The report by Ribeiro et al in this issue of Blood confirms the evolving data that DNMT3A mutations represent another common alteration in adult acute myeloid leukemia (AML) and are an important modulator of outcome.1
Within the past 2 years, the invention of next-generation sequencing (NGS) has revealed a plethora of previously undescribed genetic abnormalities affecting several different pathways, including mutations in IDH1 and IDH2,2 and DNMT3A3,4 as well as numerous less common or even patient-specific abnormalities. Clarification of the prevalence and the prognostic impact of these changes has become a critical issue in how to identify the driver lesions and in deciding which factors should be added to the set of molecular abnormalities routinely tested.
In a large and well-characterized population of adult patients with AML, Ribeiro et al investigated the prevalence and prognostic impact of DNMT3A mutations. In this series, 96 of 415 patients (23%) carried a mutation of the gene, which ranks it among the most common changes in adult AML. DNMT3A mutations were predominantly found in patients with French-American-British (FAB) M4 and M5 morphology and were significantly associated with increased white cell counts at diagnosis. More importantly and in agreement with most studies published so far, patients with DNMT3A mutations were a median 10 years older than patients with wt-DNMT3A. DNMT3A mutations were mostly found in patients with cytogenetically normal (CN)–AML and occurred together with certain abnormalities enriched in this group, most importantly NPM1 and FLT3-ITD. Although DNMT3A mutations were not associated with a specific mRNA gene expression profile (GEP), the authors could show an enrichment of DNMT3A mutant samples in a cluster associated with a specific methylation pattern; however, this cluster was mostly characterized by NPM1 mutations.
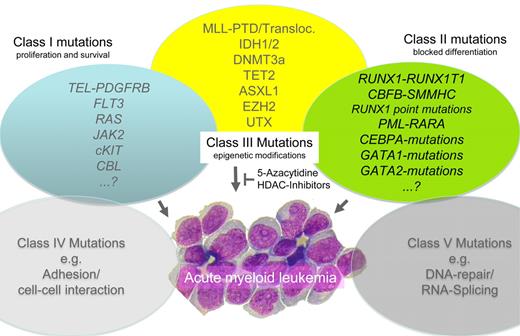
When analyzing the reported data for DNMT3A in adult AML (see table),1,3,5-10 several interesting aspects become evident. The mutation is found in approximately 15% to 25% of series reported from Europe and the United States1,3,5,9 ; however, the prevalence is somewhat lower in the 2 large unselected studies from Asia (7% and 14%),8,10 potentially indicating an effect of ethnic background. Given the association with CN-AML observed in all studies, it is not astonishing that the highest prevalence was reported in the 2 series focusing on CN-AML (29%–36%).6,7 Most reports agree in certain clinical aspects (increased patient age, high WBC) and all confirmed the association with FAB M4/M5-morphology. The important association with increased patient age is also evident in most series, which is in line with the very low prevalence in pediatric AML.11 In addition, the majority of studies could confirm the association with outcome, indicating that patients with DNMT3A mutations have a significantly shorter overall and disease-free survival. This appears not to be an effect of a decreased rate of complete remission, but reflects an increase in disease recurrence. On the biologic side, the correlation with other molecular changes (NPM1, FLT3-ITD) and the mutual exclusive mutational spectrum with genes directly involved in the regulation of DNA methylation (ie, TET2 and ASXL1) further strengthens the importance of epigenetics as a key molecular pathway for leukemic transformation (see figure). Because the majority of these mutations are associated with inferior outcome, the use of targeted treatment options (eg, demethylating agents such as 5-azacytidine or decitabine) specifically within this group of patients will be an important question for future studies. Despite the considerable number of studies reported, the impact of the location of the mutations within the DNMT3A gene is another open question. Approximately two-thirds of the mutations affect the catalytic domain, whereas the other 30% are distributed over the rest of the protein, clustering mostly in the zink finger and proline-tryptophane-tryptophane-proline (PWWP) domains.3 Because these types of mutations show considerable functional differences, their biologic consequences and potential differences in outcome observed in some studies6 but not confirmed in others,3 remain to be further clarified, which may become even more important in the context of targeted treatment strategies.
Published clinical studies on DNMT3A mutations
| . | Ley3 . | Thol9 . | Ribeiro1 . | Patel5 . | Shen10 . | Hou8 . | Marcucci6 . | Renneville7 . |
|---|---|---|---|---|---|---|---|---|
| Patients analyzed (N) | 281 | 489 | 415 | 398 | 1141 | 500 | 415 | 123 |
| DNMT3A mut (N) | 62 | 87 | 96 | 89 | 75 | 70 | 148 | 36 |
| DNMT3A mut (%) | 22 | 18 | 23 | 22 | 7 | 14 | 36 | 29 |
| AML population (all adult) | all | < 60/de novo | < 60 | < 60 | de novo | de novo | CN/de novo | CN, < 60/de novo |
| DNMT3A region analyzed | complete | E15–23 | E11–23 | complete | complete | E2–23 | E15–23 | E8–9/E11–23 |
| Median age, y (mut/wt) | 53/48* | 52/45*** | 51/41*** | ng | 54/38*** | 61/49*** | 61/62 | 48/47 |
| Median WBC (Gpt/l) (mut/wt) | 59/39*** | 38/17** | 53/23*** | ng | 38/7*** | 32/16*** | 43/22*** | 13/11 |
| Median BM blasts (%) (mut/wt) | 70/70 | 78/70* | 68/68 | ng | 78/65*** | ng | 58/57 | ng |
| FAB | M4/M5 | M4/M5 | M4/M5 | M4/M5 | M4/M5 | M4/M5 | M4/M5 | M4/M5 |
| Median CR rate (%) (mut/wt) | ng | 71/76 | 89/79 | ng | 46/62* | ns | ns | 90/80 |
| Median OS (mo mut/wt) | 12/41*** | 21/40* | 12/24 | 14/21 | P < .001 | 15/38 | P = .07 | 23/45* |
| Median DFS/RFS (mo mut/wt) | ng | P = .2 | ng | ng | P < .001 | 8/15 | P = .03 | ng |
| CN-AML (%) | 72*** | 82*** | 75*** | ng | ng | 51 | only CN | only CN |
| NPM1 mut (%)† | 60 | 64 | 76 | 64 | 54 | 75* | 67 | |
| FLT3-ITD (%)† | 41 | 39 | 41 | ng | 43 | 44* | 19 | |
| CEBPA mut (%)† | ng | ng | 3 | 7 | 4 | 5*** | 0 | |
| MLL-PTD (%)† | ng | ng | ng | 6 | 9 | 6 | ng | |
| TET2 mut (%)† | ng | ng | ng | 7 | 9 | 22 | ng |
| . | Ley3 . | Thol9 . | Ribeiro1 . | Patel5 . | Shen10 . | Hou8 . | Marcucci6 . | Renneville7 . |
|---|---|---|---|---|---|---|---|---|
| Patients analyzed (N) | 281 | 489 | 415 | 398 | 1141 | 500 | 415 | 123 |
| DNMT3A mut (N) | 62 | 87 | 96 | 89 | 75 | 70 | 148 | 36 |
| DNMT3A mut (%) | 22 | 18 | 23 | 22 | 7 | 14 | 36 | 29 |
| AML population (all adult) | all | < 60/de novo | < 60 | < 60 | de novo | de novo | CN/de novo | CN, < 60/de novo |
| DNMT3A region analyzed | complete | E15–23 | E11–23 | complete | complete | E2–23 | E15–23 | E8–9/E11–23 |
| Median age, y (mut/wt) | 53/48* | 52/45*** | 51/41*** | ng | 54/38*** | 61/49*** | 61/62 | 48/47 |
| Median WBC (Gpt/l) (mut/wt) | 59/39*** | 38/17** | 53/23*** | ng | 38/7*** | 32/16*** | 43/22*** | 13/11 |
| Median BM blasts (%) (mut/wt) | 70/70 | 78/70* | 68/68 | ng | 78/65*** | ng | 58/57 | ng |
| FAB | M4/M5 | M4/M5 | M4/M5 | M4/M5 | M4/M5 | M4/M5 | M4/M5 | M4/M5 |
| Median CR rate (%) (mut/wt) | ng | 71/76 | 89/79 | ng | 46/62* | ns | ns | 90/80 |
| Median OS (mo mut/wt) | 12/41*** | 21/40* | 12/24 | 14/21 | P < .001 | 15/38 | P = .07 | 23/45* |
| Median DFS/RFS (mo mut/wt) | ng | P = .2 | ng | ng | P < .001 | 8/15 | P = .03 | ng |
| CN-AML (%) | 72*** | 82*** | 75*** | ng | ng | 51 | only CN | only CN |
| NPM1 mut (%)† | 60 | 64 | 76 | 64 | 54 | 75* | 67 | |
| FLT3-ITD (%)† | 41 | 39 | 41 | ng | 43 | 44* | 19 | |
| CEBPA mut (%)† | ng | ng | 3 | 7 | 4 | 5*** | 0 | |
| MLL-PTD (%)† | ng | ng | ng | 6 | 9 | 6 | ng | |
| TET2 mut (%)† | ng | ng | ng | 7 | 9 | 22 | ng |
ng indicates not given.
P < .05;
P < .01; and
P < .001.
For DNMT3A mutation patients.
Although these data shed important light on the biology of AML, the individual patient picture might actually be more obscure. Because NGS data of several AML patients indicate that typically 8 to 12 somatic mutations can be found per genome in CN-AML,2,3 several additional pathways might be affected (eg, DNA repair or cellular migration). Thus, the current scheme of least 3 major pathways affected in adult AML, that is, proliferation, differentiation and epigenetic modification, might need further extension in the near future (see figure).
Conflict-of-interest disclosure: The author declares no competing financial interests. ■