Abstract
Desensitization controls G protein–dependent signaling of chemokine receptors. We investigate the physiologic implication of this process for CXCR4 in a mouse model harboring a heterozygous mutation of the Cxcr4 gene, which engenders a desensitization-resistant receptor. Such anomaly is linked to the warts, hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome, a human rare combined immunodeficiency. Cxcr4+/mutant(1013) mice display leukocytes with enhanced responses to Cxcl12 and exhibit leukopenia as reported in patients. Treatment with CXCL12/CXCR4 antagonists transiently reverses blood anomalies, further demonstrating the causal role of the mutant receptor in the leukopenia. Strikingly, neutropenia occurs in a context of normal bone marrow architecture and granulocyte lineage maturation, indicating a minor role for Cxcr4-dependent signaling in those processes. In contrast, Cxcr4+/1013 mice show defective thymopoiesis and B-cell development, accounting for circulating lymphopenia. Concomitantly, mature T and B cells are abnormally compartmentalized in the periphery, with a reduction of primary follicles in the spleen and their absence in lymph nodes mirrored by an unfurling of the T-cell zone. These mice provide a model to decipher the role of CXCR4 desensitization in the homeostasis of B and T cells and to investigate which manifestations of patients with WHIM syndrome may be overcome by dampening the gain of CXCR4 function.
Introduction
The WHIM syndrome (WS) is a rare combined immunodeficiency disorder characterized by human papilloma virus–induced warts, hypogammaglobulinemia, recurrent infections, and myelokathexis, an abnormal retention of hypermature neutrophils in the bone marrow (BM).1 The clinical manifestations of this life-threatening disorder are however variable, except for neutropenia and lymphopenia that affects circulating B and T cells. WS patients are thus susceptible to respiratory infections caused by encapsulated bacteria, resulting in transient normalization of peripheral leukocyte counts.2 This feature distinguishes WS from other congenital neutropenia, suggesting that the WS-related neutropenia arises from disturbed neutrophil trafficking rather than from a production defect. However, the mechanisms accounting for the lympho-neutropenia are still lacking.
Research into the genetic basis for WS has identified inherited heterozygous autosomal dominant mutations in the CXCR4 gene encoding for the receptor of the CXC α-chemokine stromal cell–derived factor-1 (CXCL12).3 CXCR4 engagement by CXCL12 induces typical activation of Gαi protein–dependent pathways of a chemokine receptor. These processes are regulated in a timely manner by the recruitment of β-arrestin to the receptor that precludes further G protein activation (ie, desensitization) and leads to receptor internalization. Mutants of CXCR4 associated with WS give rise to impaired desensitization and internalization of the receptor on CXCL12 exposure, leading to enhanced and prolonged G protein– and β-arrestin–dependent responses.3-8 We reported a similar pattern of aberrant CXCL12/CXCR4 responses in leukocytes from the minority of patients, who carry a wild-type (WT) CXCR4,4,9 consistent with a role for impaired CXCR4 desensitization in the WS pathophysiology. In cooperation with other chemokine receptors, Cxcr4 in mice regulates physiologic processes involved in leukocyte homeostasis, and particularly hematopoiesis,10-12 and the lymphoid and peripheral trafficking of neutrophil and lymphocyte subsets. Targeted disruption of either Cxcl12 or Cxcr4 is embryo lethal, evidencing critical functions for this couple in early development.13-15
Here, we have generated a new knockin mouse strain that harbors a WS-associated heterozygous mutation of the Cxcr4 gene (ie, Cxcr4+/1013)4 to analyze the impact of Cxcr4 desensitization on leukocyte homeostasis. We demonstrated that impaired Cxcr4 desensitization leads to a gain of receptor function and engenders peripheral leukopenia that was transiently reversed by a treatment with the CXCR4 antagonist AMD3100. These findings indicated that mutant mice exhibit striking parallels to the major immunologic features of WS. We highlighted that circulating lymphopenia results from defective thymopoiesis and B-cell development. Importantly, proper Cxcr4-dependent signaling is required for the formation of primary follicles in the spleen and lymph nodes (LNs). However, and in contrast to patients, circulating neutropenia occurred in a context of normal BM architecture. Moreover, mutant mice displayed normal granulocyte lineage maturation, revealing an unanticipated minor role for Cxcr4-dependent signaling in those processes. These data provide insight into the underlying mechanisms by which impaired Cxcr4 desensitization affects leukocyte development, compartmentalization and likely function. They demonstrate the validity of Cxcr4+/1013 mice as a model for WS-related immunohematologic manifestations to assess the potential of proposed treatments on a long term as well to develop new and chronic effective interventions.
Methods
Generation of Cxcr4+/1013 mice
Mice were generated using a knockin strategy and bred under specific pathogen-free conditions as indicated in supplemental Methods and supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The Institutional Animal Care and Use Committee d'Ile de France–Service Protection et Santé Animale approved this study.
Reagents, cell culture, and transfection
Dr F. Baleux (Unité de Chimie Organique, Institut Pasteur, Paris, France) provided CXCL12. Recombinant mouse Ccl21/6Ckine, Cxcl13/Blc/Bca-1, and Ccl25/TECK were obtained from R&D Systems. AMD3100 (or plerixafor) was from Sigma-Aldrich. Chalcone 4 was prepared as described in Gasparik et al16 and in supplemental Methods. Descriptions of the culture and transfection are provided in supplemental Methods.
Flow cytometry analyses and antibodies
Analyses were carried out on an FACSCalibur flow cytometer (BD Biosciences) as indicated in supplemental Methods using the following anti–mouse monoclonal antibodies (mAbs): CD3 (clone 145-2C11, hamster IgG1), CD4 (clone RM4-5, rat IgG2a), CD8 (clone 53-6.7, rat IgG2a), CD62L (clone MEL-14, rat IgG2a), CD44 (clone IM7, rat IgG2b), Gr1 (clone RB6-8C5, rat IgG2b), CD11b (clone M1/70, rat IgG2b), CD19 (clone 1D3, rat IgG2a), CD45R/B220 (clone RA3-6B2, rat IgG2a), CD21/C35 (clone 7G6, rat IgG2b), CD23 (clone B3B4, rat IgG2a), CD5 (clone 53-7.3, rat IgG2a), IgM (clone II/41, rat IgG2a), Cxcr2 (clone 242216, rat IgG2a), Cxcr4 (clone 2B11, rat IgG2b), Cxcr5 (clone 2G8, rat IgG2a), Ccr7 (clone 4B12, rat IgG2a), and Ccr9 (clone 242503, rat IgG2b). mAbs were conjugated to fluorescein isothiocyanate, phycoerythrin, allophycocyanin, or peridinin-chlorophyll proteins and were purchased from BD Biosciences, R&D Systems, or eBioscience.
Functional assays
Chemotaxis, receptor internalization, and apoptosis were performed as described previously 17 and in supplemental Methods.
Enzyme-linked immunosorbent assay
Total levels of IgG, IgM, and IgA were determined by ELISA, as described previously,17 with some modifications specified in supplemental Methods.
Histologic analyses
Detailed methods of organs fixation, immunohistochemistry, and immunofluorescence are described in supplemental Methods.
Immunoprecipitation assays
Immunoprecipitation and Western blot assays were performed according to Lagane et al,7 with modifications specified in supplemental Methods.
In vivo mobilization
Mice were treated with AMD3100 (5 mg/kg), chalcone 4 (12.4 mg/kg), or PBS via intraperitoneal injection. Blood and BM leukocytes were harvested and analyzed by flow cytometry as described in supplemental Methods.
Statistical analysis
Data are expressed as means ± SD. The statistical significance between groups was evaluated using the 2-tailed Student t test.
Results
Cxcr4+/1013 leukocytes exhibit a gain of Cxcr4 function
To assess the in vivo consequences of the gain of CXCR4 function on leukocyte homeostasis, we engineered heterozygous Cxcr4+/1013 mice using a gene knockin strategy by replacing the mouse WT Cxcr4 gene by a mutated gene reproducing the CXCR41013 mutation we described in WS patients4 (supplemental Figure 1A). Cxcr4+/1013 mice were fertile, and births followed an expected Mendelian ratio. Young mice from both sexes developed without apparent morphologic abnormality. Genetic uniformity was reached by a 10-generation backcross to the C57BL6/J background (> 98%). Genotyping and expression analyses of the Cxcr4 receptor permitted to distinguish heterozygous (Cxcr4+/1013) animals from their WT (Cxcr4+/+) littermates (supplemental Figure 1B-D).
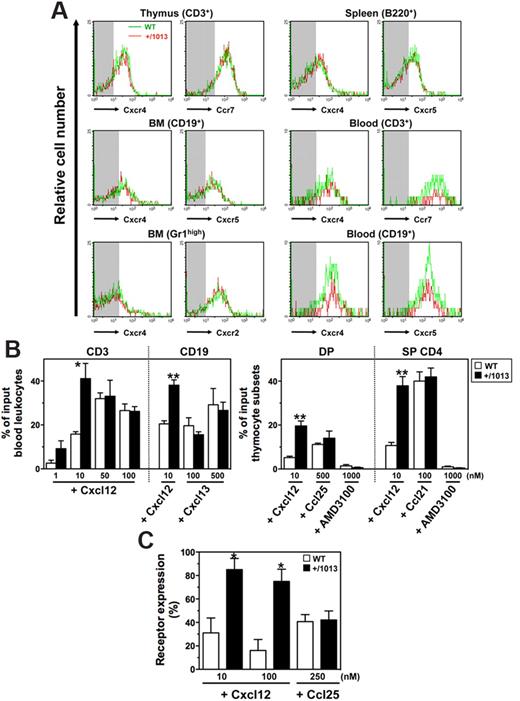
Circulating leukocytes from WS patients display normal expression of CXCR4 but enhanced migratory responses to CXCL12.4 Membrane expression levels of Cxcr4 and other chemokine receptors, including Ccr7, Cxcr2, and Cxcr5, were in the same range between Cxcr4+/1013 and WT neutrophils, and lymphocytes from blood and lymphoid tissues (Figure 1A). Moreover, all tested leukocyte subsets from Cxcr4+/1013 mice, as shown for blood T and B cells, double-positive (CD4+CD8+), and single-positive (CD4+CD8−) thymocytes, displayed increased sensitivity to Cxcl12-promoted chemotaxis (Figure 1B). The maximal effective concentration of the chemokine decreased in Cxcr4+/1013 cells, indicating higher potency of Cxcl12 toward these cells. The increased responsiveness of Cxcr4+/1013 thymocytes to Cxcl12 was abolished by the specific Cxcr4 antagonist AMD3100 (Figure 1B) and was associated with impaired Cxcr4 internalization following Cxcl12 stimulation (Figure 1C). In contrast, other chemokine receptors remained fully sensitive to their cognate ligand (Figure 1B-C). These data indicate that the gain of Cxcr4 function affects neither the expression nor the function of these receptors, at least ex vivo, thus extending observations made in patient-derived cells.4,5,9
Exacerbated Cxcl12-induced chemotaxis of Cxcr4+/1013 leukocytes. (A) Cxc and Cc receptors expression on gated thymocytes (CD3+), BM B cells (CD19+) and neutrophils (SSChighGr1high), spleen B cells (B220+), and blood T (CD3+) and B (CD19+) cells from WT and Cxcr4+/1013 (+/1013) mice. Background fluorescence is shown (shaded area). (B) Migration of blood T and B cells (left) and of double-positive (DP) and single-positive (SP) CD4+ thymocytes (right) in response to Cxcl12 (with or without AMD3100), Cxcl13, Ccl21, or Ccl25. (C) Cell surface expression of Cxcr4 and Ccr9 in DP thymocytes on exposure to Cxcl12 and Ccl25, respectively. Receptor expression on thymocytes incubated in medium alone was set as 100%. Results represent the means ± SD of 3 to 5 independent experiments (B-C) or are representative of 3 to 5 independent determinations (A), (*P < .05; **P < .005 compared with WT leukocytes).
Exacerbated Cxcl12-induced chemotaxis of Cxcr4+/1013 leukocytes. (A) Cxc and Cc receptors expression on gated thymocytes (CD3+), BM B cells (CD19+) and neutrophils (SSChighGr1high), spleen B cells (B220+), and blood T (CD3+) and B (CD19+) cells from WT and Cxcr4+/1013 (+/1013) mice. Background fluorescence is shown (shaded area). (B) Migration of blood T and B cells (left) and of double-positive (DP) and single-positive (SP) CD4+ thymocytes (right) in response to Cxcl12 (with or without AMD3100), Cxcl13, Ccl21, or Ccl25. (C) Cell surface expression of Cxcr4 and Ccr9 in DP thymocytes on exposure to Cxcl12 and Ccl25, respectively. Receptor expression on thymocytes incubated in medium alone was set as 100%. Results represent the means ± SD of 3 to 5 independent experiments (B-C) or are representative of 3 to 5 independent determinations (A), (*P < .05; **P < .005 compared with WT leukocytes).
Distal truncations of the WS-associated receptor remove the last 10 to 19 residues of the carboxyl-terminal tail (C-tail), including phospho-acceptor serine/threonine sites, that account for the failure of CXCR4 to be desensitized and internalized18 and contribute, together with abnormal β-arrestin–dependent signaling, to enhanced CXCL12-promoted chemotaxis.7,8 However, the complete deletion of the Cxcr4 C-tail leads to a loss of G protein–dependent function that causes developmental defects similar to those of Cxcr4-null mice.19 This suggests that the remaining residues in the C-tail of WS-associated CXCR4 mutants that are targeted by numerous regulators8,9,18 contribute to the enhanced CXCL12-dependent signaling. In lysates from WT mice, Cxcr4+/1013 mice, and Cxcr41013/1013 mice obtained by intercrossing heterozygous mice, immunodetection of Cxcr4 similarly revealed 1 band, suggesting that mutant and WT receptors cannot be distinguished in size (supplemental Figure 1D). Collectively, these data strongly suggest that the Cxcr41013 receptor is expressed with its WT counterpart in Cxcr4+/1013 mice and exerts a functional prevalence through a dominant effect.
Cxcr4+/1013 mice develop normally but are profoundly lympho-neutropenic
Cxcl12 and Cxcr4 play an essential role in embryogenesis and are notably critical for heart, brain, lung, and blood vessel development.13 In contrast to mice with targeted deletion of Cxcl12 or Cxcr4, which die between embryonic days 15 and 18 of gestation, Cxcr4+/1013 mice were viable and their development seemed normal. Histologic analyses of Cxcr4+/1013 mice showed no apparent morphologic abnormality in the myocardium architecture, cerebellum organization, Peyer patches architecture and distribution, and Malpighi's glomerulus structure (supplemental Figure 2). Collectively, together with the fact that Cxcr4+/1013 mice display no alterations in weight and growth rate, these data indicate that the gain of Cxcr4 function has no deleterious developmental effects.
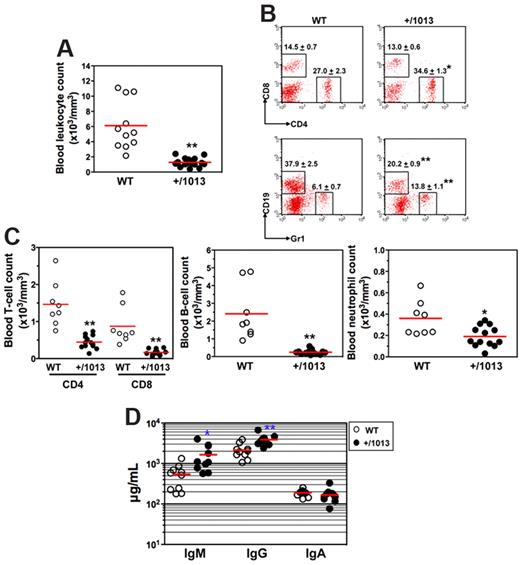
We then investigated how impaired Cxcr4 desensitization impacts on leukocyte homeostasis. In the peripheral blood, Cxcr4+/1013 mice had ∼ 80% fewer leukocytes than their WT littermates (Figure 2A). In Cxcr4+/1013 mice, although the frequency of circulating CD8+ T cells was unaltered, that of CD4+ T cells and Gr1+ neutrophils increased, whereas the proportion of the CD19+ B cells decreased (Figure 2B). However, the absolute number of each blood leukocyte subpopulation was significantly reduced in Cxcr4+/1013 mice (Figure 2C). Cxcr4+/1013 mice also displayed a significant monocytopenia (data not shown). All Cxcr4+/1013 mice tested exhibited a severe leukopenia, demonstrating a complete penetrance of the mutated allele in mice. We also assessed to what extent the gain of Cxcr4 function affected basal humoral immunity. Serum levels of natural IgA were similar in nonmanipulated Cxcr4+/1013 and WT mice, whereas IgM and IgG increased in Cxcr4+/1013 mice (Figure 2D). Combined with the reduced frequency and number of circulating B cells, these results suggest that B-cell trafficking or compartmentalization is compromised in Cxcr4+/1013 mice.
Disturbed leukocyte homeostasis in the blood of Cxcr4+/1013 mice. (A) Absolute numbers of leukocytes were determined in blood samples. (B-C) Blood samples were stained with indicated mAbs, and the frequencies (mean ± SD, n = 8-13; B) and absolute numbers (C) of total CD4+ or CD8+ T cells (gated CD3+), B cells (gated CD19+), and neutrophils (gated Gr1+) are shown. (D) Sera total Igs were analyzed. Results of all analyzed littermates are shown (A,C,D). Lines indicate the mean and each circle indicates an individual mouse. (B) Each dot-plot represents one mouse (*P < .05; **P < .005 compared with WT mice).
Disturbed leukocyte homeostasis in the blood of Cxcr4+/1013 mice. (A) Absolute numbers of leukocytes were determined in blood samples. (B-C) Blood samples were stained with indicated mAbs, and the frequencies (mean ± SD, n = 8-13; B) and absolute numbers (C) of total CD4+ or CD8+ T cells (gated CD3+), B cells (gated CD19+), and neutrophils (gated Gr1+) are shown. (D) Sera total Igs were analyzed. Results of all analyzed littermates are shown (A,C,D). Lines indicate the mean and each circle indicates an individual mouse. (B) Each dot-plot represents one mouse (*P < .05; **P < .005 compared with WT mice).
Impaired Cxcr4 desensitization does not affect granulopoiesis
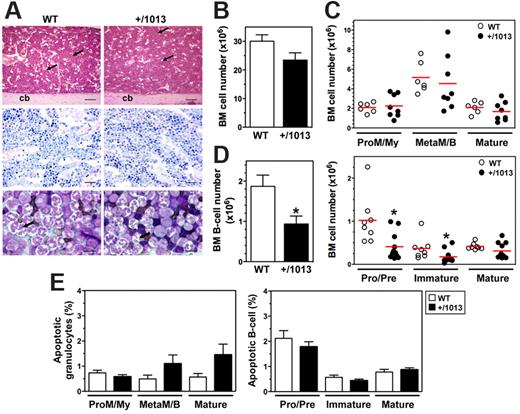
In contrast to other forms of inherited neutropenia, WS is not usually linked to life-threatening infections because acute infection or prophylactic treatment (eg, granulocyte colony-stimulating factor) transiently corrects the peripheral neutropenia.20 This suggests that the neutropenia results from a defect in BM release of neutrophils, rather than from a production defect, a mechanism that fits well with the regulation of neutrophil homing and egress from the BM by the Cxcl12–Cxcr4 interaction.21 Thus, the WS-associated gain of CXCR4 function may cause the exaggerated retention of neutrophils in the BM. We found no difference in BM architecture and cellularity between WT and Cxcr4+/1013 mice using histologic analyses and microscopic cell enumeration (Figure 3A-B; Table 1). Similarly, analysis of CD11b and Gr1 expression in BM aspirates did not show differences in both the number and frequency of granulocyte subsets (Figure 3C and supplemental Figure 3A). These results suggest that the gain of Cxcr4 function does not disturb BM granulocyte development, as has been reported in patients.1,22
B-cell, but not granulocyte, development is altered in Cxcr4+/1013 mice. (A) H&E and Giemsa staining of longitudinal sections of paraffin-embedded decalcified tibia. Shown are some venous sinuses (arrows) and the cortical bone (cb). In the representative May-Grünwald-Giemsa (MGG) staining of BM aspirates (bottom), most of the cells are neutrophils and some have hypersegmented nuclei (arrows). Original magnification, ×40 (H&E), ×100 (Giemsa), and ×400 (MGG); scale bars denote 500, 250, and 25 μm, respectively. (B-D) Absolute numbers (mean ± SD, n = 14-19) of BM cells were determined from 2 femurs (B) and analyzed for the content (C) in promyelocytes/myelocytes (ProM/My), metamyelocytes/band forms (MetaM/B), and mature neutrophils (Mature), and (D) in total B cells (mean ± SD, n = 8-11; left) and (right) pro/pre-B cells, immature, and mature B-cell subsets (Mature). (E) Caspase-3 activation was used to detect apoptotic BM neutrophils and B cells. Results are from all the analyzed littermates (B-D), are from 3 to 5 independent experiments (E), or are representative of 3 to 5 independent determinations (A), (*P < .05 compared with WT mice).
B-cell, but not granulocyte, development is altered in Cxcr4+/1013 mice. (A) H&E and Giemsa staining of longitudinal sections of paraffin-embedded decalcified tibia. Shown are some venous sinuses (arrows) and the cortical bone (cb). In the representative May-Grünwald-Giemsa (MGG) staining of BM aspirates (bottom), most of the cells are neutrophils and some have hypersegmented nuclei (arrows). Original magnification, ×40 (H&E), ×100 (Giemsa), and ×400 (MGG); scale bars denote 500, 250, and 25 μm, respectively. (B-D) Absolute numbers (mean ± SD, n = 14-19) of BM cells were determined from 2 femurs (B) and analyzed for the content (C) in promyelocytes/myelocytes (ProM/My), metamyelocytes/band forms (MetaM/B), and mature neutrophils (Mature), and (D) in total B cells (mean ± SD, n = 8-11; left) and (right) pro/pre-B cells, immature, and mature B-cell subsets (Mature). (E) Caspase-3 activation was used to detect apoptotic BM neutrophils and B cells. Results are from all the analyzed littermates (B-D), are from 3 to 5 independent experiments (E), or are representative of 3 to 5 independent determinations (A), (*P < .05 compared with WT mice).
Bone marrow morphology
| . | Cxcr4+/+ . | Cxcr4+/1013 . |
|---|---|---|
| Cell type | ||
| Myeloblasts | 1* | 0.9 |
| Promyelocytes | 1.3 | 0 |
| Myelocytes | 8.8 | 8 |
| Metamyelocytes/band cells | 7.1 | 6.5 |
| Neutrophils | 21.5 | 31.7 |
| Eosinophils | 0 | 0 |
| Basophils | 0 | 0 |
| Total myeloid cells | 39.7 | 47.1 |
| Proerythroblasts | 0 | 0 |
| Basophilic | 1.5 | 0.5 |
| Polychromatophilic | 17 | 16.2 |
| Acidophilic | 9.2 | 4.7 |
| Total erythroid cells | 27.7 | 21.4 |
| Lymphocytes | 32.6 | 31.5 |
| Plasmocytes | 0 | 0 |
| Monocytes | 0 | 0 |
| Reticulum cells | 0 | 0 |
| M/E ratio† | 1.4 | 2.2 |
| . | Cxcr4+/+ . | Cxcr4+/1013 . |
|---|---|---|
| Cell type | ||
| Myeloblasts | 1* | 0.9 |
| Promyelocytes | 1.3 | 0 |
| Myelocytes | 8.8 | 8 |
| Metamyelocytes/band cells | 7.1 | 6.5 |
| Neutrophils | 21.5 | 31.7 |
| Eosinophils | 0 | 0 |
| Basophils | 0 | 0 |
| Total myeloid cells | 39.7 | 47.1 |
| Proerythroblasts | 0 | 0 |
| Basophilic | 1.5 | 0.5 |
| Polychromatophilic | 17 | 16.2 |
| Acidophilic | 9.2 | 4.7 |
| Total erythroid cells | 27.7 | 21.4 |
| Lymphocytes | 32.6 | 31.5 |
| Plasmocytes | 0 | 0 |
| Monocytes | 0 | 0 |
| Reticulum cells | 0 | 0 |
| M/E ratio† | 1.4 | 2.2 |
Results are expressed as percentage of total cells.
M/E ratio indicates myeloid:erythroid ratio.
Next, we searched for the presence of vacuolated neutrophils with hypersegmented nuclei connected by long filaments, the morphologic features of WS patients BM smears (ie, the pathognomonic myelokathexis). We did not detect any vacuolated neutrophils in WT and Cxcr4+/1013 BM (Figure 3A). Although hypersegmented neutrophils were easily detected in the BM smears from Cxcr4+/1013 mice, their frequency (∼ 7% of total neutrophils) was similar in WT mice, suggesting that they do not feature mutant mice but are part of neutrophil homeostasis. This phenomenon is actually not specific to the mouse model, because some hypermature neutrophils with pyknotic nuclei also can be recovered from the BM of humans (3%-14% and ∼ 55% in healthy and WS individuals, respectively).23 The histologic pattern of BM biopsies from WS patients is consistent with apoptosis of mature myeloid cells as a consequence of their abnormal BM sequestration, although the formal demonstration has never been made.20 Thus, we searched for these cells in Cxcr4+/1013 mice using another experimental approach based on the quantification by flow cytometry of cleaved caspase-3. A sizable pool of apoptotic neutrophils was detected similarly in the BM of Cxcr4+/1013 and WT mice (Figure 3E). Therefore, the gain of Cxcr4 function results in a circulating neutropenia that occurs in the context of normal granulocyte maturation and is associated with neither a significant increase of mature neutrophils nor their exacerbated apoptosis in the BM. These data further indicate that impaired Cxcr4 desensitization does not cause myelokathexis.
Altered B- and T-cell development accounts for chronic lymphopenia
In the postnatal life, the CXCL12/CXCR4 axis regulates the BM homing and retention of hematopoietic progenitor cells (HPCs) and is involved in B-cell lymphopoiesis. Because CXCR4 is virtually expressed on all B-cell subpopulations, changes in CXCL12 responsiveness that occur throughout B-cell ontogeny were proposed to result from differences in the activation of CXCR4-mediated signaling.24 Thus, the gain of Cxcr4 function could affect B-cell development. Both frequency and absolute number of total CD19+ B cells were slightly, but significantly, lower in the BM from Cxcr4+/1013 mice (Figure 3D and supplemental Figure 3B). In contrast to the myeloid series, B-cell differentiation was altered in Cxcr4+/1013 mice. Indeed, although the frequency of immature B cells was unchanged, that of pro/pre-B cells was reduced, and that of mature recirculating B cells was increased (supplemental Figure 3B). Consequently, the absolute number of pro/pre-B cells, and to a lesser extent of immature B cells, was reduced in Cxcr4+/1013 mice (Figure 3D). Pro/pre-B cells and other B-cell subsets from Cxcr4+/1013 mice displayed a low rate of apoptosis similar to that of the corresponding WT B-cell subpopulation (Figure 3E). Therefore, the alteration of early B-cell development in Cxcr4+/1013 mice was not associated with enhanced cell death.
In mammals, T-cell development mainly occurs in the thymus and is governed by a chemokine-dependent homing of hematopoietic progenitors, and relies on the cooperative action of the Ccl25/Ccr9, Ccl21/Ccr7, and Cxcl12/Cxcr4 pairs.12 We found no impact of the gain of Cxcr4 function on thymus wet weight and architecture (supplemental Figure 4A-B). Double-positive and single-positive thymocytes localized to the cortex and the medulla, respectively, suggesting that thymocyte segregation occurred normally in mutant mice. Moreover, Cxcr4+/1013 and WT mice displayed similar frequencies of double-negative, double-positive, and single-positive thymocytes (supplemental Figure 4C). However, in accordance with the ∼ 30% reduction in thymus cellularity (supplemental Figure 4A), the absolute number of each thymic subset (from progenitors to mature single-positive thymocytes) was significantly decreased in Cxcr4+/1013 mice (supplemental Figure 4D). Therefore, impaired Cxcr4 desensitization does not disturb thymus organization but affects the homing, differentiation, or expansion of early T-cell progenitors. Together, these findings reveal that altered Cxcr41013-driven development of B and T cells leads to chronic circulating lymphopenia.
Cxcr4 activation governs peripheral lymphocyte compartmentalization
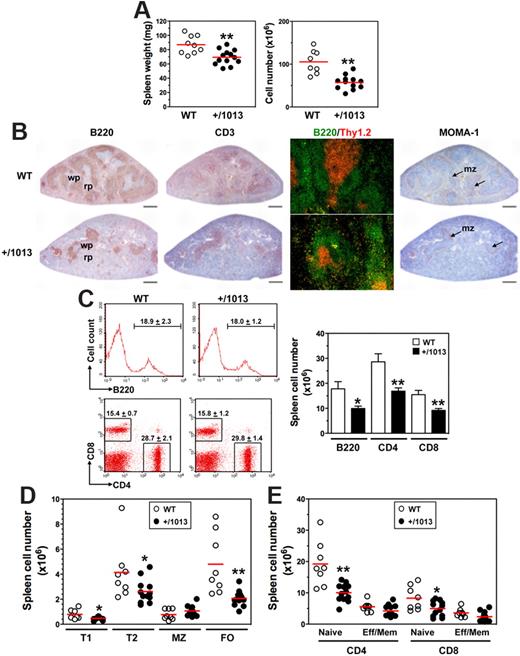
We sought to delineate how the disturbed lymphopoiesis in Cxcr4+/1013 mice alters trafficking and distribution of B and T cells in the spleen, LNs, and peritoneal cavity (PerC), processes that involve the Cxcl12/Cxcr4 axis.25-27 Cxcr4+/1013 mice had ∼ 20% and 45% lower spleen wet weight and cellularity, respectively, than WT mice (Figure 4A). Histologic analyses revealed a dramatic reduction of primary B-cell follicles in the spleen of Cxcr4+/1013 mice (Figure 4B). However, the few B and T cells detected were adequately compartmentalized within the white pulp, with T cells being surrounded by B-cell follicles. In addition, the marginal zone (MZ) was preserved in Cxcr4+/1013 mice, as revealed by the normal distribution of MOMA-1+ metallophilic macrophages. This context suggested reduced B- and T-cell numbers. Consistently, the total number of B220+ B cells and CD4+ or CD8+ T cells decreased by ∼ 40% to 45% in Cxcr4+/1013 mice (Figure 4C). Immature B cells from the BM emerge into the spleen as transitional B cells of type 1 and then develop into B cells of type 2 that give rise to 2 subsets of long-lived mature B cells, MZ and follicular (FO) B cells. Numbers of both type 1 and type 2 B cells were reduced as well as that of FO B cells (> 50%), whereas the MZ B-cell subset was maintained (Figure 4D). Thus, in the spleen of Cxcr4+/1013 mice, FO B cells represented a smaller fraction that is counterbalanced by the MZ B-cell subpopulation (supplemental Figure 5A). Analysis of T-cell subsets indicated that the total numbers of naive CD4+ and CD8+ T cells decreased by ∼ 50% and 40%, respectively, in Cxcr4+/1013 mice, whereas the decrease in effector/memory cells was lower (Figure 4E). No imbalance in the proportion of each T-cell population was detected in the spleen of Cxcr4+/1013 mice (supplemental Figure 5B).
Decreased numbers of splenic B and T cells in Cxcr4+/1013 mice. (A) Spleen wet weight (left) and total cell number (right) were determined. (B) Spleen sections were immunostained for B220, CD3, or MOMA-1 (brown) or for B220 (green) and Thy1.2 (red). Marginal zone (mz), white pulp (wp), and red pulp (rp) are indicated. Original magnification, ×40 (B220, CD3 and MOMA-1) and ×200 (B220/Thy1.2); scale bars denote 500 μm. (C) Frequencies (mean ± SD, n = 8-12; left) and absolute numbers (right) of total spleen B cells (gated B220+CD3−) and T cells (gated B220−CD3+CD4+ or CD8+) are shown. (D-E) Absolute numbers of (D) transitional (type T1 and T2), marginal zone (MZ), and follicular (FO) B cells, and of (E) naive and effector/memory (Eff/Mem) CD4+ or CD8+ T cells, as determined in supplemental Figure 5. Results are from all the analyzed littermates (A,C-E), are from 1 representative mouse (C, left) or are representative of 3 independent determinations (B). Lines indicate the mean and each circle indicates an individual mouse (A,D-E), (*P < .05; **P < .005 compared with WT mice).
Decreased numbers of splenic B and T cells in Cxcr4+/1013 mice. (A) Spleen wet weight (left) and total cell number (right) were determined. (B) Spleen sections were immunostained for B220, CD3, or MOMA-1 (brown) or for B220 (green) and Thy1.2 (red). Marginal zone (mz), white pulp (wp), and red pulp (rp) are indicated. Original magnification, ×40 (B220, CD3 and MOMA-1) and ×200 (B220/Thy1.2); scale bars denote 500 μm. (C) Frequencies (mean ± SD, n = 8-12; left) and absolute numbers (right) of total spleen B cells (gated B220+CD3−) and T cells (gated B220−CD3+CD4+ or CD8+) are shown. (D-E) Absolute numbers of (D) transitional (type T1 and T2), marginal zone (MZ), and follicular (FO) B cells, and of (E) naive and effector/memory (Eff/Mem) CD4+ or CD8+ T cells, as determined in supplemental Figure 5. Results are from all the analyzed littermates (A,C-E), are from 1 representative mouse (C, left) or are representative of 3 independent determinations (B). Lines indicate the mean and each circle indicates an individual mouse (A,D-E), (*P < .05; **P < .005 compared with WT mice).
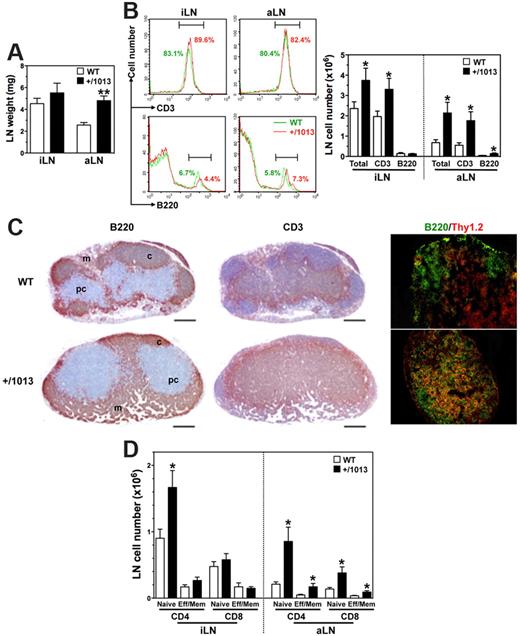
In contrast to the follicular hypoplasia detected in the spleen, LNs displayed an increase in the absolute number of total cells, mostly composed of CD3+ T cells (Figure 5A-B). Immunostaining revealed a disorganized architecture characterized by the absence of B-cell follicles and an unfurling of the T-cell zone at the expense of the B-cell one (Figure 5C). As expected, high endothelial venules (HEVs) in WT mice were predominantly localized in T-cell zones such as the paracortical area, but they also were found at the periphery of primary follicles. Although HEVs were readily detected in Cxcr4+/1013 mice, their distribution was abnormally scattered, thus mirroring the disorganization of T- and B-cell zones (supplemental Figure 5C). Surprisingly, fewer and abnormally localized vessels were positive for the LN lymphatic vessels (LNLVs) marker LYVE-1 in mutant mice. The architecture of Peyer patches, the formation of which follows a scheme highly similar to that of LNs, was found to be normal in Cxcr4+/1013 mice (supplemental Figure 2F). Flow cytometric analyses of the LN T-cell compartment indicated an increased frequency of CD4+ T cells at the expense of that of CD8+ T cells (supplemental Figure 5D). The proportions of naive and effector/memory T cells remained unchanged in Cxcr4+/1013 mice, whereas their number tended to be higher with a significant increase in naive CD4+ T cells (Figure 5D). Similarly to the spleen and LNs, the gain of Cxcr4 function impaired B-cell compartmentalization in the PerC, where B2 and B1b cells, which primarily derive from BM precursors, were reduced in favor of B1a cells that are known to develop from fetal liver precursors (supplemental Figure 6). Taken together, these data unveil altered Cxcr41013-mediated trafficking of lymphocytes between lymphoid organs and the peripheral blood.
Increased numbers of LN T cells in Cxcr4+/1013 mice. (A-B) The wet weights (mean ± SD, n = 6-10; A) of inguinal (i) and axillary (a) LNs were determined as well as (B) total cell numbers, frequencies (left), and absolute numbers (right) of total B and T cells. (C) aLN sections were immunostained for B220, CD3 (brown), or for B220 (green) and Thy1.2 (red). Cortex (c), paracortex (pc), and medullary (m) areas are indicated. Original magnification, ×40 (B220 and CD3) and ×200 (B220/Thy1.2); scale bars denote 500 μm. (D) Absolute numbers (mean ± SD) of naive and effector/memory (Eff/Mem) CD4+ or CD8+ T cells in peripheral LNs are shown. Results are from all the analyzed littermates (A,B,D), are from 1 representative mouse (B, left), or are representative of 3 independent determinations (C), (*P < .05; **P < .005 compared with WT mice).
Increased numbers of LN T cells in Cxcr4+/1013 mice. (A-B) The wet weights (mean ± SD, n = 6-10; A) of inguinal (i) and axillary (a) LNs were determined as well as (B) total cell numbers, frequencies (left), and absolute numbers (right) of total B and T cells. (C) aLN sections were immunostained for B220, CD3 (brown), or for B220 (green) and Thy1.2 (red). Cortex (c), paracortex (pc), and medullary (m) areas are indicated. Original magnification, ×40 (B220 and CD3) and ×200 (B220/Thy1.2); scale bars denote 500 μm. (D) Absolute numbers (mean ± SD) of naive and effector/memory (Eff/Mem) CD4+ or CD8+ T cells in peripheral LNs are shown. Results are from all the analyzed littermates (A,B,D), are from 1 representative mouse (B, left), or are representative of 3 independent determinations (C), (*P < .05; **P < .005 compared with WT mice).
Blockade of Cxcr4-dependent signaling reverses leukopenia
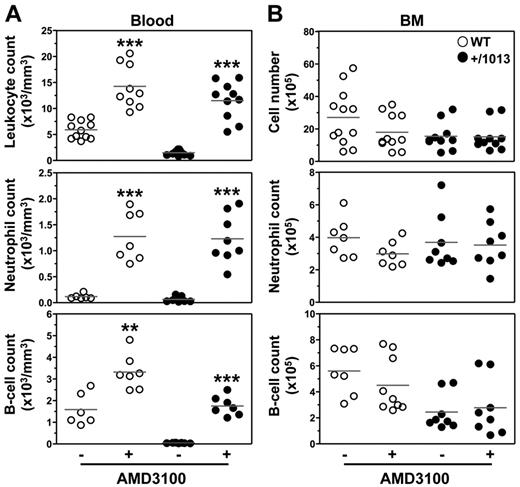
The main challenge in understanding the WS pathogenesis is to develop selective and safe therapies for chronic use aimed at correcting the gain of CXCR4 function and associated clinical manifestations. To this end, we analyzed the impact of the first licensed CXCR4 antagonist AMD3100 (or plerixafor) approved for BM HPC mobilization and transplantation28 but also, of chalcone- 4, a CXCL12-neutralizing molecule that has shown efficacy in vivo in a mouse model of asthma.29 Whereas AMD3100 is a selective and competitive CXCR4 antagonist, chalcone 4 binds CXCL12 and prevents signaling through CXCR4; thus, it is expected to leave the basal activity of the receptor unchanged. With others, we reported previously that AMD3100 rescued the gain of CXCR4 function in patient-derived cells.4,30 Here, we show that single intraperitoneal injection of either AMD3100 or chalcone 4 increased within 3 hours the absolute numbers of total leukocytes in the blood of WT mice, including neutrophils, B and T cells (Figure 6A and supplemental Figures 7 and 8). Importantly, in Cxcr4+/1013 mice, both drugs reversed circulating panleukopenia. Strikingly, AMD3100-induced leukocytosis was mirrored by a measurable but not significant reduction (P ≥ .1) in BM neutrophil and B-cell numbers in WT, but not in mutant mice (Figure 6B). Although the precise location of the mobilizable pool of leukocytes remains to be delineated, the BM could constitute a source of AMD3100-mobilized neutrophils and B cells.21,31 The relative short half-life (0.9 hour) of AMD3100 in rodents together with the gain of Cxcr4 function, which may force the homing of peripheral leukocytes to the BM, could account for the unexpected preserved leukocyte count in the BM of mutant mice. Alternatively, but not exclusively, this stability might reflect the recruitment of leukocytes from additional sources than the BM (eg, LNs). Collectively, these results together with 2 recent studies revealing that acute administration of plerixafor to WS patients corrected leukopenia provide evidence for the causal role of the gain of CXCR4 function in the panleukopenia.32,33 They support the validity of this mouse as a preclinical model of WS-related immunohematologic manifestations for testing new pharmacologic agents for chronic use and to gain insight into their mode of action.
AMD3100-promoted reversion of lymphoneutropenia in Cxcr4+/1013 mice. (A) Absolute numbers of total leukocytes, CD19+ B cells, and Gr1+ neutrophils were determined in the blood 3 hours after intraperitoneal injection of AMD3100 (+) or PBS (−). (B) Absolute numbers of total BM cells, CD19+ B cells, and SSChighGr1+ neutrophils were simultaneously determined from 1 tibia. Results of all analyzed littermates are shown (A-B). Lines indicate the mean and each circle indicates an individual mouse (**P < .005; ***P < .0005 compared with PBS-treated mice).
AMD3100-promoted reversion of lymphoneutropenia in Cxcr4+/1013 mice. (A) Absolute numbers of total leukocytes, CD19+ B cells, and Gr1+ neutrophils were determined in the blood 3 hours after intraperitoneal injection of AMD3100 (+) or PBS (−). (B) Absolute numbers of total BM cells, CD19+ B cells, and SSChighGr1+ neutrophils were simultaneously determined from 1 tibia. Results of all analyzed littermates are shown (A-B). Lines indicate the mean and each circle indicates an individual mouse (**P < .005; ***P < .0005 compared with PBS-treated mice).
Discussion
Using combined genetic, biochemical, and pharmacologic approaches, we established that proper desensitization of Cxcr4 is required for B- and T-cell development and the trafficking of lymphocytes and neutrophils between the peripheral blood and lymphoid organs under homeostatic conditions. Because of the exacerbated Cxcl12-promoted chemotaxis of Cxcr4+/1013 leukocytes, lympho-neutropenia in mutant mice might primarily result from impaired trafficking that skews the tissue distribution of leukocyte subsets.
Patients carrying the orthologous CXCR4 mutation that causes the WS commonly exhibit a severe B- and T-cell lymphopenia.20,34 In 3 patients, the proportions of circulating switched and unswitched memory B cells were reduced.5 In search of a potential central defect, analyses were extended to BM B-cell subsets in one patient, but they indicated that B-cell precursors, immature, and mature B cells were normally represented.5 In contrast, the BM of Cxcr4+/1013 mice displays a defect in CD19+ B cells that selectively affects the number of B-cell precursors and that is not the consequence of an increased rate of apoptosis. We presume that the population of recirculating mature B cells that is quantitatively preserved in the BM probably originates from the periphery. Adoptive cell transfer experiments would help to delineate this issue. Mice deficient in Cxcl12 or Cxcr4 lack B-cell lymphopoiesis,13 and selective inactivation of Cxcr4 in B cells results in the premature migration of B-cell precursors from the BM and their abnormal accumulation in follicles of the spleen and intestinal lamina propria.26 Conversely, we found that the gain of Cxcr4 function correlates with a decreased number of immature B cells in the spleen and the PerC, underscoring a pivotal role for the Cxcl12/Cxcr4-dependent chemotaxis in regulating B-cell homeostasis. The observation that the B1a-cell subset is maintained, and even increased, in the PerC indicates that antenatal B-cell lymphopoiesis occurs efficiently in the fetal liver of mutant mice.
Cxcr4+/1013 mice also exhibited a diminished number of spleen B-cell follicles in a context of unaltered lymphocyte compartmentalization and preserved integrity of the MZ, suggesting that impaired Cxcr4 desensitization alters entry of transitional B cells through the marginal sinus. Although mature FO B cells also were reduced, the population of MZ B cells was surprisingly preserved. Thus, the gain of Cxcr4 function may increase retention or survival of MZ B cells into Cxcl12-expressing MZ bridging channels25 and differentially affect signals that control the generation of MZ versus FO B cells (eg, B-cell receptor signal strength, Cxcr7 expression, Notch-2 or E proteins).35,36 Hypogammaglobulinemia, which most frequently involves IgG but also may affect IgA and IgM, constitutes a clinical sign variable among WS patients with moderate deficiency to normal levels of Igs.20,34 Nonmanipulated mutant mice exhibited normal or even higher serum titers of IgG and IgM than their WT littermates. Serum levels of natural IgA also were preserved in Cxcr4+/1013 mice, consistent with the normal architecture, distribution, and number of Peyer patches. Thus, hypogammaglobulinemia did not seem related to the gain of Cxcr4 function or to the degree of B-cell lymphopenia. It might manifest in a higher susceptibility to respiratory infections, as suggested by the beneficial effect of intravenous Ig therapy in a subset of patients.20 Challenging the mice with pathogens will help to delineate this issue.
WS patients generally exhibit robust responses after immune challenges, indicating that humoral immunity is not completely defective in this context of B-cell lymphopenia. However, evidence also suggest that patients fail to maintain antigen-specific Ab production, which is manifested by reduced circulating memory B cells.5,34,37 Impaired B-cell memory responses (ie, somatic hypermutation and isotype switching) were reported in one patient on experimental immunization.38 Moreover, poorly formed lymphoid follicles and reduced B-cell numbers were reported on inguinal LN biopsies after diphtheria and tetanus vaccine in 2 patients.22 Here, we uncovered a new role for Cxcr4-dependent signaling in the organization of LNs. Nonmanipulated Cxcr4+/1013 mice bred under pathogen-free conditions displayed disorganized LNs, featured by an abnormal expansion of the T-cell zone and the absence of B-cell follicles. This suggests that T- and B-cell contacts are not restricted to anatomically defined structures and thus may interfere with B-cell fate and maturation. Interestingly, follicular dendritic cells ablation leads to disrupted follicular organization and, in the course of immunization, the disappearance of germinal centers and the dispersal and death of associated B cells.39 Immunization of Cxcr4+/1013 mice will help determine whether the gain of Cxcr4 function affects germinal center formation, T- and B-cell maturation, and hence production and trafficking of memory B and plasma cells.
T-cell lymphopenia in WS patients, which was thought to result from an abnormal egress from the thymus or trapping into secondary lymphoid organs, is associated with a marked reduction of circulating naive T cells and TCR excision circles.5 However, here we unraveled that all thymic cell subsets—from progenitors to mature single-positive thymocytes—were reduced in mutant mice, indicating that the gain of Cxcr4 function disturbs thymus homing, development, or expansion of early T-cell progenitors. Also, mice reconstituted with T-cell progenitors expressing a Cxcl12 intrakine that retains Cxcr4 intracellularly display a T-cell lymphopenia and an impaired intrathymic maturation.40 This supports the finding that Cxcr4 contributes to the homing and settling of T-cell progenitors in the thymus.12 Cxcl12 expressed by the thymic epithelium plays a role in the survival, expansion, and subsequent differentiation of CXCR4-expressing early T-lymphoid progenitors.10,11 Analyses of Cxcr4+/1013 mice designate a developmental defect at one of the early double-negative stages of thymocyte maturation as causative in the decreased peripheral T-cell numbers. HPC transfer and immune reconstitution in immunodeficient mice will establish whether the lymphopenia displayed by Cxcr4+/1013 mice results from a hematopoietic cell–intrinsic defect. In the periphery, the situation is probably complex as the spleen and LNs display opposite phenotypes. In the spleen, one can speculate that T-cell reduction reflects either the lymphopenia in the bloodstream or a defective entry of residual circulating T cells through the marginal sinus. By contrast, an increased number of naive, and to a lesser extent of effector/memory, T cells was detected in LNs. Because the HEV network was apparently preserved, we anticipate that the T-cell entry into LNs is not affected in Cxcr4+/1013 mice. However, the abnormal pattern of LYVE-1+ LNLVs suggests an impaired egress as the main mechanism accounting for T-cell accumulation in this organ. The inverse correlation between T cells and LNLVs extends the negative role of T cells in LNLV formation41 and further underscores the dependence of LN organization on proper Cxcr4 activation. Whether disturbance of additional organizers such as the reticular network, which expresses Cxcl12 and others chemokines (ie, Ccl-19 and -21), might contribute to the phenotype of Cxcr4+/1013 mice and ultimately affect T-cell homeostasis will be the focus of future investigations.
Two animal models have analyzed the consequences of the gain of CXCR4 function on neutrophil biology. First, in the zebrafish, expression of a WS-associated CXCR4 induced a peripheral neutropenia concomitant to a normal neutrophil development.42 Second, expressing a WS truncation mutation of CXCR4 into healthy human HPCs enhanced their engraftment into the BM of NOD/SCID mice and thereafter decreased recovery of CD45+ leukocytes from the blood.43 Neither neutrophil aggregation at the sites of high CXCL12 expression in the transgenic zebrafish nor the accumulation of leukocytes in the BM of engrafted mice was associated with a severe cell death.42,43 Cxcr4+/1013 mice, which are the first model mimicking the “homeostatic” coexpression of WT and mutant Cxcr4 receptors of WS patients, unambiguously demonstrate the linkage between the gain of Cxcr4 function and the WS-associated neutropenia. Peripheral neutropenia was found despite normal representation of the different cellular stages of granulocyte development. Moreover, neutrophils exhibited a normal rate of apoptosis in the BM. These observations suggest that proper desensitization of Cxcr4 is not critical for granulopoiesis and clearance of senescent neutrophils under homeostatic conditions proceeds normally in the BM of Cxcr4+/1013 mice. A corollary is that impaired CXCR4 desensitization is unlikely to be the primary cause of the patient-associated myelokathexis. The basis for this phenotype remains to be characterized but could rely on the fact that neutrophil clearance by BM macrophages reported in mice44 might be less robust or differentially regulated in man.45 In vivo studies of aged neutrophils clearance could help to determine the relative contribution of the BM versus spleen and liver in this process. Alteration of additional mechanisms involved in neutrophil retention (ie, Rac2)46 or egress (ie, Cxcr2)47 also can be invoked. Although granulopoiesis was maintained in mutant mice, impaired Cxcr4 desensitization impacted on B- and natural killer cell development, as we found that immature Cxcr4+/1013 natural killer cells abnormally accumulated in the BM parenchyma area.48 Thus, these findings unveil differential requirements for these leukocyte lineages on Cxcr4-mediated signaling.
The first pharmacologic evidence of the causal role of CXCR4 hyperactivity in WS-associated panleukopenia recently came from 2 independent phase 1 clinical trials based on acute administration of AMD3100.32,33 It is unclear from which compartment leukocytes were mobilized, although studies suggested the BM as a possible source. Here, we found that blockade of the gain of Cxcr4 function was effective in releasing leukocytes in the blood. However, even though neutrophils and B cells seemed to be preferentially mobilized from the BM in WT mice, this was apparently not the case for Cxcr4+/1013 mice, suggesting other sources from which leukocytes are mobilized. This interesting difference with regard to treatment responses indicates that Cxcr4+/1013 mice will be useful for dissecting the distinct mechanisms governing the sequestration and mobilization of the different leukocyte subsets. Because management of WS will need chronic treatment aimed to normalize, but not abolish, CXCR4 signaling, these mice set a model for investigation of known CXCR4 antagonists, the long-term safety of which may be questioned (ie, AMD310049,50 ), and for the characterization of alternative new inhibitory compounds. Finally, exploration in Cxcr4+/1013 mice of the mechanisms of leukocyte development and trafficking between lymphoid organs will help to define how proper CXCR4 signaling contributes to these processes under homeostatic conditions and in the course of immune responses.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank F. Arenzana-Seisdedos (Laboratoire de Pathogénie Virale, Institut Pasteur, Paris, France) who encouraged them to perform the mouse mutant line. They are grateful to M. Huerre (Institut Curie, Paris, France) for his invaluable advice, and F. Baleux (Unité de Chimie Organique, Institut Pasteur, Paris, France) for providing CXCL12. They also thank P. Avé (Histopathologie Humaine et Modèles Animaux, Institut Pasteur, Paris, France) for excellent technical assistance in the histologic analysis as well as F. Gaudin and P. Hemon (Plateforme d'Histologie, Institut Paris-Sud d'Innovation Thérapeutique, Châtenay-Malabry, France) and J. Harriague (4Clinics, Paris, France) for editing the manuscript.
This work was supported by the Agence Nationale de la Recherche (ANR; 2010 JCJC 1104 01) and the European Union FP6 (INNOCHEM, LSHB-CT-2005-518167, K.B.); and ANR (ANR-07-MRAR-029), AP-HP, and E-rare (07 E-RARE 013-01, F.B.). K.B., E.B., V.B., L.B.-D., D.E., and F.B. are members of the Laboratory of Excellence in Research on Medication and Innovative Therapeutics supported by a grant from ANR (Investissements d'Avenir). The mouse mutant line was established at the Mouse Clinical Institute (Illkirch, France) in the Targeted Mutagenesis and Transgenesis Department with funds from GIS–Institut des Maladies Rares, Inserm, and the Consortium National de Recherche en Génomique.
Authorship
Contribution: K.B. and F.B. designed research; E.B., V.B., L.B.-D., and E.L. performed research; D.B. provided chalcone 4; K.B., F.B., E.B., V.B., L.B.-D., E.L., O.F., and L.F. analyzed data; O.F. and D.E. contributed analytic tools; E.B., V.B., L.B.-D., D.E., and L.F. edited the manuscript; and K.B. and F.B. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
D.E. is deceased.
Correspondence: Karl Balabanian or Françoise Bachelerie, Inserm UMR S996, 32 rue des Carnets, 92140 Clamart, France; e-mail: karl.balabanian@u-psud.fr or francoise.bachelerie@u-psud.fr.
References
Author notes
E.B., V.B., and L.B.-D. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal