Abstract
In the present study, surface CD1d, which is involved in immune cell interactions, was assessed for effects on hematopoiesis. Mouse BM hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) express CD1d. The numbers and cycling status of HPCs in the BM and spleen of different strains of cd1d−/− mice were enhanced significantly, suggesting that CD1d is a negative regulator of HPCs. In support of this, CD1d was required for the SCF and Flt3 ligand synergistic enhancement of CSF induction of HPC colony formation and for HPC response to myelosuppressive chemokines. Colony formation by immature subsets of HPCs was greatly enhanced when normal, but not cd1d−/−, BM cells were pretreated with CD1d Abs in vitro. These effects required the full CD1d cytoplasmic tail. In contrast, long-term, but not short-term, repopulating HSC engraftment was impaired significantly, an effect that was minimally influenced by the presence of a truncated CD1d cytoplasmic tail. Pretreatment of normal BM cells with CD1d Abs greatly enhanced their engraftment of HSCs. The results of the present study implicate CD1d in a previously unrecognized regulatory role of normal and stressed hematopoiesis.
Introduction
Hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs), which give rise to all blood cells, have been used to practical advantage for treating blood cell systems compromised by genetic disease, cancer, and chemotherapy.1,2 A better understanding of HSC/HPC regulation would enhance HSC/HPC transplantation and disease treatment. Proteins on the surface of HSCs/HPCs, such as Sca1, CD34, CD90, CD150, and CD48, have been used to identify and purify these cells,3 but little is known about the possible roles of these cell-surface markers in regulating HSC/HPC function. MHC class I and II antigens on HSCs/HPCs, which are crucial for matching donor cells with recipients for optimal HSC transplantation, have in only limited situations been linked to responsiveness of HPCs to regulatory cytokines/chemokines.4-6 Based on our previous studies showing a role for both natural killer T (NKT) cells and CD1d7 in hematopoietic effects in mice infected with CMV and linking cells of the immune system to regulation of hematopoiesis,8-12 along with our interest in CD1d-NKT cell inter-actions under normal conditions and in several different disease models,13-15 in the present study, we evaluated the expression of CD1d on HSCs and HPCs. After identifying this expression, we tested the hypothesis that cell-surface expression of CD1d on HSCs and HPCs plays a regulatory role in their proliferation and engraftment. Our findings suggest novel and previously unrecognized roles for CD1d in the regulation of hematopoiesis.
Methods
Mice
Normal C57Bl/6 and Balb/c mice were purchased from The Jackson Laboratory. C57BL/6 CD1d-deficient (CD1d−/−) mice were obtained from 2 sources. The laboratory of Luc van Kaer (Vanderbilt University, Nashville, TN) generated cd1d1−/− mice on the C57BL/6 background.16 Mice deficient in both cd1d1 and cd1d217 (on the C57Bl/6 background) were generated by Stephen Balk (Harvard University, Cambridge, MA) and purchased from The Jackson Laboratory. Therefore, these CD1d−/− mice lines were generated by a different laboratory and the mice are distinct. cd1d−/− mice on a Balb/c background were also obtained from Dr van Kaer. Jα18−/− mice18 were originally generated on a C57BL/6 background by Masaru Taniguchi (Chiba University, Chiba, Japan). These mice were backcrossed 8 times onto a Balb/c background and obtained from Ram Singh (University of California, Los Angeles) with permission from Dr Taniguchi. CD1d−/− mice with a knock-in of a truncated CD1d cytoplasmic tail19 were generated at the University of Chicago (Chicago, IL) and kindly provided by Dr Albert Bendelac. All animal experiments were approved by the Indiana University School of Medicine institutional review board.
Reagents
CSFs, SCF, FL, MIP-1α/CCL3, ENA-78/CXCL5, IL-8/CXCL8, TNF-α, and IFN-γ were purchased from R&D Systems. Pokeweed mitogen mouse spleen cell conditioned medium (PWMSCM) was prepared as described previously.8 α- and β-galactosylceramides (GalCer) were purchased from Enzo Life Sciences. Anti-CD1d Abs were: 1B1,20 1H6,21 1A8,22 1E2,22 6F7,22 and 9G122 and isotype-matched controls were generated as described previously.
HPC colony assays and HSC engrafting studies
The numbers of HPCs per femur or spleen, and the percentage of HPCs in cycle (S phase of the cell cycle) were performed and calculated as described previously.23,24 Competitive and noncompetitive engraftment of lethally irradiated primary recipients and noncompetitive engraftment of lethally irradiated, secondary, congenic mouse recipients were performed as described previously.23,24
Statistics
Statistically significant differences were calculated by a 2-tailed t test, with a P value of at least < .05 considered significant.
Results
CD1d−/− mice under steady-state conditions manifest enhanced HPC numbers and proliferation
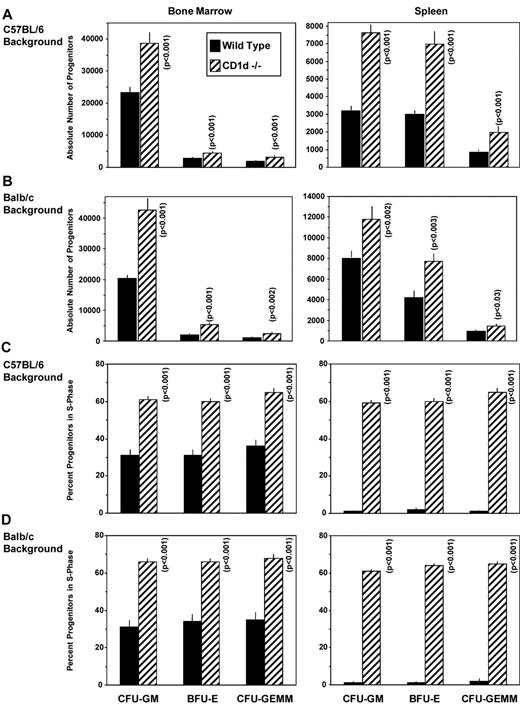
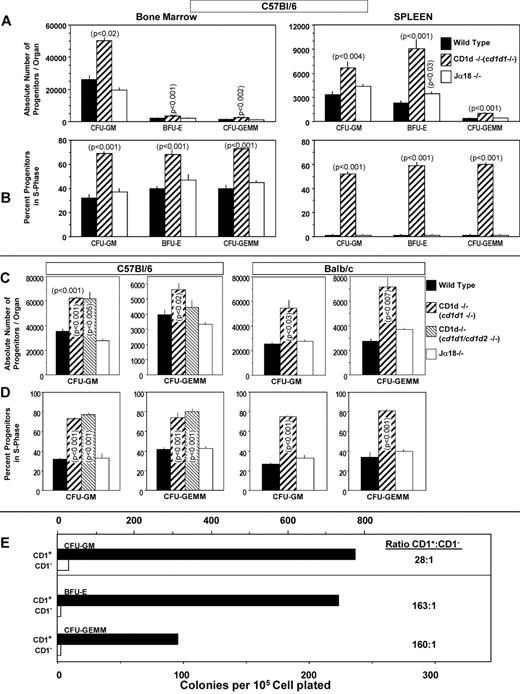
To investigate the possibility that CD1d might be involved in the regulation of hematopoiesis, we analyzed numbers of myeloid progenitor cells and their proliferation status in femoral BM and spleens of CD1d−/− (cd1d1−/−) mice compared with that of control mice. As seen in Figure 1A and B, the absolute numbers of granulocyte-macrophage (CFU-GM), erythroid (BFU-E), and multipotential (CFU-GEMM) progenitors in the BM and spleen of CD1d−/− mice were significantly higher than in the control mice. This observation was reproducible in CD1d−/− mice on either a C57Bl/616 (Figure 1A) or Balb/c (Figure 1B) background and on BM cells from a completely different CD1d-knockout mouse (cd1d1 and cd1d2 deletion17 ) on a C57Bl/6 background (Figure 2C-D), suggesting that the observed effects were not because of mouse strain or genetic CD1d−/− background differences. That this was because of increased proliferation of the myeloid progenitors in BM and spleen is apparent from the results shown in Figure 1C (C57Bl/6 mice), Figure 1D (Balb/c mice), and Figure 2D (C57Bl/6 mice). The percentage of HPCs in the S phase (the DNA-synthesis phase) of the cell cycle, as estimated by the high specific activity tritiated thymidine kill assay,23,24 demonstrates an approximately 2-fold increase in cycling of HPCs in the BM of CD1d−/− mice compared with that in the CD1d+/+ mice. HPCs in the spleens of normal mice are usually in a slow or noncycling state, as was observed herein, and this cycling rate was greatly enhanced in the spleens of CD1d−/− mice for the 2 different mouse strains (Figure 1C-D). That the enhanced hematopoiesis noted at the HPC level in CD1d−/− mice was not a reflection of type 1 NKT cell effects was shown in additional experiments. CD1d−/− mice are deficient in both type I and type II NKT cells.25 However, Jα18−/− mice, which are deficient in type I NKT cells but express normal levels of CD1d, demonstrated normal absolute numbers (Figure 2A) and cycling (Figure 2B) of HPCs in the BM and spleen of C57Bl/6 mice and in the BM of Balb/c mice (Figure 2C-D). Therefore, CD1d expression acts in a negative manner to control proliferation of HPCs in mice, reflecting either direct effects through CD1d on myeloid progenitors and/or indirect effects perhaps mediated through CD1d-expressing accessory cells. However, it does not reflect type I NKT cell activity and instead suggests a role for type II NKT cells or CD1d in the observed effects. We evaluated expression of CD1d on HSCs and HPCs in the BM of CD1d+/+ mice.
Influence of CD1d on hematopoietic progenitor cells in C57B1/6 and Balb/c mice. Absolute numbers of immature subsets of CFU-GM, BFU-E, and CFU-GEMM in BM (femur) and spleens of WT littermate controls (WT = +/+) and CD1d−/− mice on a C57Bl/6 (A) and Balb/c (B) background. Cycling status (percentage of cells in the S phase) of immature subsets of CFU-GM, BFU-E, and CFU-GEMM in the BM (femur) and spleen in WT and CD1d−/− mice on a C57Bl/6 (C) and Balb/c (D) background. Results are expressed as means ± 1SEM for 17 mice each from a total of 4 experiments for C57Bl/6 mice (A and C) and 8 mice each from a total of 2 experiments for Balb/c mice (B and D). Significance values of CD1d−/− compared with WT cells are shown.
Influence of CD1d on hematopoietic progenitor cells in C57B1/6 and Balb/c mice. Absolute numbers of immature subsets of CFU-GM, BFU-E, and CFU-GEMM in BM (femur) and spleens of WT littermate controls (WT = +/+) and CD1d−/− mice on a C57Bl/6 (A) and Balb/c (B) background. Cycling status (percentage of cells in the S phase) of immature subsets of CFU-GM, BFU-E, and CFU-GEMM in the BM (femur) and spleen in WT and CD1d−/− mice on a C57Bl/6 (C) and Balb/c (D) background. Results are expressed as means ± 1SEM for 17 mice each from a total of 4 experiments for C57Bl/6 mice (A and C) and 8 mice each from a total of 2 experiments for Balb/c mice (B and D). Significance values of CD1d−/− compared with WT cells are shown.
Hematopoiesis in CD1d−/− and Jα18−/− mice. Comparative analysis of absolute numbers (A,C) and cycling status (B,D) of HPCs in BM (femur) and spleen of CD1d−/− (cd1d1; C57Bl/6) and Jα18−/− (C57Bl/6) mice and in the BM of CD1d1 (cd1d1 and cd1d2; C57Bl/6)−/− (C-D) and Jα18−/− (Balb/c) mice (C-D) compared with WT mice and sorting of myeloid progenitors mainly into the CD1d+ cell populations (E). Results in panels A and B are expressed as means ± SEM for 5 mice in each group and for 4 mice/group for panels C and D. For panel E, CD1+ or CD1− BM cells were each plated at 5, 2.5, and 1.25 × 104 cells/mL. Results are calculated from 1.25 × 104 CD1+ and 5 × 104 CD1− cells/mL. Note that colony formation of 1.25 × 104 CD1+ plus 1.25 × 104 CD1− cells/mL was equal to the sum of the individual populations alone (data not shown), suggesting that the results were not because of interactions of CD1+and CD1− cells that either enhanced or suppressed colony formation. Significance levels compared with WT controls are shown in panels A through D; others are not significant (P > .05).
Hematopoiesis in CD1d−/− and Jα18−/− mice. Comparative analysis of absolute numbers (A,C) and cycling status (B,D) of HPCs in BM (femur) and spleen of CD1d−/− (cd1d1; C57Bl/6) and Jα18−/− (C57Bl/6) mice and in the BM of CD1d1 (cd1d1 and cd1d2; C57Bl/6)−/− (C-D) and Jα18−/− (Balb/c) mice (C-D) compared with WT mice and sorting of myeloid progenitors mainly into the CD1d+ cell populations (E). Results in panels A and B are expressed as means ± SEM for 5 mice in each group and for 4 mice/group for panels C and D. For panel E, CD1+ or CD1− BM cells were each plated at 5, 2.5, and 1.25 × 104 cells/mL. Results are calculated from 1.25 × 104 CD1+ and 5 × 104 CD1− cells/mL. Note that colony formation of 1.25 × 104 CD1+ plus 1.25 × 104 CD1− cells/mL was equal to the sum of the individual populations alone (data not shown), suggesting that the results were not because of interactions of CD1+and CD1− cells that either enhanced or suppressed colony formation. Significance levels compared with WT controls are shown in panels A through D; others are not significant (P > .05).
CD1d is expressed on phenotypically defined populations of mouse BM HSCs/HPCs and sorted populations of myeloid colony-forming cells
We first assessed the expression of CD1d on highly purified, phenotypically defined populations of mouse BM cells using flow cytometry. More than 61% of BM mononuclear cells isolated from C57Bl/6 mice expressed CD1d. CD1d was expressed on 97.9% of Sca-1+Lin− and on 99.8% of Sca-1+c-kit+Lin− cells (n = 3 experiments). Because all HSCs can be found in these populations, this suggests that CD1d is expressed on all, or essentially all, HSCs. Myeloid progenitor cells are also found in the Sca-1+Lin− population, demonstrating that essentially all HPCs also express CD1d. To determine the expression of CD1d on the functional subsets of myeloid progenitors, mouse BM was sorted into CD1d+ and CD1d− populations of cells. As seen in Figure 2E, most CFU-GM, BFU-E, and CFU-GEMM, defined here as immature subsets of these cells because they were stimulated in vitro by multiple growth factors,2,25 sorted into the CD1d+ population of cells. These results suggest that the effects seen on HPC numbers and cycling status in the CD1d−/− mice (Figures 1A-D and 2A-D) might reflect, at least in part, direct effects involving expression of CD1d on HPCs.
Anti-CD1d stimulation increases numbers of BM myeloid progenitor cell–derived colonies in vitro
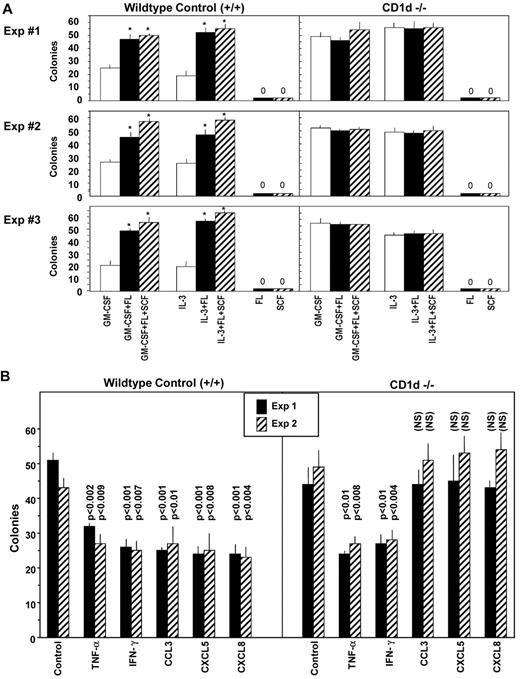
To determine whether blocking CD1d would influence colony formation by myeloid progenitor cells in vitro, unseparated mouse BM cells were preincubated in suspension culture with control medium, isotype control Abs, or CD1d-specific Abs, and the cells were plated in methylcellulose culture medium in the presence of erythropoietin, PWMSCM, SCF, and hemin to determine the effects on immature subsets of CFU-GM, BFU-E, or CFU-GEMM or in agar culture medium with GM-CSF, IL-3, or M-CSF for the respective assessment of mature subsets of GM-CSF–responsive CFU-GM, IL-3–responsive CFU-GM, or M-CSF–responsive macrophage progenitors (CFU-M). As shown in Figure 3A, treatment of unseparated BM cells in vitro with a CD1d-specific (1H6) Ab significantly increased the numbers of cytokine-stimulated immature CFU-GM–, BFU-E– and CFU-GEMM–derived colonies, as well as more mature populations of CFU-GM– and CFU-M–derived colonies. This anti-CD1d–mediated enhancement in the proliferation of immature subsets of CFU-GM, BFU-E, and CFU-GEMM (Figure 3A) and mature subsets of CFU-GM and CFU-M (data not shown) was seen with control (CD1d+/+) BM cells, but not with BM cells from CD1d−/− mice (Figure 3B). No colonies of any type formed in the absence of CSFs or other growth factors, regardless of whether anti-CD1d Abs were present (data not shown). The proliferation-enhancing effects of several different CD1d-specific Abs on BM CFU-GM from both wild-type (WT) C57Bl/6 and Balb/c mice, but not on C57Bl/6 or Balb/c CD1d−/− cells, are shown in Figure 3C. The anti-CD1d Abs enhanced colony formation of HPCs from Jα18−/− mice, which express CD1d but lack type I NKT cells (Figure 3C). Therefore, type I NKT cells are not (and perhaps type II NKT cells are) involved in this enhancing effect. As additional specificity controls, neither Sca1 Ab nor MHC class I– or class II–specific Abs influenced colony formation under the same conditions used for the anti-CD1d Abs (data not shown). That the anti-CD1d enhancement is likely through CD1d on the myeloid progenitors themselves is suggested by information presented in Figure 3D. Treatment of Sca1+Lin− cells at low cell-plating concentrations (250 cells/mL) with anti-CD1d manifested the same level of enhancement in colony numbers (Figure 3D) as when unseparated cells were plated with anti-CD1d at much higher cell concentrations (5 × 104 cells/mL; Figure 3A-C).
Influence of CD1 Abs on colony formation in vitro by hematopoietic progenitor cells. Immature subsets of CFU-GM, BFU-E, and CFU-GEMM from unseparated mouse BM cells were stimulated by a combination of cytokines and mature subsets of CFU-GM and CFU-M were stimulated with single cytokines from normal (A-C) and CD1d−/− cells (B-C) in unseparated BM plated at 5 × 104 cells/mL or in Sca1+Lin− cells plated at 250 cells/mL (D). Colonies were scored after 7 days of incubation. Results are expressed as the means ± SD of 3 plates/point. For panel A, the asterisk designates P < .001 compared with cells treated with control medium. Isotype Ab control values were not significantly different from the control group (P > .05). Significance values are shown for panel B. (C) Significant differences compared with each specific isotype control: aP < .001, bP < .002, cP < .004, and dP < .01. Other differences are not significant (P > .05).
Influence of CD1 Abs on colony formation in vitro by hematopoietic progenitor cells. Immature subsets of CFU-GM, BFU-E, and CFU-GEMM from unseparated mouse BM cells were stimulated by a combination of cytokines and mature subsets of CFU-GM and CFU-M were stimulated with single cytokines from normal (A-C) and CD1d−/− cells (B-C) in unseparated BM plated at 5 × 104 cells/mL or in Sca1+Lin− cells plated at 250 cells/mL (D). Colonies were scored after 7 days of incubation. Results are expressed as the means ± SD of 3 plates/point. For panel A, the asterisk designates P < .001 compared with cells treated with control medium. Isotype Ab control values were not significantly different from the control group (P > .05). Significance values are shown for panel B. (C) Significant differences compared with each specific isotype control: aP < .001, bP < .002, cP < .004, and dP < .01. Other differences are not significant (P > .05).
Colony formation of BM myeloid progenitor cells is not influenced by αGalCer
CD1d molecules are similar to MHC class I molecules based on structure and noncovalent association with β2-microglobulin, although their intracellular trafficking is similar to MHC class II molecules.21,26-29 There are 2 groups of CD1 molecules, and most CD1 molecules possess a tyrosine-based endosomal-targeting motif that allows intracellular trafficking of CD1 molecules to compartments used by MHC class II molecules.21 CD1d comprises the group II CD1 molecules.21,27 NKT cells play important roles in various immune responses, as assessed in autoimmune models, various infectious diseases, and cancer.27,30,31 Antigen presentation by CD1d molecules is essential for NKT cell activity in these disorders. Although there have been reports of actual natural ligands in very low abundance presented to NKT cells by CD1d molecules,21,32,33 a synthetic glycolipid, α-GalCer, likely mimics the probable natural ligands.34 Cytokines are important in hematopoietic and immune regulation,2,35 and stimulation of CD1d+ cells with αGalCer enhances production of both Th1 and Th2 cytokines by NKT cells.30,36 Th1 and Th2 cytokines can directly and indirectly influence the proliferation of myeloid progenitor cells.8-11 Therefore, we were interested in determining whether stimulation of cells with the CD1d-binding glycolipid αGalCer would influence colony formation of myeloid progenitor cells. To determine whether αGalCer would stimulate, enhance, or inhibit colony formation by HPCs, BM cells from control (CD1d+/+) mice were plated in vitro for colony formation by immature subset populations of CFU-GM, BFU-E, and CFU-GEMM (stimulated in methylcellulose by erythropoietin, PWMSCM, SCF, and hemin), by immature populations of CFU-GM and CFU-M (stimulated in agar by GM-CSF, IL-3, or M-CSF, each in the presence of SCF), by more mature populations of CFU-GM or CFU-M (respectively stimulated in agar by only GM-CSF, IL-3, or M-CSF), or BM cells plated without any growth factors. As seen in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article), neither αGalCer nor the control βGalCer enhanced or suppressed colony formation in vitro by immature or mature subsets of myeloid progenitor cells. Moreover, neither αGalCer nor βGalCer stimulated colony formation by any of these progenitor cells in the absence of exogenously added growth factors (no colonies formed with or without only αGalCer or βGalCer; data not shown). In addition, neither αGalCer nor βGalCer influenced colony formation by CFU-GM in a sorted population of 250 purified mouse BM Sca1+Lin− cells per mL stimulated by PWMSCM and SCF (24 ± 5, 23 ± 1, and 23 ± 2 colonies, respectively, for control medium, αGalCer, and βGalCer). Therefore, neither a CD1d-binding glycolipid, αGalCer, nor βGalCer stimulated or enhanced cytokine-stimulated colony formation by myeloid progenitor cells in vitro. However, this does not rule out the possibility that αGalCer may influence hematopoiesis indirectly in vivo through CD1d-mediated NKT cell production of cytokines, as has been suggested previously.36
Neither FL nor SCF enhance colony formation in vitro of CD1d−/− BM myeloid progenitor cells
FL and SCF are potent costimulating molecules that act through their respective tyrosine kinase receptors, Flt3 and C-kit.2,35,37-39 These receptors and ligand-receptor interactions are important components of hematopoiesis.2,35 In the process of evaluating colony numbers from control (CD1d+/+) and CD1d−/− BM cells that were stimulated in vitro with either a single cytokine (GM-CSF, IL-3, FL, or SCF) or with GM-CSF or IL-3 each in combination with either FL or SCF, we observed that the usual synergism in colony numbers apparent when either FL or SCF was used along with a colony-stimulating factor for CD1d+/+ BM cells (Figure 4A left panels) was not apparent when CD1d−/− BM cells were assayed (Figure 4A right panels). Colony numbers for CFU-GM in CD1d−/− BM cells that were stimulated with either GM-CSF or IL-3 were already increased in frequency (number of colonies formed per number of BM cells plated) compared with those in the CD1d+/+ BM (Figure 4A right vs left panels). Colony numbers in CD1d−/− BM stimulated by either GM-CSF or IL-3 were not further enhanced by the addition of either FL or SCF to GM-CSF or IL-3. Therefore, numbers of single cytokine-response CFU-GM are elevated on a frequency basis in CD1d−/− compared with CD1d+/+ BM, but CD1d−/− CFU-GM do not respond to the usual costimulating/enhancing effects of FL or SCF.
Influence of costimulating factors FLT3 ligand (FL) and SCF and negative regulators on colony formation by CFU-GM from BM of WT control (CD1d+/+) and CD1d−/− mice on a C57Bl/6 background. (A) Co-stimulating factors, and (B) negative regulators. The average results ± SEM from 3 separate experiments each for CD1d+/+ and CD1d−/− mice are shown. In panel A, asterisk designates significant differences of GM-CSF or IL-3 in combination with SCF and/or FL compared with either GM-CSF– or IL-3 alone–stimulated culture, P < .003. Colony formation for panel B was stimulated by erythropoietin (Epo), PWMSCM, SCF, and hemin, and cells plated in the absence and presence of 10 ng/mL of TNF-α, 10 ng/mL of IFN-γ, or 10 ng/mL of the chemokines MIP-1α/CCL3, ENA-78/CXCL5, or IL-8/CXCL8. NS indicates not significant (P > .05).
Influence of costimulating factors FLT3 ligand (FL) and SCF and negative regulators on colony formation by CFU-GM from BM of WT control (CD1d+/+) and CD1d−/− mice on a C57Bl/6 background. (A) Co-stimulating factors, and (B) negative regulators. The average results ± SEM from 3 separate experiments each for CD1d+/+ and CD1d−/− mice are shown. In panel A, asterisk designates significant differences of GM-CSF or IL-3 in combination with SCF and/or FL compared with either GM-CSF– or IL-3 alone–stimulated culture, P < .003. Colony formation for panel B was stimulated by erythropoietin (Epo), PWMSCM, SCF, and hemin, and cells plated in the absence and presence of 10 ng/mL of TNF-α, 10 ng/mL of IFN-γ, or 10 ng/mL of the chemokines MIP-1α/CCL3, ENA-78/CXCL5, or IL-8/CXCL8. NS indicates not significant (P > .05).
Myeloid progenitor cells from CD1d−/− mouse BM are sensitive to the myelosuppressive effects of TNF-α and IFN-γ, but not to that of the chemokines CCL3, CXCL5, or CXCL8
Several cytokines, including TNF-α, IFN-γ, and members of the chemokine family, act to suppress proliferation of myeloid progenitor cells in vitro and in vivo.2 Because the suppressive activity of chemokines is manifest on immature subsets of progenitors that are responsive to stimulation by multiple cytokines (including either FL or SCF with a CSF), whereas the suppressive effects of TNF-α and IFN-γ are manifest on the immature as well as mature populations of progenitors, we evaluated the effects of TNF-α, IFN-γ, and the CC and CXC chemokines CCL3, CXCL5, and CXCL8 on in vitro colony formation by myeloid progenitors from BM of control (CD1d+/+) and CD1d−/− mice stimulated with the combination of GM-CSF plus SCF. As shown in Figure 4B left, TNF-α, IFN-γ, CCL3, CXCL5, and CXCL8 each suppressed colony formation significantly by CD1d+/+ CFU-GM. However, of these 5, only TNF-α and IFN-γ suppressed colony formation of the CD1d−/− CFU-GM significantly (Figure 4B right). Therefore, CD1d−/− CFU-GM are not responsive in vitro to the synergistic proliferation effects of a CSF plus a costimulating cytokine such as SCF (Figure 4A), nor to inhibition in vitro by myelosuppressive chemokines CCL3, CXCL5, or CXCL8 (Figure 4B).
Role of cytoplasmic domain of CD1d on HPCs
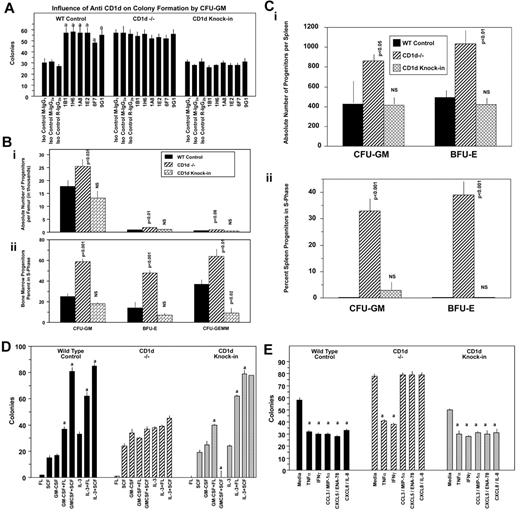
The cytoplasmic domain of CD1d is involved in intracellular signaling mediated through the activation of CD1d,27,40 and multiple defects in antigen presentation and T-cell development are apparent in mice expressing cytoplasmic tail–truncated CD1d.19 These latter effects were identified through the use of mice in which the CD1d cytoplasmic tail deletion was knocked into CD1d−/− mice.19 We assessed a role for the cytoplasmic tail of CD1d, first in CD1d Ab–stimulated CD1d+/+ BM cells. As seen in Figure 5A, treatment of BM cells in vitro with 6 different CD1d Abs enhanced colony formation of CFU-GM from WT (CD1d+/+), but not CD1d−/−, mice stimulated with combinations of cytokines, which is consistent with the results seen in Figure 3. However, truncated CD1d cytoplasmic domain knock-in BM cells (referred to herein as CD1d knock-in) did not respond to CD1d Ab treatment with enhanced colony formation (Figure 5A). This demonstrates that the enhancement of HPC proliferation by CD1d Abs requires the presence of the truncated portion of the cytoplasmic tail of CD1d. However, CD1d knock-in mice were similar to WT control mice under steady-state conditions in terms of absolute numbers (Figure 5Bi) and cycling status (Figure 5Bii) of BM CFU-GM, BFU-E, and CFU-GEMM, in contrast to the enhanced hematopoiesis noted in CD1d−/− mice (Figure 5Bi-ii). The CD1d knock-in mice were also similar to WT control mice in terms of absolute numbers and cycling status of HPCs in the spleen (Figure 5Ci-ii). In addition, BM CFU-GM, BFU-E, and CFU-GEMM from CD1d knock-in mice responded exactly as these progenitors from WT control mice to synergistic stimulation by the combinations of GM-CSF or IL-3 with either FL or SCF (Figure 5D) and to inhibition by selected myelosuppressive chemokines (Figure 5E). The C-terminal CD1d cytoplasmic domain sequence removed for the knock-in mice was SAYQDIR, with only 3 membrane-proximal R's left for the entire intracytoplasmic region after this deletion.19 Therefore, the last 7 amino acids (SAYQDIR) of the cytoplasmic tail of CD1d is necessary for CD1d Ab enhancement of colony formation by CFU-GM, which is likely akin to a CD1d-activated state, but not for in vivo steady-state hematopoiesis (absolute numbers and cycling status of BM and splenic myeloid progenitors) or for responsiveness to synergistic stimulation of the proliferation of the progenitors or their inhibition by suppressive chemokines. This suggests that other intracellular or extracellular factors are involved in mediating abnormalities in the cytokine-chemokine modulation of HPC proliferation noted with the CD1d−/− mice.
Influence of CD1d-truncated cytoplasmic tail on hematopoiesis. Effects of anti-CD1d Abs on colony formation by BM CFU-GM from (WT) control, CD1d−/−, and CD1d-truncated cytoplasmic tail domain knock-in mice stimulated with PWMSCM, SCF, and hemin (A) and comparative hematopoiesis in the BM (B) and spleen (C) of WT control, CD1d−/−, and truncated cytoplasmic tail CD1d knock-in mice. Results for panel A are for 1 representative of 2 similar experiments expressed as means ± SEM. a indicates enhancement of colony formation by different CD1d Abs compared with their isotype control Abs (P < .001); all other comparisons are not significant (P > .05). Results for panels B (BM) and C (spleen) shown are means ± SEM of 3 mice/group for absolute numbers (i) and cycling (ii) of hematopoietic progenitor cells (CFU-GM, BFU-E, and CFU-GEMM). In panels B and C, P values are compared with WT controls. NS indicates not significant (P > .05). (D) Influence of the combinations of GM-CSF or IL-3 with either FL or SCF on colony formation by BM CFU-GM from control, CD1d−/−, or CD1d truncated cytoplasmic domain knock-in mice. (E) Influence of suppressive cytokines/chemokines on colony formation by BM CFU-GM from control, CD1d−/−, or CD1d-truncated cytoplasmic domain knock-in mice. For panel D, a indicates synergistic stimulation of colony formation by the combination of murine GM-CSF (10 ng/mL) or IL-3 (10 ng/mL) with either human FL (100 ng/mL) or murine SCF (50 ng/mL); P ≤ .05. For panel E, a indicates significant suppression of colony formation. Results for panels D and E are for 1 representative of 2 similar experiments ± SEM.
Influence of CD1d-truncated cytoplasmic tail on hematopoiesis. Effects of anti-CD1d Abs on colony formation by BM CFU-GM from (WT) control, CD1d−/−, and CD1d-truncated cytoplasmic tail domain knock-in mice stimulated with PWMSCM, SCF, and hemin (A) and comparative hematopoiesis in the BM (B) and spleen (C) of WT control, CD1d−/−, and truncated cytoplasmic tail CD1d knock-in mice. Results for panel A are for 1 representative of 2 similar experiments expressed as means ± SEM. a indicates enhancement of colony formation by different CD1d Abs compared with their isotype control Abs (P < .001); all other comparisons are not significant (P > .05). Results for panels B (BM) and C (spleen) shown are means ± SEM of 3 mice/group for absolute numbers (i) and cycling (ii) of hematopoietic progenitor cells (CFU-GM, BFU-E, and CFU-GEMM). In panels B and C, P values are compared with WT controls. NS indicates not significant (P > .05). (D) Influence of the combinations of GM-CSF or IL-3 with either FL or SCF on colony formation by BM CFU-GM from control, CD1d−/−, or CD1d truncated cytoplasmic domain knock-in mice. (E) Influence of suppressive cytokines/chemokines on colony formation by BM CFU-GM from control, CD1d−/−, or CD1d-truncated cytoplasmic domain knock-in mice. For panel D, a indicates synergistic stimulation of colony formation by the combination of murine GM-CSF (10 ng/mL) or IL-3 (10 ng/mL) with either human FL (100 ng/mL) or murine SCF (50 ng/mL); P ≤ .05. For panel E, a indicates significant suppression of colony formation. Results for panels D and E are for 1 representative of 2 similar experiments ± SEM.
Influence of CD1d on HSCs
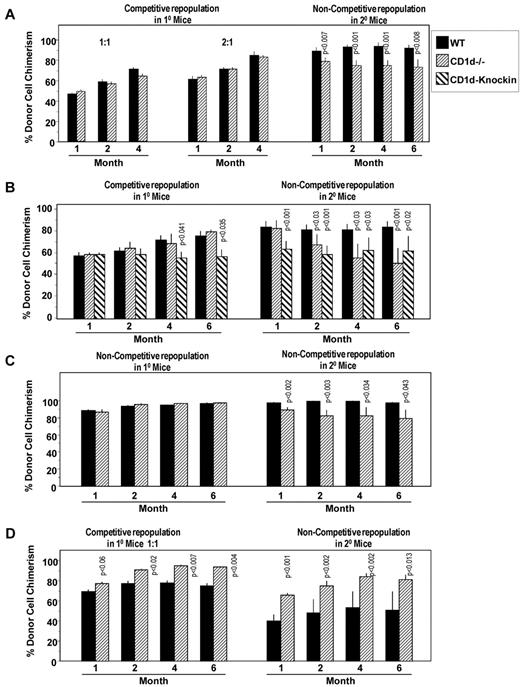
To determine whether CD1d on HSCs might play a role in the engrafting capability of HSCs, we first performed a competitive repopulating assay comparing engraftment of CD1d−/− (CD45.2+) with CD1d+/+ (C57Bl/6, CD45.2+) BM cells with nonirradiated B6.BoyJ (CD45.1+) BM competitor cells, when these cells were transplanted into lethally irradiated B6.BoyJ mice (Figure 6A). Whereas no significant differences in engraftment were detected at 2:1 or 1:1 donor-to-competitor ratios in primary mouse recipients, BM cells taken from primary mice 4 months after mice were transplanted with the 2:1 cell ratio engrafted secondary lethally irradiated mice in a noncompetitive assay, with CD1d−/− cells modestly (approximately 20%), but significantly and reproducibly over the time of the secondary transplantation time course, decreased in secondary mice compared with cells from those primary mice engrafted with CD1d+/+ cells (Figure 6A). Similar results were seen in a second competitive assay (1:1 donor-to-competitor cell ratio; Figure 6B) and in a third experiment in which the primary and secondary mice were both transplanted in a noncompetitive assay (Figure 6C). Interestingly, BM cells from the CD1d knock-in mice with a truncated CD1d cytoplasmic domain behaved almost the same as CD1d−/− cells with decreased repopulation of secondary mice, although CD1d-knock-in cells, in contrast to CD1d−/− cells, also showed some modest decreases in repopulation of primary mice 4-6 months after the transplantation (Figure 6B). Therefore, even HSCs with a CD1d cytoplasmic domain truncation manifested decreased secondary mouse repopulating capacity, suggesting the need for even the truncated CD1d cytoplasmic domain portion for optimal engraftment. The ability of HSCs to repopulate secondary mice is a measure of the long-term repopulating ability and self-renewal capacity of HSCs. Therefore, the results of the present study suggest a modest decreased number and/or self-renewal capacity of long-term-repopulating CD1d−/− HSCs. Because stimulation of CD1d+/+ BM cells with CD1d Abs enhances colony formation of HPCs (Figure 3), we also evaluated the effects of pretreating BM cells in vitro with anti-CD1d or isotype control Ab before assessing the primary and secondary engrafting capability of HSCs in these treated cell preparations. As seen in Figure 6D, pretreatment of HSCs in a mixed population of cells in vitro with anti-CD1d enhanced the engrafting capability of HSCs in the primary mice, an enhancement even more apparent in the secondary in vivo repopulation studies. The results shown in Figure 6 suggest a role for CD1d on long-term-repopulating HSC engraftment and repopulation of lethally irradiated mouse recipients.
Influence of CD1d on hematopoietic stem cells. Comparative engrafting capacities of WT control and CD1d−/− HSCs in lethally irradiated primary mice in a competitive transplantation setting (A,B,D) and a noncompetitive setting (C) without (A-C) and with (D) pretreatment of donor cells with anti-CD1d Ab. All secondary recipient transplantations were done in a noncompetitive setting. Results are expressed as CD45.2+ cell chimerism shown as mean ± SEM with significant differences from WT or untreated cells noted and are based on individual analysis of BM from 3 WT or 3 CD1d−/− mice, each injected intravenously into 5 lethally irradiated recipient B6.BoyJ (CD45.1+) mice along with 5 × 105 nonirradiated B6.BoyJ competitor cells for a total of 15 recipient mice/group (A and B). For secondary transplantations (noncompetitive situation), cells from 9 primary recipients of each group (WT or CD1d−/−) were each injected into 3 lethally irradiated B6.BoyJ mice for a total of 27 recipients per group (A and B). For panel C, 5 × 105 WT or CD1d−/− cells were transplanted as noted in panels A and B in primary B6.BoyJ recipients and secondary transplantations. For panel D, results of CD45.2+ cell chimerism (mean ± SEM) are shown with significant differences noted compared with isotype control Ab–treated cells. 10 × 106 CD45.2+ BM cells were treated with Ab for 30 minutes at 4°C, washed, and injected intravenously at a 1:1 donor-to-nonirradiated (B6.BoyJ, CD45.1+) competitor cell ratio into lethally irradiated B6.BoyJ mice for engraftment analysis of primary mice. Treated donor cells from 3 mice/group were each transplanted intravenously into 5 recipients for a total of 15 recipients/group. For secondary transplantations (noncompetitive situation), cells from 9 primary recipients in each group (control Ab or anti-CD1d treated) were each injected into 3 lethally irradiated B6.BoyJ mice for a total of 27 recipients/group.
Influence of CD1d on hematopoietic stem cells. Comparative engrafting capacities of WT control and CD1d−/− HSCs in lethally irradiated primary mice in a competitive transplantation setting (A,B,D) and a noncompetitive setting (C) without (A-C) and with (D) pretreatment of donor cells with anti-CD1d Ab. All secondary recipient transplantations were done in a noncompetitive setting. Results are expressed as CD45.2+ cell chimerism shown as mean ± SEM with significant differences from WT or untreated cells noted and are based on individual analysis of BM from 3 WT or 3 CD1d−/− mice, each injected intravenously into 5 lethally irradiated recipient B6.BoyJ (CD45.1+) mice along with 5 × 105 nonirradiated B6.BoyJ competitor cells for a total of 15 recipient mice/group (A and B). For secondary transplantations (noncompetitive situation), cells from 9 primary recipients of each group (WT or CD1d−/−) were each injected into 3 lethally irradiated B6.BoyJ mice for a total of 27 recipients per group (A and B). For panel C, 5 × 105 WT or CD1d−/− cells were transplanted as noted in panels A and B in primary B6.BoyJ recipients and secondary transplantations. For panel D, results of CD45.2+ cell chimerism (mean ± SEM) are shown with significant differences noted compared with isotype control Ab–treated cells. 10 × 106 CD45.2+ BM cells were treated with Ab for 30 minutes at 4°C, washed, and injected intravenously at a 1:1 donor-to-nonirradiated (B6.BoyJ, CD45.1+) competitor cell ratio into lethally irradiated B6.BoyJ mice for engraftment analysis of primary mice. Treated donor cells from 3 mice/group were each transplanted intravenously into 5 recipients for a total of 15 recipients/group. For secondary transplantations (noncompetitive situation), cells from 9 primary recipients in each group (control Ab or anti-CD1d treated) were each injected into 3 lethally irradiated B6.BoyJ mice for a total of 27 recipients/group.
Discussion
With our increasing knowledge of the cell-surface markers currently being used to define and distinguish HSCs and HPCs from each other1-3 and from other cells in the BM, there is a paucity of information on what role, if any, these cell-surface markers may play in the regulation of the functions of HSCs and HPCs. In the present study, we not only demonstrated expression of CD1d on HSCs and HPCs, but we also found that this cell-surface protein plays a role in the proliferation of HPCs and the numbers of or engrafting capability of HSCs. That CD1d plays a negative role in HPC proliferation was clear from the enhanced numbers and cell-cycling status of HPCs of CD1d−/− mice from 2 different CD1d gene knockouts (cd1d1 and cd1d2), both on a C57Bl/6 background, and cd1d1−/− mice (also on a Balb/c background), and that different CD1d-specific Abs could enhance numbers of HPCs in an in vitro setting of control, but not CD1d−/−, cells.
Exactly how CD1d mediates these proliferative effects on CD1d on HPCs is not completely clear, but the fact that the in vivo effects of CD1d−/− mice and in vitro effects of anti-CD1d Abs were not manifest when only the truncated form of CD1d was present and the C-terminal sequence SAYQDIR was deleted makes it clear that this truncated portion of the CD1d molecule was critical for the negative role of CD1d on proliferation in steady-state conditions, in vivo, and during CD1d stimulation of cytokine-induced HPC proliferation, the latter of which is akin to an activated or stressed condition. It is known that the cytoplasmic tail of CD1d is important for expression of functional CD1d,20,27,32,40-42 and that cross-linking of CD1d activates CD1d cells, resulting in the production of cytokines.43,44 Whereas CD1d+ cells in the BM can produce cytokines,35 and such cytokines produced may have an effect on hematopoiesis,2,35 it does not seem that the phenomenon we observed is related to changes in cytokine production, at least from type I NKT cells. Jα18−/− mice on either a C57Bl/6 or Balb/c strain background, which are deficient in type I NKT cells but express normal levels of CD1d, behaved as do normal mice with no changes in absolute numbers and cycling status of HPCs in vivo compared with control mice, and Jα18−/− HPCs respond as do HPCs from control mice with regard to synergistic stimulation with a CSF plus a potent costimulating cytokine such as SCF or FL to anti-CD1d Ab stimulation and to negative regulation by selected members of the myelosuppressive chemokine family. This latter effect of chemokine inhibition may in part reflect the need for the presence of cells responsive to cytokine synergy, as well as to the presence or absence of expression of cyclin-dependent kinase inhibitors.45 That anti-CD1d Ab stimulation of HPCs in normal Sca1+Lin− BM occurred at low cell-plating numbers (250 cells/mL) of a population of cells with a highly enriched frequency of HPCs and in the presence of high levels of exogenously added cytokine, and that this effect was similar to that noted when much higher cell-plating concentrations (5 × 104 c/mL) of unseparated BM cells were used, greatly decreases the likelihood that the anti-CD1d stimulation of enhanced colony numbers in vitro was due to CD1d-specific Ab-induced release of cytokines.
Our present results are somewhat disparate from other studies46 regarding hematopoiesis in Jα18−/− mice, in which impaired progenitor cell numbers was noted.46 Whereas it is not possible to determine the reason for these differences, we have reported previously that infection of CD1d−/− and Jα18−/− mice with murine CMV resulted in decreased hematopoiesis.7 Therefore, it is possible, but not yet clear, that the decreased hematopoiesis in the Jα18−/− mice observed by others45 could have been due, at least in part, to a virus infection or other stress-induced effects.
The results of the present study also implicated CD1d expression in the engrafting capability of murine BM HSCs. Three separate experiments, 2 performed in a competitive HSC setting and 1 in a noncompetitive HSC setting, demonstrated little or no differences in CD1d−/−- compared with control CD1d–expressing HSC engraftment into lethally irradiated primary recipients. However, a reproducible decrease in the secondary repopulating capacity of these CD1d−/− cells in the 3 experiments, whether the entire CD1d molecule was absent or if only the truncated CD1d cytoplasmic domain was present, clearly demonstrate that CD1d is necessary for optimal engraftment of longer-term engrafting HSCs as opposed to shorter-term engrafting HSCs or the self-renewal capacity of these longer-term engrafting HSCs. Whereas the engrafting capacity of HSCs is at least a 2-step event, with the first being the process of homing of the HSCs to the appropriate hematopoietic niche in the BM microenvironment,23 it appears unlikely, based on the similar engrafting capabilities of CD1d−/− and control CD1d-expressing cells in primary recipients, that the homing capacity of CD1d−/− HSCs was compromised.
Our present results also demonstrate that, in contrast to the CD1d C-terminal SAYQDIR amino acid sequence role in HPC proliferation, this region was minimally, if at all, involved in the engrafting capability of HSCs. Consistent with the positive effects of CD1d on engraftment of at least long-term repopulating HSCs, activation of HSCs ex vivo with a CD1d-specific Ab modestly increased the primary engrafting capacity of control HSCs, with an even larger effect on enhancement of the HSCs that could repopulate secondary mouse recipients. Because the test population of BM cells was only subjected to anti-CD1d stimulation shortly before infusion of the cells into primary mice, and the cells from primary recipients that were transplanted into secondary recipients were not exposed to CD1d Abs again, the effects of the anti-CD1d Ab stimulation were initiated before transplantation into the primary mouse recipients. Whereas several of the differences seen in the secondary repopulation of CD1d−/− compared with control cells were modest, the reproducibility of these findings and the greater effects noted after CD1d Ab stimulation of control cells indicate that CD1d plays a role in HSC engraftment. How CD1d-mediated engraftment of murine BM HSCs is mediated, whether this information can be used to enhance the engrafting capacity of human HSCs in a transplantation setting, or if knowledge of the negative regulation effects of CD1d on HPC proliferation can be modulated for clinical benefit remains to be determined. The results of the present study of CD1d expression on HSCs/HPCs and its influence on HSC/HPC function provide a new, heretofore unrecognized role for this particular cell-surface molecule that is already well known as an important component of the innate immune response.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Luc Van Kaer, Ram Singh, Masaru Taniguchi, and Albert Bendelac for providing the mice and Jessica Montgomery for help with animal husbandry.
This study was supported by Public Health Service grants RO1 HL05641, RO1 HL67384, and PO1 DK090948 to H.E.B. and RO1 AI46455 and RO1 CA89026 to R.R.B. G.J.R. was supported by National Institutes of Health Training Grant T32 DK007519.
National Institutes of Health
Authorship
Contribution: H.E.B. planned and scored the experiments, supported the study, and wrote the manuscript; K.C. planned, set up, and scored the experiments and helped with manuscript preparation; G.H., S.C., and C.M. set up and scored experiments; G.J.R. planned and set up the experiments; and R.R.B. planned the experiments, supported the study, and helped with manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hal E. Broxmeyer, PhD, Department of Microbiology and Immunology, Indiana University School of Medicine, 950 W Walnut St, R2 Bldg, Rm 302, Indianapolis, IN 46202-5181; e-mail: hbroxmey@iupui.edu.