.GFP (H-2b) mice. On day 8 after transplantation, CD45.1+ CD4+ T cells (right plot) and CD8+ T cells (left plot) were analyzed for expression of Helios. (D) GFP− Tconvs (CD4+ and CD8+) were sorted from CD45.1+ FoxP3.GFP (H-2b) mice, combined with RAG−/− splenocytes (H-2b) and T cell–depleted BM (H-2b), and injected into irradiated B6D2F1 mice (H-2bxd). A representative plot examining donor T cells (CD45.1+) is shown for CD8+ (left plot) and CD4+ (middle plot) T cells in the spleen on day 8 after transplantation. The right plot was gated on all GFP+ cells and shows the relative contribution of CD8+ and CD4+ Tregs to the iTreg pool. One representative of 3 independent experiments is shown. (E) CD45.1+ GFP− CD4+ and CD8+ Tconvs were sorted from FoxP3.GFP (CD45.1+):WT and Scurfy (CD45.1+):WT mixed BM chimeras. 0.6 × 106 CD4+ Tconvs (WT GFP− or Scurfy) were mixed with 0.4 × 106 CD8+ Tconvs (WT GFP− or Scurfy) and used as effectors for GVHD induction in irradiated B6D2F1 mice. Survival was monitored over an 8-week period. Two independent experiments were combined with a total of n = 9 or 10 mice/group to generate the survival curve. *P < .05 by log-rank test.
CD8+ Tregs represent a large fraction of iTregs that are generated in the recipient during GVHD. (A) GFP+ Tregs (CD4+ and CD8+) were sorted from CD45.1+ FoxP3.GFP mice (H-2b) and mixed with GFP− Tconvs (CD4+ and CD8+) sorted from CD45.2+ FoxP3.GFP mice (H-2b). The T cells were combined with RAG−/− splenocytes (H-2b) and T cell–depleted BM (H-2b) and injected into irradiated CD45.1/CD45.2 heterozygous B6D2F1 mice (H-2bxd). (A) Representative plot examining CD45.1 positivity of donor-derived GFP+ Tregs (host cells are also gated out by CD45.1/CD45.2 double positivity) is shown for CD4+ (top panels) and CD8+ (bottom panels) Tregs in the spleen on day 8 after transplantation. (B) The percentage of CD4+ and CD8+ Tregs derived from Tconvs (CD45.1−) was determined in the spleen and inguinal and mesenteric LNs of mice (n = 3) on day 8 after transplantation. Results are expressed as mean ± SEM. (C) Irradiated CD45.2+ B6D2F1 (H-2bxd) mice were injected with splenocytes and T cell–depleted BM from CD45.1+ FoxP3.GFP (H-2b) mice. On day 8 after transplantation, CD45.1+ CD4+ T cells (right plot) and CD8+ T cells (left plot) were analyzed for expression of Helios. (D) GFP− Tconvs (CD4+ and CD8+) were sorted from CD45.1+ FoxP3.GFP (H-2b) mice, combined with RAG−/− splenocytes (H-2b) and T cell–depleted BM (H-2b), and injected into irradiated B6D2F1 mice (H-2bxd). A representative plot examining donor T cells (CD45.1+) is shown for CD8+ (left plot) and CD4+ (middle plot) T cells in the spleen on day 8 after transplantation. The right plot was gated on all GFP+ cells and shows the relative contribution of CD8+ and CD4+ Tregs to the iTreg pool. One representative of 3 independent experiments is shown. (E) CD45.1+ GFP− CD4+ and CD8+ Tconvs were sorted from FoxP3.GFP (CD45.1+):WT and Scurfy (CD45.1+):WT mixed BM chimeras. 0.6 × 106 CD4+ Tconvs (WT GFP− or Scurfy) were mixed with 0.4 × 106 CD8+ Tconvs (WT GFP− or Scurfy) and used as effectors for GVHD induction in irradiated B6D2F1 mice. Survival was monitored over an 8-week period. Two independent experiments were combined with a total of n = 9 or 10 mice/group to generate the survival curve. *P < .05 by log-rank test.
We next investigated whether CD4+ and CD8+ iTregs contributed to protection against GVHD. WT and Scurfy CD4+ and CD8+ Tconvs were mixed in different combinations and injected into irradiated B6D2F1 mice. Compared with mice receiving Scurfy CD4+ and CD8+ Tconvs, mice receiving WT CD4+ or CD8+ Tconvs displayed less mortality (Figure 5E). However, no significant differences in weight loss were observed among the groups (supplemental Figure 6). These data suggest that the conversion of CD4+ and CD8+ Tconvs into FoxP3+ iTregs represents a sizeable component of the total Treg pool and might contribute to protection against GVHD mortality.
CD4+ and CD8+ iTregs require TGFβR for their conversion
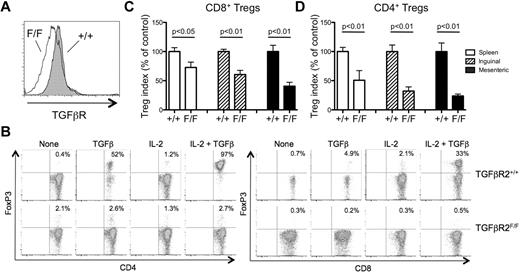
We next sought to determine the mechanism by which FoxP3 was induced in CD4+ and CD8+ iTregs during GVHD. TGFβ has been implicated as an important soluble mediator in the conversion of Tconvs into iTregs, because the addition of TGFβ and a TCR stimulus has been shown to be sufficient for the induction of FoxP3 in CD4+ and CD8+ T cells in vitro.30-32 Indeed, FoxP3 was detected in CD4+ and CD8+ T cells that were stimulated by anti-CD3 in the presence of TGFβ and IL-2 (Figure 6B). To determine the role of TGFβR without perturbing T-cell development, we crossed TGFβR2F/F mice to CreT2 mice with a YFP Cre-reporter to delete the TGFβR on T cells after their maturation. Administration of tamoxifen to these mice resulted in the inducible deletion of detectable surface TGFβR expression on YFP+ T cells from TGFβR2F/F mice (Figure 6A). The addition of IL-2 and TGFβ induced FoxP3 expression by TCR-stimulated YFP+ CD4+ and CD8+ T cells from TGFβR2+/+ mice, but not those from TGFβR2F/F mice, confirming that YFP+ T cells from TGFβR2F/F mice lost their ability to respond to TGFβ (Figure 6B). To determine the role of TGFβR in iTreg generation in vivo, we adoptively transferred mixed populations of FACS-sorted Tconvs (CD45RBhiCD25−) from B6.PL (Thy1.1+; WT competitor) and YFP+ TGFβR2F/F or YFP+ TGFβR2+/+mice into irradiated B6D2F1 mice. On day 8, the secondary lymphoid organs were harvested and the expression of FoxP3 by CD4+ and CD8+ T cells was determined. The absence of TGFβR diminished the ability of CD4+ T cells and, to a lesser extent CD8+ T cells, to convert to iTregs significantly (Figure 6C-D). These data suggest that both CD4+ and CD8+ iTregs require TGFβR for optimal induction of FoxP3.
TGFβR expression is required for the optimal generation of CD4+ and CD8+ iTregs during GVHD. YFP+ CD4+ and CD8+ naive (CD25−CD45RBhi) T cells were FACS sorted from tamoxifen-treated TGFβR2F/F/ROSA-YFP/CreT2 and control TGFβR2+/+/ROSA-YFP/CreT2 mice. (A) The TGFβR expression level on YFP+ T cells from TGFβR2F/F/ROSA-YFP/CreT2 (open histogram) and TGFβR2+/+/ROSA-YFP/CreT2 (shaded histogram) was determined by flow cytometry. (B) The YFP+ Tconvs were treated with plate-bound anti-CD3 and anti-CD28 with or without IL-2 and/or TGFβ for 4 days. FoxP3 expression by CD4+ (top panels) and CD8+ (bottom panels) T cells in each of the conditions was determined by flow cytometry. (C) The sorted CD45.2+YFP+ Tconvs (H-2b) were mixed with Thy1.1+ WT competitor CD25−CD45RBhi Tconvs (H-2b) and combined with RAG−/− splenocytes (H-2b) and T cell–depleted BM (H-2b) and injected into irradiated CD45.1/CD45.2 heterozygous B6D2F1 mice (H-2bxd). On day 8 after transplantation, FoxP3 expression by the CD45.2+YFP+ and Thy1.1+ WT competitor CD8+ (A) and CD4+ (B) T cells in the spleen (open bars), inguinal LNs (hatched bars), and mesenteric LNs (filled bars) was determined by flow cytometry. A Treg generation index was calculated as described in “Characterization of Treg populations during GVHD,” and the results are represented as the mean percentage of control ± SEM (n = 8 mice/group). One representative of 3 independent experiments is shown.
TGFβR expression is required for the optimal generation of CD4+ and CD8+ iTregs during GVHD. YFP+ CD4+ and CD8+ naive (CD25−CD45RBhi) T cells were FACS sorted from tamoxifen-treated TGFβR2F/F/ROSA-YFP/CreT2 and control TGFβR2+/+/ROSA-YFP/CreT2 mice. (A) The TGFβR expression level on YFP+ T cells from TGFβR2F/F/ROSA-YFP/CreT2 (open histogram) and TGFβR2+/+/ROSA-YFP/CreT2 (shaded histogram) was determined by flow cytometry. (B) The YFP+ Tconvs were treated with plate-bound anti-CD3 and anti-CD28 with or without IL-2 and/or TGFβ for 4 days. FoxP3 expression by CD4+ (top panels) and CD8+ (bottom panels) T cells in each of the conditions was determined by flow cytometry. (C) The sorted CD45.2+YFP+ Tconvs (H-2b) were mixed with Thy1.1+ WT competitor CD25−CD45RBhi Tconvs (H-2b) and combined with RAG−/− splenocytes (H-2b) and T cell–depleted BM (H-2b) and injected into irradiated CD45.1/CD45.2 heterozygous B6D2F1 mice (H-2bxd). On day 8 after transplantation, FoxP3 expression by the CD45.2+YFP+ and Thy1.1+ WT competitor CD8+ (A) and CD4+ (B) T cells in the spleen (open bars), inguinal LNs (hatched bars), and mesenteric LNs (filled bars) was determined by flow cytometry. A Treg generation index was calculated as described in “Characterization of Treg populations during GVHD,” and the results are represented as the mean percentage of control ± SEM (n = 8 mice/group). One representative of 3 independent experiments is shown.
IL-2R signaling is required for CD4+ and CD8+ iTreg generation during GVHD
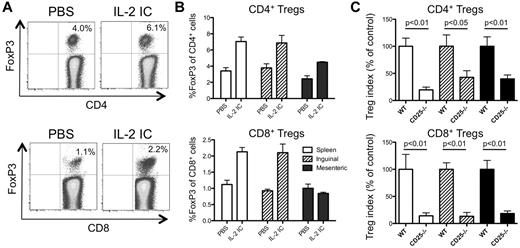
In addition to TGFβ, IL-2 was also required for the efficient generation of CD4+ and CD8+ iTregs in vitro (Figure 6B), prompting us to question whether IL-2 was required for iTreg generation during GVHD in vivo. We first took a gain-of-function approach using IL-2/anti–IL-2 ICs, which have been shown previously to preferentially expand CD4+ Tregs in vivo.28 Administration of IL-2 ICs during GVHD increased the proportion of both CD4+ and CD8+ Tregs in the spleen and inguinal LNs, but not in the mesenteric LNs (Figure 7A-B), suggesting that IL-2 signaling enhances the proliferation/generation of both Treg subsets in some but not all secondary lymphoid organs. To determine whether IL-2R signaling was required for the generation of iTregs, FACS-sorted Thy1.1+CD45RBhi Tconvs were mixed with FACS-sorted Thy1.2+CD45RBhi Tconvs from B6 or CD25−/− mice. On day 8, the secondary lymphoid organs were harvested and the expression of FoxP3 was determined in CD4+ and CD8+ T cells. Compared with T cells from WT mice, CD25−/− T cells were less effective at giving rise to CD4+ and CD8+ iTregs (Figure 7C). These data suggest that IL-2R signaling is required for the optimal generation of CD4+ and CD8+ iTregs during GVHD.
IL-2 is required for optimal generation of CD4+ and CD8+ iTregs during GVHD. Irradiated B6D2F1 mice (H-2bxd) were injected with splenocytes and T cell–depleted BM from CD45.1+ FoxP3.GFP mice (H-2b) and injected with vehicle (PBS) or with IL-2 IC. (A) Representative plots demonstrating FoxP3 expression in donor CD4+ (top plots) and CD8+ (bottom plots) T cells from the spleen on day 8 after transplantation is shown. (B) The fraction of FoxP3+ cells of CD4+ (top graph) and CD8+ (bottom graph) T cells in the spleen (open bars), inguinal LNs (hatched bars), and mesenteric LNs (filled bars) was determined by flow cytometry on day 8 after transplantation. Results are expressed as means ± SEM (n = 3 mice/group). (C) Sorted CD45.2+CD45RBhi Tconvs from B6 or CD25−/− mice (H-2b) were mixed with Thy1.1+ CD45RBhi WT competitor Tconvs (H-2b) and combined with RAG−/− splenocytes (H-2b) and T cell–depleted BM (H-2b) and injected into irradiated CD45.1/CD45.2 heterozygous B6D2F1 mice (H-2bxd). On day 8 after transplantation, FoxP3 expression by the CD45.2+ B6 or CD25−/− and Thy1.1+ WT competitor T cells in the spleen (open bars), inguinal LNs (hatched bars), and mesenteric LNs (filled bars) was determined by flow cytometry. A Treg generation index was calculated as described in “Characterization of Treg populations during GVHD,” and the results are represented as the mean percentage of control ± SEM (n = 3 mice/group). One representative of 2 independent experiments is shown.
IL-2 is required for optimal generation of CD4+ and CD8+ iTregs during GVHD. Irradiated B6D2F1 mice (H-2bxd) were injected with splenocytes and T cell–depleted BM from CD45.1+ FoxP3.GFP mice (H-2b) and injected with vehicle (PBS) or with IL-2 IC. (A) Representative plots demonstrating FoxP3 expression in donor CD4+ (top plots) and CD8+ (bottom plots) T cells from the spleen on day 8 after transplantation is shown. (B) The fraction of FoxP3+ cells of CD4+ (top graph) and CD8+ (bottom graph) T cells in the spleen (open bars), inguinal LNs (hatched bars), and mesenteric LNs (filled bars) was determined by flow cytometry on day 8 after transplantation. Results are expressed as means ± SEM (n = 3 mice/group). (C) Sorted CD45.2+CD45RBhi Tconvs from B6 or CD25−/− mice (H-2b) were mixed with Thy1.1+ CD45RBhi WT competitor Tconvs (H-2b) and combined with RAG−/− splenocytes (H-2b) and T cell–depleted BM (H-2b) and injected into irradiated CD45.1/CD45.2 heterozygous B6D2F1 mice (H-2bxd). On day 8 after transplantation, FoxP3 expression by the CD45.2+ B6 or CD25−/− and Thy1.1+ WT competitor T cells in the spleen (open bars), inguinal LNs (hatched bars), and mesenteric LNs (filled bars) was determined by flow cytometry. A Treg generation index was calculated as described in “Characterization of Treg populations during GVHD,” and the results are represented as the mean percentage of control ± SEM (n = 3 mice/group). One representative of 2 independent experiments is shown.
Discussion
In the present study, we have provided data demonstrating that, in addition to nTregs, the donor-derived Treg pool during acute GVHD is composed of iTregs that were converted in the recipient from donor Tconvs through a TGFβ– and IL-2–dependent mechanism. Using different combinations of T cells from WT and FoxP3-deficient mice as effectors, our data suggest that both donor-derived nTregs and the generation of iTregs in the recipient mice might contribute to protection against GVHD-induced weight loss. In addition to CD4+ iTregs, our data suggest that CD8+ Tregs with suppressive activity are a major contributor to the iTreg pool. Both iTreg subsets appeared to contribute to protection against GVHD-induced mortality. These data illustrate the complex makeup of the donor-derived Treg pool in allogeneic recipients.
The presence of both nTregs and iTregs appeared to be required for maximal disease attenuation, suggesting that the 2 distinct Treg subsets play nonredundant roles in protecting against Tconv-mediated attack of the recipient. Alternatively, both subsets might be required to stably achieve adequate numbers of Tregs to prevent allogeneic Tconv activation. The addition of nTregs increased the total Treg proportion by approximately 3-fold, which may explain the added benefit of the adoptively transferred nTregs. In support of this argument, anti–IL-21 Ab treatment in a GVHD model was shown to double the CD4+ iTreg fraction,19 which was sufficient to almost completely protect against GVHD-induced weight loss and mortality. In addition to Treg numbers, the instability of the iTreg population may also play a role, because CD8+ and CD4+ iTregs decreased significantly after day 21 after BMT. This notion is supported by a recent study demonstrating that adoptively transferred, in vitro–generated iTregs revert to Tconvs and fail to protect against lethal GVHD.33 Therefore, nTregs might be required to maintain a stable population of donor-derived Tregs in the allogeneic recipient. Although recipient-derived nTregs play a role in protection against GVHD in some settings,21,34 especially with less myeloablative regimens, we did not detect any host Tregs in our GVHD model.
The fraction of the iTregs that were generated in the allogeneic recipient was consistently in the 1%-2% range, which is less than what is thought to be required for Tconv suppression. It is possible that this small fraction of iTregs was still protective because of the biased TCR specificity of the iTregs. Because the iTregs were generated in response to allogeneic MHC stimulation in vivo, the iTregs most likely possess TCRs of relevant specificity, which could markedly enhance their efficacy in preventing allogeneic Tconv activation. Indeed, activation of Tregs by their cognate antigen during GVHD dramatically increases their effectiveness in preventing GVHD.35 However, it should be reiterated that whereas we attempted to equalize the activation potential of WT and Scurfy Tconvs by taking a mixed BM chimera approach, Scurfy Tconvs produced more IFNγ on TCR stimulation compared with WT Tconvs. Therefore, we cannot entirely exclude the possibility that the differences seen between GVHD induced by Scurfy Tconvs and that induced by WT Tconvs was due to inherent differences in their ability to induce GVHD rather than their inability to give rise to iTregs.
One unexpected finding in our analyses was that CD8+ Tregs comprised approximately 70% of the total iTreg pool during GVHD. These CD8+ Tregs, which are a minor component of the Treg pool in nontransplanted WT mice, displayed similarities to CD4+ Tregs and exhibited suppressive function toward Tconv proliferation. CD8+ T cells with inhibitory activity, originally coined as suppressor T cells, were described before CD4+ Tregs. The study of this cell type fell out of favor in the late 1980s partially because of the inability to map molecularly the restricting elements of this cell.36 Since the identification of FoxP3, interest in CD8+ Tregs has been resurrected, with several recent studies demonstrating their presence in infectious,37-39 neoplastic,40,41 and autoimmune settings.31,42 Although most studies have implied that the CD8+FoxP3+ T cells are iTregs, CD8+ Tregs can also found in the thymus, suggesting that CD8+ Tregs could also be nTregs.43 During GVHD, we found that the CD8+FoxP3+ cells were generated from CD8+FoxP3− T cells in the host and lacked expression of Helios, suggesting that the CD8+ Tregs were iTregs in this setting.
TGFβ has been shown to be a major factor in driving the conversion of Tconvs to iTregs in vitro.30-32 However, few studies have investigated the role of TGFβ in inducing iTreg formation in vivo. One obstacle in testing the involvement of TGFβR expression by Tconvs is that the T cell–specific manipulation of TGFβR results in perturbed development of the T cells.44,45 An inducible deletion system involving the CreT2 system enabled us to test the cell-autonomous role of this receptor in T cells that have developed normally. CD4+ and CD8+ Tconvs isolated from these mice did not express FoxP3 after stimulation with anti-CD3, IL-2, and TGFβ in vitro, suggesting that successful deletion of TGFβR had occurred in these cells. Using these T cells as effectors in GVHD, we found decreased iTreg formation in Tconvs lacking the TGFβR. However, iTreg formation by TGFβR2F/F Tconvs was not abolished completely, because the fraction of CD4+ and CD8+ iTregs among Tconvs lacking the TGFβR was reduced only by approximately 50%-70% and 20%-50%, respectively, compared with TGFβR2+/+ controls. It is possible that these FoxP3+ T cells represented an expansion of contaminating nTregs in our inoculum, which typically contained < 0.2% FoxP3+ Tregs after sorting (data not shown). Alternatively, factors other than TGFβ could have induced FoxP3 expression in Tconvs in a TGFβ-independent manner. This was especially true for CD8+ Tregs, in which the expansion of preexisting CD8+ nTregs did not contribute to the CD8+ Treg pool during GVHD (Figure 4). Therefore, factors other than TGFβ are also likely to contribute to CD8+ iTreg formation in vivo. Although IL-6 was reported to be important for the generation of CD8+ iTregs in a colitis model,31 anti–IL-6 Ab treatment did not alter CD8+ iTreg formation in the present study (data not shown), suggesting that IL-6 may not be required for CD8+ iTreg generation during GVHD.
A markedly reduced fraction of CD8+ and, to a lesser extent, CD4+ iTregs was detected when the Tconvs lacked expression of CD25. This signifies that iTregs are highly dependent on IL-2R signaling for their generation. Alternatively, IL-2 may be required for the stability of FoxP3 expression and the expansion of the newly formed iTregs.46 Consistent with this observation, the administration of IL-2 ICs increased the fraction of CD8+ and CD4+ iTregs in the spleen and inguinal LNs (Figure 7B). The reason that IL-2 ICs failed to increase mesenteric LN Tregs is unclear. Tconvs lacking CD25 gave rise to significantly fewer Tregs even in the mesenteric LNs, suggesting that these Tregs are also dependent on IL-2. Therefore, it is possible that either the IL-2 ICs have restricted access in these areas or that IL-2 is found at saturating amounts in the mesenteric LNs such that further IL-2 signaling is unable to augment Treg numbers. Although it is difficult to determine precisely how and where IL-2 works, it is clear that IL-2 signaling increases the number of Tregs relative to Tconvs, suggesting that it could potentially be used for therapy against GVHD. Indeed, although the mechanism was unclear at the time, earlier studies performed in the 1990s by Sykes et al demonstrated that IL-2 pretreatment prevents GVHD.47 More recently, IL-2 was shown to increase Treg numbers, which correlated with protection in mouse models of GVHD and in humans with chronic GVHD.48,49 Therefore, drugs that trigger or enhance the TGFβ and IL-2 signaling pathways could lead to an increase in Treg number and subsequent protection against GVHD.
In summary, in the present study, we have demonstrated that iTregs are generated during GVHD and may play a role in attenuating disease. Surprisingly, CD8+ iTregs with potent suppressive function were a major contributor to the total Treg pool. The generation of CD8+ and CD4+ iTregs occurred at least in part by a cell-autonomous IL-2R– and TGFβR-dependent mechanism. Further studies examining the involvement of other factors and signaling pathways in the generation of iTregs will be necessary for full therapeutic manipulation of iTreg formation during GVHD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Theresa Leichner and Awo Aboagye for reading the manuscript and the staff of the Koretzky, Nichols, Behrens, and Kambayashi laboratories for discussions.
This work was supported by grants from the National Blood Foundation, the American Society of Hematology, University of Pennsylvania internal funds, and the National Heart, Lung, and Blood Institute of the National Institutes of Health (R01HL107589, R01HL111501, and K08HL086503).
National Institutes of Health
Authorship
Contribution: N.S., A.S, A.M.S., I.T.L., and P.O. performed the experiments; Y.T. provided valuable reagents; N.S., A.S., and T.K. designed the research and analyzed the data; and T.K. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Taku Kambayashi, Division of Transfusion Medicine, Dept of Pathology and Laboratory Medicine, University of Pennsylvania, 288 John Morgan Bldg, 3620 Hamilton Walk, Philadelphia, PA 19104; e-mail: taku.kambayashi@uphs.upenn.edu.
References
Author notes
N.S. and A.S. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal