Abstract
Involution of the thymus results in reduced production of naive T cells, and this in turn is thought to contribute to impaired immunity in the elderly. Early T-cell progenitors (ETPs), the most immature intrathymic T-cell precursors, harvested from the involuted thymus exhibit a diminished proliferative potential and increased rate of apoptosis and as a result their number is significantly reduced. In the present study, we show that these age-induced alterations result in part from increased expression of the Ink4a tumor-suppressor gene in ETPs. We also show that repression of Ink4a in aged ETPs results in their partial rejuvenation and that this can be accomplished by in vivo fibroblast growth factor 7 administration. These results define a genetic basis for thymocyte progenitor aging and demonstrate that the senescence-associated gene Ink4a can be pharmacologically repressed in ETPs to partially reverse the effects of aging.
Introduction
T-cell development in the thymus is a highly regulated process in which immature, BM derived progenitors progress through various intermediate stages before generating mature T lymphocytes.1,2 Early T-cell progenitors (ETPs), defined by their lineage-negative (Lin−) CD44+CD25−CD117(c-kit)high phenotype,3 are thought to be the most immature intrathymic population. ETPs in turn differentiate into the double-negative (DN) progeny DN2, DN3, and DN4 in which TCR rearrangements occur. The latter cells then generate CD4+CD8+ double-positive thymocytes that mature into CD4+ or CD8+ single-positive T lymphocytes.
Declines in thymopoiesis can result from various physiologic and pathologic processes, and this may ultimately contribute to immunodeficiency and increased susceptibility to life-threatening infections. For example, a hallmark of immune system aging is involution of the thymus, resulting in a reduced production of naive T cells that has been proposed to underlie, at least in part, the impaired immunity observed in the elderly.4,5 The causes of thymus involution are multifactorial and complex,6 but the effects are manifest in 2 distinct cellular compartments. First, it is known that thymic epithelial cells that constitute a key element of the thymus microenvironment decline in number with age.7 In addition, the number of ETPs in the aged thymus is significantly reduced because of their diminished proliferative potential and increased rate of apoptosis.8,9
A decline in proliferation is a hallmark of stem and progenitor cell aging in multiple tissues, and recent studies have implicated the p16Ink4a and p19Arf tumor-suppressor proteins, which are encoded by the Cdkn2a locus, in this process.10,11 Expression of Ink4a results in activation of retinoblastoma, which in turn leads to inhibition of cell-cycle progression, and p19Arf promotes the activity of p53, which induces cell-cycle arrest and/or apoptosis.12-14 Evidence from loss- and gain-of-function studies indicates that Ink4a is an important regulator of thymopoiesis. For example, thymus size is increased in Ink4a-deficient mouse strains,15,16 and mice engineered to express Ink4a under the control of an lck promoter have a block in T-cell differentiation at the DN3 stage.17 More recently, Liu et al conditionally deleted Ink4a in thymocytes by mating mice with a floxed Ink4a allele to lck-Cre mice and demonstrated that thymic involution was delayed significantly.18 Whereas these studies established a role for Ink4a in thymopoiesis and thymic involution, the precise stage(s) of thymocyte development in which Ink4a and Arf are expressed during aging has not been established.
Identification of the genetic programs responsible for thymocyte progenitor aging is relevant to the design of strategies to rejuvenate the involuted thymus. There has been considerable interest in devising a way to do so in view of the key role of T cells in the immune response.6 With this goal in mind, the potential of various agents has been evaluated in both preclinical studies and clinical trials, and these fall into 3 general categories. Some, like IL-7,19 bind to receptors expressed primarily on lymphoid progenitors in the BM and thymus. Others, including growth hormone,20 insulin-like growth factor 1 (IGF-1),21 and androgen inhibitors,22 have more widespread effects that may include actions on lymphocyte progenitors and components of the hematopoietic microenvironment. A third category, typified by fibroblast growth factor 7 (Fgf7; also referred to as keratinocyte growth factor), binds to receptors expressed on thymic stromal cells but not thymocytes.23-25 Of the above factors, Fgf7 is particularly effective, because thymus cellularity in old mice administered multiple rounds of Fgf7 is restored to levels observed in the young.24
The goals of the present study were to define the stages of thymopoiesis in which expression of Ink4a and/or Arf occurs during aging and to determine whether their expression can be targeted pharmacologically. We report herein that age-related increases in Ink4a, but not Arf, expression occur in ETPs and their downstream DN progeny, contributing to the impaired proliferation of these immature thymocyte progenitors during aging. Using Fgf7 as an example, we also demonstrate the potential of pharmacologic mediators in modulating the expression of Ink4a and other cell-cycle regulators in aging T-cell progenitors.
Methods
Mice
Four- to 8-week-old C57BL/6J (B6) mice were obtained from the Division of Laboratory Animal Medicine of the University of California, Los Angeles (UCLA). Eighteen-month-old B6 mice were purchased from the National Institute on Aging (National Institutes of Health [NIH], Bethesda, MD). Timed pregnant Swiss Webster mice were purchased from Taconic Farms. Animals were housed in the UCLA Division of Laboratory Animal Medicine, and experiments were conducted according to UCLA Institutional Animal Care and Use Committee guidelines.
In vivo Fgf7 treatment
Each round of Fgf7 treatment consisted of 3 daily injections of 5 mg/kg/d of recombinant human Fgf7 (a generous gift from Biovitrum) solubilized in Ca2+- and Mg2+-free PBS; control animals received vehicle (PBS) only. In some experiments, multiple rounds of Fgf7 treatment were administered monthly according to this schedule.
Immunofluorescence and flow cytometry
ETPs and DN thymocyte subpopulations were resolved as described previously in detail.8 Abs to CD3ϵ (145-2C11), CD8α (53-6.7), NK-1.1 (PK136), TCRβ (H57-597), TCRγδ (UC7-13D5), Gr-1 (RB6-8C5), CD11b (M1/70), CD45R (B220; RA3-6B2), and TER-119 (TER119), all from eBiosciences, and goat anti–mouse IgM (Southern Biotechnology) were used to define lineage-positive cells. Thymocyte subpopulations were analyzed on an LSRII or sorted using a FACSAria flow cytometer (BD Biosciences) located in the Broad Stem Cell Research Center and the Jonsson Cancer Center Flow Cytometry Core at UCLA, respectively.
qRT-PCR
For quantitative RT-PCR (qRT-PCR), RNA was extracted with the RNeasy Plus micro- or minikit and cDNA was synthesized with the RT2 First Strand kit (both from QIAGEN). Reactions were run in 25 μL with SYBR Green qPCR Master Mix (Bio-Rad) as recommended by the manufacturer. The presence of single RT-PCR products was confirmed by melt curve analysis. Data were analyzed with Bio-Rad IQ5 2.0 software using the Pfaffl method with β-actin or gapdh as a reference gene. Amplification efficiencies were routinely found to be between 95% and 105%. All reactions were run at least twice in duplicate. Ink4a primer sequences were as follows: GTGTGCATGACGTGCGGG (forward) and GCAGTTCGAATCTGCACCGTAG (reverse). Arf primer sequences were as follows: GCTCTGGCTTTCGTGAACATG (forward) and TCGAATCTGCACCGTAGTTGAG (reverse). qRT-PCR primer sets for Bmi1, Ets1, Ets2, Id1, Hmga2, Ccnd1, Egr1, Gsk3b, β-Actin, Ezh2, and gapdh were purchased from QIAGEN. Let7b and Sno202 expression was assessed using TaqMan MicroRNA assays from Applied Biosystems. The RT2 profiler PCR array PAMM-050A-2 from QIAGEN was used to assess mouse senescence gene expression in sorted ETPs. The complete list of genes tested can be found at http://www.sabiosciences.com/ArrayList.php. RT2 Profiler PCR Array results were analyzed with RT2 Profiler PCR Array Version 3.5 data analysis software located on the QIAGEN Web site.
Retroviral transduction
The wells of 24-well plates were precoated overnight at 4°C with 32 μg/mL of RetroNectin (Takara Shuzo), 0.4 mL of retroviral supernatant was added to each retronectin-coated well, plates were centrifuged at 700g for 2 hours at 30°C. Next, 3-8 × 103 ETPs were added per well in RPMI 1640 medium containing 10% FCS, 5 × 10−5M 2ME, 1mM l-glutamine, 100 U/mL of streptomycin, 100 μg/mL of penicillin, and 50 μg/mL of gentamycin supplemented with 10 ng/mL of IL-7 and Flt3 ligand (Biosource International), and cultures were placed in a humidified 37°C, 5% CO2/air incubator. An additional 0.4 mL of retroviral supernatant was added to the cultures at 18 hours.
After 48 hours of culture in these conditions, the transduced cells were harvested and transferred either to fetal thymic organ culture (FTOC) or seeded over OP9-Δ1 stromal cells (a generous gift of Dr J. C. Zuñiga-Pflücker, University of Toronto, Toronto, ON). Ink4a/GFP was a generous gift of Dr Charles Sherr (St Jude Children's Research Hospital, Memphis, TN) and Ink4a/GFP shRNA was a generous gift from Dr Scott Lowe (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY) and both were used as described previously.26
Thymocyte cultures
Thymocytes were either seeded onto confluent OP9-Δ1 stromal cells in RPMI medium supplemented with 5% FCS and 5 × 10−5M 2-ME or placed in FTOC. Thymic lobes were harvested from 15-day-old Swiss Webster mouse embryos and depleted of endogenous thymocytes by treatment with deoxyguanosine (Sigma-Aldrich) for 1 week, as described previously.8 Ten to 15 lobes per treatment were preloaded with 200-300 cells/lobe in hanging-drop cultures for 48 hours, followed by culture on nucleopore filters for 14 days. Thymocytes were recovered by mechanical disruption of the thymic lobes.
Statistical analysis
Unless indicated otherwise, data are expressed as means ± SD or SEM, as indicated in the figure legends. Differences between groups were determined by a 2-tailed, unpaired Student t test (α = 0.05).
Results
Expression of Ink4a, but not Arf, is increased in aging thymocyte progenitors
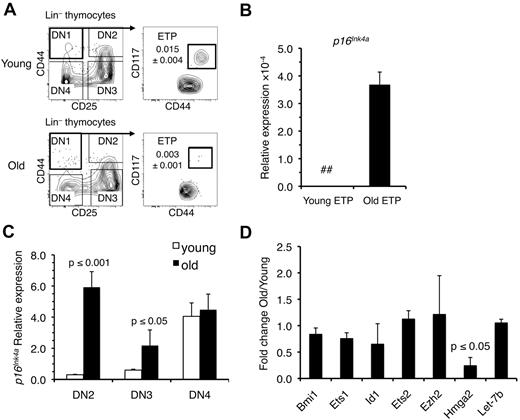
To determine whether increases in Ink4a and Arf expression are associated with the reduced number of ETPs in the involuted thymus (Figure 1A), we harvested these progenitors from young (4 weeks) and old (18 months) B6 mice and examined their Ink4a and Arf expression. The results demonstrated that Ink4a was expressed at increased levels in ETPs from old mice (Figure 1B). However, Arf was not detected in ETPs at any age (data not shown). We also examined ETP progeny and observed significant increases in Ink4a expression in old DN2 and DN3 thymocytes, whereas no differences were observed between young and old DN4 cells (Figure 1C). Although low levels of Arf could be detected in DN2, DN3, and DN4 cells from young and old mice, differences in expression between these populations were not significant (data not shown).
Ink4a, but not Arf, is up-regulated in old ETPs. (A) Representative immunostaining profiles used to purify ETPs and DN2, DN3, and DN4 thymocytes from young and old mice. The frequency of ETPs in young and old thymi is indicated. (B) Expression of Ink4a relative to gapdh in ETPs from young (4-6 weeks) and old (18-20 months) mice. Values are reported as the mean ratio of expression in old versus young cells. The mean ± SEM based on 3 independent experiments is shown. (C) Changes in expression of Ink4a relative to gapdh in the indicated DN populations. Values are reported as the mean ratio of expression in old versus young cells. The mean ± SEM based on 3 independent experiments is shown. (D) Expression of Bmi-1, Ets-1, Id-1, Ets-2, Ezh2, and Hmga2 relative to gapdh and Let-7b relative to Sno202 in ETPs. Values are reported as the mean ratio of expression in old versus young cells. The mean ± SEM based on 3 independent experiments is shown. The P values are based on comparison of relative levels between old and young cells. ## indicates not detectable.
Ink4a, but not Arf, is up-regulated in old ETPs. (A) Representative immunostaining profiles used to purify ETPs and DN2, DN3, and DN4 thymocytes from young and old mice. The frequency of ETPs in young and old thymi is indicated. (B) Expression of Ink4a relative to gapdh in ETPs from young (4-6 weeks) and old (18-20 months) mice. Values are reported as the mean ratio of expression in old versus young cells. The mean ± SEM based on 3 independent experiments is shown. (C) Changes in expression of Ink4a relative to gapdh in the indicated DN populations. Values are reported as the mean ratio of expression in old versus young cells. The mean ± SEM based on 3 independent experiments is shown. (D) Expression of Bmi-1, Ets-1, Id-1, Ets-2, Ezh2, and Hmga2 relative to gapdh and Let-7b relative to Sno202 in ETPs. Values are reported as the mean ratio of expression in old versus young cells. The mean ± SEM based on 3 independent experiments is shown. The P values are based on comparison of relative levels between old and young cells. ## indicates not detectable.
Multiple genetic regulators of Ink4a expression have been defined, including Bmi-1.27 We previously demonstrated decreased expression of Bmi-1 and increased levels of Ink4a in pro-B cells from old mice.26 To determine whether elevated Ink4a expression in aging T-cell progenitors was similarly correlated with reduced Bmi-1 expression, we compared Bmi-1 levels in young and old ETPs. In contrast to what occurs in aging B-cell progenitors, Bmi-1 levels did not decline with age in ETP (Figure 1D). This result prompted us to examine expression of other regulators of Ink4a that included Ets-1, Ets-2, Id-1,28 Hmga2,29-31 and Ezh2.32 Only levels of Hmga2 were significantly reduced in old ETPs (Figure 1D). Because Hmga2 expression can be repressed by Let7b microRNA, we examined its expression in young and old ETPs. Let7b levels remained constant between young and old ETPs (Figure 1D), even though its expression has been reported to increase with age in some tissues.33
Ink4a is a negative regulator of ETP proliferation
Our results thus far indicated that Ink4a is a biomarker of T cell–progenitor aging. To investigate whether the expression of Ink4a induces an aging phenotype in immature thymocytes, we isolated ETPs from young, 4- to 8-week-old mice and transduced them with an Ink4a/GFP-containing retroviral vector.
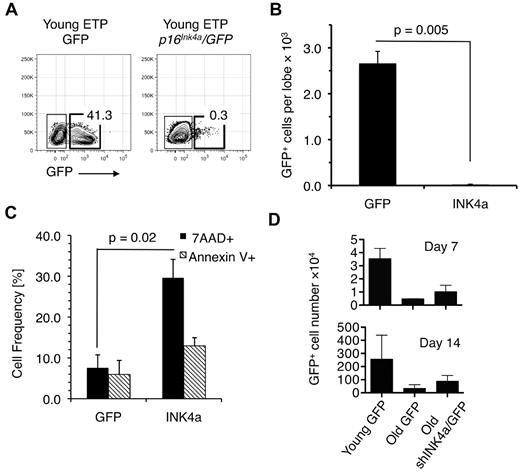
The GFP-only– or Ink4a/GFP-expressing cells were then seeded into fetal thymic lobes that were placed in FTOC for 2 weeks. Whereas ETPs transduced with GFP-only vectors expanded in FTOC (Figure 2A-B), Ink4a/GFP-transduced cells failed to grow, likely because they underwent cell-cycle arrest and apoptosis. The fact that Ink4a-transduced cells harvested 7 days after seeding on OP9-Δ1 stromal cells (which have been shown to support early T-cell development in vitro34 ) exhibited significantly higher levels of death compared with the green fluorescent protein (GFP)–expressing control populations is consistent with this conclusion (Figure 2C). These results indicate that increased expression of Ink4a in young ETPs results in their diminished proliferative potential and increased apoptosis, and that Ink4a is both a biomarker and effector of T-lineage aging.
Ectopic expression of Ink4a in young ETPs causes reduced cell production. (A) Representative FACS plots showing that young ETPs transduced with Ink4a/GFP did not generate thymocytes in fetal thymic cultures compared with young ETPs transduced with GFP alone. Plots are representative of 3 independent experiments. (B) Number of GFP- and p16Ink4a/GFP-expressing thymocytes recovered from the fetal thymic cultures shown in panel A. (C) Frequencies of 7-amino-actinomycin D+ (dead) and annexin V+ (apoptotic) cells in cell suspensions harvested from cultures established by seeding Ink4a/GFP- or GFP-only–transduced young ETPs on OP9-Δ1 stromal cells 2 weeks after seeding. Average frequencies ± SEM are based on data pooled from 3 independent experiments. (D) GFP+ cell recovery from cultures established by seeding young (4-6 weeks) and old (18 months) ETPs transduced with shInk4a/GFP- or GFP-only–containing retroviral vectors onto OP9-Δ1 stromal cell layers. Cell recovery was determined 7 and 14 days after seeding. The mean ± SEM based on 3 independent experiments is shown.
Ectopic expression of Ink4a in young ETPs causes reduced cell production. (A) Representative FACS plots showing that young ETPs transduced with Ink4a/GFP did not generate thymocytes in fetal thymic cultures compared with young ETPs transduced with GFP alone. Plots are representative of 3 independent experiments. (B) Number of GFP- and p16Ink4a/GFP-expressing thymocytes recovered from the fetal thymic cultures shown in panel A. (C) Frequencies of 7-amino-actinomycin D+ (dead) and annexin V+ (apoptotic) cells in cell suspensions harvested from cultures established by seeding Ink4a/GFP- or GFP-only–transduced young ETPs on OP9-Δ1 stromal cells 2 weeks after seeding. Average frequencies ± SEM are based on data pooled from 3 independent experiments. (D) GFP+ cell recovery from cultures established by seeding young (4-6 weeks) and old (18 months) ETPs transduced with shInk4a/GFP- or GFP-only–containing retroviral vectors onto OP9-Δ1 stromal cell layers. Cell recovery was determined 7 and 14 days after seeding. The mean ± SEM based on 3 independent experiments is shown.
It is likely that the levels of In4ka expression achieved in these transduction experiments are far above those observed in old T-cell progenitors. We therefore used a loss-of-function approach in which ETPs isolated from old mice were transduced with a previously validated Ink4a/GFP shRNA26 or GFP-only–containing retroviral vectors to determine whether down-regulation of Ink4a expression would restore their proliferative potential. Cells were then seeded on OP9-Δ1 stromal cells and examined 1 and 2 weeks later. As shown in Figure 2D, the number of cells harvested from cultures established with Ink4a shRNA-transduced old ETPs was approximately 2-fold higher than that in cultures established with old ETPs transduced with the GFP-only vector at the 1week time point. After 2 weeks of culture, approximately 1 million cells were recovered from cultures seeded with shRNA-transduced cells, whereas those seeded with cells transduced with GFP-only–containing vectors contained approximately 5-fold fewer cells. However, cell production in cultures initiated with Ink4a shRNA-transduced cells did not reach levels observed with young ETPs. This may be because Ink4a levels in the former population, which had now matured to the DN2-DN4 stages of development, had been reduced by only 20% compared with levels in DN cells directly harvested from old thymus. This level of Ink4a down-regulation was observed consistently in each of 3 independent experiments. Nevertheless, these results indicate that Ink4a contributes to the age-related decline in ETP proliferation and that these aging deficits can be partially reversed.
Fgf7 treatment increases the number of ETPs and DN cells in the involuted thymus
There has been considerable interest in using various hormones and growth factors to reverse thymic involution, and one of these, Fgf7, has been shown to be particularly effective in stimulating thymopoiesis and increasing the number of ETPs in young mice.25 Consistent with these previous findings, in the present study, a single round of Fgf7 administration increased the total number of thymocytes in old mice, and this effect was further accentuated with 3 rounds of treatment (Figure 3A). However, whether this occurs because Fgf7 increases the number of ETPs in the involuted thymus has not been examined.
The number and proliferation of ETPs and DN thymocytes in the involuted thymus is increased after Fgf7 treatment. (A) Mean ± SD number of cells in the thymi of 15- to 18-month-old mice 15 days after 1 round (Fgf7 × 1, n = 8 mice) or 3 rounds (Fgf7 × 3, n = 6 mice) of Fgf7 or PBS treatment. The average number of total cells in the thymi of young (4-6 weeks) mice is shown for comparison. (B) Average ± SD number of ETPs in the thymi of the mice shown in panel A. (C) Frequency of Ki-67–expressing ETPs in the thymi of 15- to 18-month-old Fgf7-treated mice described in panel A. (D) Mean ± SD number of DN2, DN3, and DN4 cells in the thymi of the mice described in panel A. (E) Frequency of Ki-67–expressing DN2, DN3, and DN4 cells in the thymi of mice described in panel A. Mean ± SD values presented above are based on data pooled from 4 independent experiments. The P values are based on comparison between cells isolated from Fgf7- and PBS-treated old mice.
The number and proliferation of ETPs and DN thymocytes in the involuted thymus is increased after Fgf7 treatment. (A) Mean ± SD number of cells in the thymi of 15- to 18-month-old mice 15 days after 1 round (Fgf7 × 1, n = 8 mice) or 3 rounds (Fgf7 × 3, n = 6 mice) of Fgf7 or PBS treatment. The average number of total cells in the thymi of young (4-6 weeks) mice is shown for comparison. (B) Average ± SD number of ETPs in the thymi of the mice shown in panel A. (C) Frequency of Ki-67–expressing ETPs in the thymi of 15- to 18-month-old Fgf7-treated mice described in panel A. (D) Mean ± SD number of DN2, DN3, and DN4 cells in the thymi of the mice described in panel A. (E) Frequency of Ki-67–expressing DN2, DN3, and DN4 cells in the thymi of mice described in panel A. Mean ± SD values presented above are based on data pooled from 4 independent experiments. The P values are based on comparison between cells isolated from Fgf7- and PBS-treated old mice.
As shown in Figure 3B, 1 round of Fgf7 treatment increased the number of ETPs in the involuted thymus significantly. There was also a slight increase in the frequency of ETPs (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Further increases in ETP number were observed after 3 rounds of drug administration (Figure 3B), although their total number still remained below that in young mice. This expansion of ETPs was likely because of their increased proliferation, because the number of Ki-67–expressing ETPs was increased significantly (Figure 3C).
DN thymocytes undergo extensive expansion before acquisition of the CD4 and CD8 cell-surface determinants and expression of the TCR.35 Therefore, we examined the effects of Fgf7 treatment on DN2, DN3, and DN4 cells from old mice. As shown in Figure 3D, the number of DN2, DN3, and DN4 cells was significantly increased after Fgf7 treatment and, as observed with ETPs, was correlated with a significant increase in the number of proliferating DN cells (Figure 3E). However, the frequencies of DN2, DN3, and DN4 cells were not changed by Fgf7 treatment (supplemental Figure 1B).
Ink4a expression is repressed in ETPs from Fgf7 treated old mice
In view of the results described in the previous section, we considered the possibility that Fgf7 treatment resulted in a repression of Ink4a expression in ETPs and DN cells, allowing their increased proliferation and survival. ETPs and their downstream DN2, DN3, and DN4 progeny were harvested from old mice 15 days after the cessation of a single round of Fgf7 treatment and levels of Ink4a were measured. This analysis showed that Ink4a expression was decreased in ETPs (Figure 4A) and in DN2, DN3, and DN4 thymocytes (Figure 4B). In addition, we observed increased expression of cell cycle–promoting genes such as Cyclin D1 (Ccnd1), which is downstream of Ink4a,12 and its transcriptional activator early growth response protein 1 (Egr-1)36 and reduced expression of serine/threonine kinase and glycogen synthase kinase-3b (Gsk3b), which triggers cyclin D1 proteasomal degradation37 (Figure 4C-D).
Expression of Ink4a is down-regulated in ETPs isolated from old Fgf7-treated mice. Levels of Ink4a expression in ETPs (A) and the indicated DN populations (B) after Fgf7 treatment. Cells were isolated from 15- to 18-month-old mice 15 days after 1 round of Fgf7 treatment and gene expression was examined by qRT-PCR. (C) Expression of cell cycle–related genes in ETPs from the mice described in panel A as determined with the mouse cellular senescence RT2 PCR array PAMM-050A-2 (QIAGEN). (D) The changes in gene expression observed with the array were validated by qRT-PCR. P values for the differences in expression levels between ETPs isolated from Fgf7- and PBS-treated mice are indicated. All experiments were performed in triplicate and data are expressed as means ± SD. ## indicates not detectable.
Expression of Ink4a is down-regulated in ETPs isolated from old Fgf7-treated mice. Levels of Ink4a expression in ETPs (A) and the indicated DN populations (B) after Fgf7 treatment. Cells were isolated from 15- to 18-month-old mice 15 days after 1 round of Fgf7 treatment and gene expression was examined by qRT-PCR. (C) Expression of cell cycle–related genes in ETPs from the mice described in panel A as determined with the mouse cellular senescence RT2 PCR array PAMM-050A-2 (QIAGEN). (D) The changes in gene expression observed with the array were validated by qRT-PCR. P values for the differences in expression levels between ETPs isolated from Fgf7- and PBS-treated mice are indicated. All experiments were performed in triplicate and data are expressed as means ± SD. ## indicates not detectable.
ETPs from Fgf7-treated mice exhibit impaired differentiation
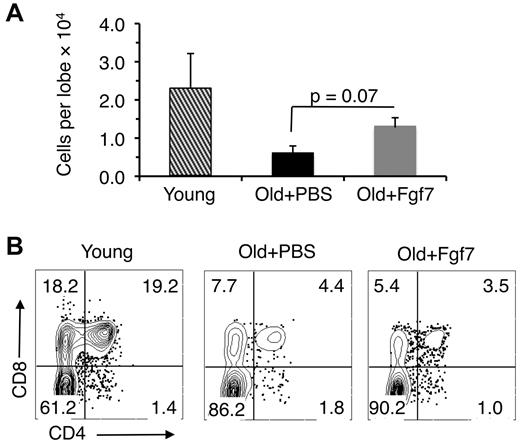
The results described in the previous section indicate that Fgf7 treatment can partially reverse declines in proliferation exhibited by old ETPs. In addition to proliferative defects, ETPs from old mice do not differentiate as efficiently as their young counterparts,8 but whether Fgf7 treatment reverses this deficiency has not been evaluated previously. To do so, ETPs were harvested from mice treated previously with 1 round of Fgf7 and seeded into FTOC. Cells in the lobes were then enumerated and phenotyped 2 weeks later.
Consistent with the in vivo data shown in Figure 3, higher numbers of thymocytes were recovered from lobes seeded with ETPs from Fgf7-treated old mice than from lobes seeded with ETPs from PBS-treated control animals (Figure 5A). However, ETPs obtained from the old Fgf7-treated mice did not differentiate into CD4- and/or CD8-expressing cells with the same efficiency as young ETPs (Figure 5B). These data indicate that whereas Fgf7 treatment partially reverses age-related deficiencies in proliferation, it does not restore the differentiation potential of aged ETPs.
Fgf7 does not correct age-related declines in differentiation. (A) Cell recovery from fetal thymic organ cultures 2 weeks after seeding with ETPs isolated from 15- to 18-month-old Ffg7-treated mice. Cells were harvested from thymic lobes 15 days after treatment with 1 round of Fgf7. Average ± SD total number of cells shown is pooled from 3 independent experiments. (B) Representative FACS plots from 1 of the experiments described in panel A showing CD4 and CD8 expression by cells harvested from the fetal thymic lobes.
Fgf7 does not correct age-related declines in differentiation. (A) Cell recovery from fetal thymic organ cultures 2 weeks after seeding with ETPs isolated from 15- to 18-month-old Ffg7-treated mice. Cells were harvested from thymic lobes 15 days after treatment with 1 round of Fgf7. Average ± SD total number of cells shown is pooled from 3 independent experiments. (B) Representative FACS plots from 1 of the experiments described in panel A showing CD4 and CD8 expression by cells harvested from the fetal thymic lobes.
Discussion
Involution of the thymus is the most well-recognized age-related change in the hematopoietic system. Whereas multiple events contribute to this process, there is strong evidence that the ability of BM38 and intrathymic progenitors8 to generate T lymphocytes becomes increasingly impaired with age. These declines are cell autonomous even if they were triggered by external influences such as changes in the aging microenvironment, because ETPs from the involuted thymus still exhibit impaired T-lymphopoietic potential when they are transferred to a young environment.8 One goal of the present study was to identify the genetic alterations that contribute to this aging phenotype.
Particular focus was placed on Ink4a and Arf, because an age-related increase in expression of both of these tumor-suppressor genes has been reported in numerous tissues,13 and we observed previously that this was also the case in the B-cell lineage.26 Our present results demonstrate that expression of Ink4a is increased significantly with age in ETPs ands in DN2 and DN3 thymocytes. However, in contrast to what we observed in aging pro-B cells,26 no significant increases in Arf expression were observed in old ETPs or their DN progeny. The regulation of Ink4a expression in aging B- and T-cell progenitors also appears to differ. Whereas loss of Bmi-1 was shown to be correlated with increased expression of Ink4a in pro-B cells from old mice,26 its levels remained constant in old ETPs. The precise basis for the age-related decline in Ink4a expression in ETPs and DN cells is unclear. Of the various Ink4a regulators examined, only expression of Hmga2 was reduced in old ETPs. However, whether this reduction is the basis for increased Ink4a expression remains to be determined.
The results of the present study establish Ink4a as a biomarker of thymocyte progenitor aging that may be useful in measuring the efficacy of antiaging therapies. In addition, the data suggest that p16Ink4a is also a mediator of that process. Overexpression of Ink4a impaired the proliferation of young ETPs, and down-regulation of Ink4a in old ETPs resulted in enhanced growth. The ability to significantly delay thymic involution by conditionally deleting Ink4a in thymocytes provides further support for its involvement in T-lineage aging.18 However, the thymus of these mice ultimately underwent involution. This may have occurred because expression of lck used to drive Cre expression is most robust starting at the DN2 stage of development,2 and the deleterious effects of Ink4a expression in ETP function still occur. Factors in addition to Ink4a that contribute to thymic involution are likely to be operative.
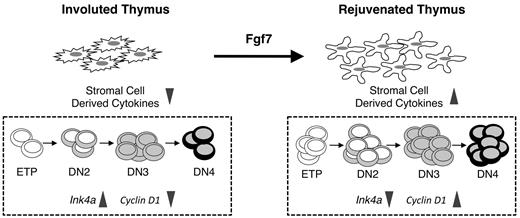
Our data support a model in which Fgf7 treatment results in down-regulation of Ink4a in thymocyte progenitors and partial restoration of ETP and DN proliferation (Figure 6). However, because the Fgf7 receptor (FγfR2IIIb) is expressed by thymic epithelial cells and not by thymocytes,24,25 the precise mechanisms by which Fgf7 affects thymocyte progenitors remain to be defined. Fgf7 is known to stimulate the secretion of various cytokines by thymic epithelial cells.25 Therefore, one possibility is that these factors bind to ETPs and DN cells and promote down-regulation of Ink4a expression in them (Figure 6). Interestingly, IGF-1, which stimulates thymocyte proliferation, may act through a similar mechanism.21 Alternatively, aged thymic epithelial cells may acquire a senescence-associated secretory phenotype (SASP)39,40 and Fgf7 may alter it. The SASP is characterized by the increased production of inflammatory mediators that can subsequently induce senescence in surrounding cells. Therefore, such mediators secreted by aged thymic stromal cells could contribute to thymic involution by inducing Ink4a expression in thymocytes, and Fgf7 may in turn reverse this process. Interestingly, Fgf7 has been reported to reduce the expression of inflammatory mediators in the thymus of mice undergoing acute GVHD.41 Whether suppression of the SASP results in decreased Ink4a expression in ETPs remains to be determined.
Model for Fgf7 actions in the involuted thymus. (Left) The number and quality of thymic epithelial cells are reduced in the involuted thymus. As a result, the production of epithelial-derived thymopoietic factors is attenuated. In this environment, ETPs and their downstream progenitors display an aging phenotype characterized by increased Ink4a expression and reduced proliferation. (Right) Fgf7 binds to thymic epithelial cells and induces them to secrete various cytokines. These in turn act on thymocytes and mediate down-regulation of Ink4a expression. As a result, the proliferative potential of ETPs and DN cells is partially restored and the number of T-cell progenitors is increased.
Model for Fgf7 actions in the involuted thymus. (Left) The number and quality of thymic epithelial cells are reduced in the involuted thymus. As a result, the production of epithelial-derived thymopoietic factors is attenuated. In this environment, ETPs and their downstream progenitors display an aging phenotype characterized by increased Ink4a expression and reduced proliferation. (Right) Fgf7 binds to thymic epithelial cells and induces them to secrete various cytokines. These in turn act on thymocytes and mediate down-regulation of Ink4a expression. As a result, the proliferative potential of ETPs and DN cells is partially restored and the number of T-cell progenitors is increased.
A key tenet of the model shown in Figure 6 is that exogenous factors in the microenvironment can affect the senescent state of target cells by regulating gene expression in them. Such a mechanism has been shown recently to operate in the pancreas, where PDGF regulates the levels of Ink4a in pancreatic β cells.42 If Fgf7 acts by stimulating thymic epithelial cell production of factors that in turn target gene expression in ETPs and their DN progeny, it will ultimately be of interest to identify these microenvironmental signals because they may prove to be of value in targeting aged ETPs directly in a therapeutic context.
Fgf7 did not correct all of the age-related deficiencies in thymopoiesis. Whereas proliferation of old ETPs was enhanced, these cells still did not differentiate as efficiently as their young counterparts. Therefore, Fgf7 does not completely reverse the effects of aging to make old thymocytes young again. Rather, its thymopoietic effects result from the fact that the number of ETPs and DN cells is significantly increased, resulting in an increase in total thymus cellularity. Nevertheless, this does not exclude the possibility that altering Ink4a expression with factors such as Fgf7 may still be clinically useful. Repressing the expression of this tumor-suppressor protein may stimulate thymopoiesis to levels sufficient to boost the peripheral pool of naive T cells and improve impaired immune function in the elderly.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grant AG034875 from NIH. The UCLA Flow Cytometry Core Facility is supported by NIH grants CA16042 and AI28697.
National Institutes of Health
Authorship
Contribution: B.B.-M., E.M.-R., and R.A.J.S. designed and conducted the experiments and wrote the manuscript; and K.D. supervised and had overall responsibility for the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for R.A.J.S. is Children's Research Institute, University of Texas Southwestern Medical Center, Dallas, TX.
Correspondence: Kenneth Dorshkind, Pathology and Laboratory Medicine 173216, David Geffen School of Medicine at UCLA, 10833 Le Conte Ave, Los Angeles, CA 90095; e-mail: kdorshki@mednet.ucla.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal